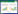扩展功能
文章信息
- Torsten Blanck, 周婷, 李艺, Tomáš Protiva, Paul Crow, Ralph Tiedemann
- Torsten Blanck, ZHOU Ting, LI Yi, Tomáš Protiva, Paul Crow, Ralph Tiedemann
- 越南三线闭壳龟、中国三线闭壳龟和金头闭壳龟的新亚种
- New Subspecies of Cuora cyclornata (Blanck, Mccord & Le, 2006), Cuora trifasciata (Bell, 1825) and Cuora aurocapitata (Luo & Zong, 1988)
- 四川动物, 2017, 36(4): 368-385
- Sichuan Journal of Zoology, 2017, 36(4): 368-385
- 10.11984/j.issn.1000-7083.20160249
-
文章历史
- 收稿日期: 2016-09-16
- 接受日期: 2017-04-26
2. 海南省林业科学研究所, 海口 571100;
3. 惠州李艺金钱龟生态 发展有限公司, 广东惠州 516157;
4. 查尔斯大学动物学系理学部, 布拉格-Vininá 7, 12844布拉格, 捷克共和国;
5. 嘉道理农场&植物园-林锦公路, 大浦, 新界, 香港特别行政区, 中国;
6. 波茨坦大学, 生物和生物化学学院, 进化生物学/系统动物学, 卡尔-李卜克内西街24-25, 豪斯26, 德国波茨坦 14476
2. Hainan Forestry Science Institute, Haikou 571100, China;
3. Huizhou Liyi Golden Coin Turtle Farm Co. Ltd, Huizhou, Guangdong Province 516157, China;
4. Department of Zoology, Faculty of Science, Charles University Prague-ViniČná 7, 12844 Prague 2, Czech Republic;
5. Kadoorie Farm & BotanicGarden-Lam Kam Road, Tai Po, New Territories, Hong Kong SAR, China;
6. University of Potsdam, Institute for Biology andBiochemistry, Unit of Evolutionary Biology/Systematic Zoology, Karl-Liebknecht-Str. 24-25, Haus 26, 14476 Potsdam, Germany
The Asian box turtle genus Cuora (Gray, 1855) includes 13 species, with 6-7 recognized subspecies (TTWG, 2014). All but one species are listed as Critically Endangered by the IUCN, and most of them rank in the World's 25+ Most Endangered Tortoises and Freshwater Turtles (TCC, 2011). Two species, Cuora mccordi and C. zhoui, might already be extirpated in the wild, but all members of the genus are highly threatened by habitat destruction and collection for human consumption and as pets. The genus Cuora includes species that are aquatic as well as some that are essentially terrestrial. They inhabit the rainforests of south and east Asia and range in size from 12-35 cm straight carapace length (SCL).
The taxonomy of this genus has remained controversial (Blanck et al., 2006; Spinks & Shaffer, 2007; Spinks et al., 2009, 2012) despite numerous recent molecular studies. Whereas mitochondrial DNA (mtDNA) studies appeared to provide a sound framework for taxonomic decisions (Spinks et al., 2004; Stuart & Parham, 2004), recent work with a few nuclear single copy loci identified some putative "species" as hybrids (Stuart & Parham, 2007). Taxonomic uncertainty has been particularly apparent for the clade including C. trifasciata sensu lato, which was recently suggested to consist of two species (Blanck et al., 2006), i.e., C. trifasciata sensu stricto (=Clade A; Fig. 1) and C. cyclornata (=Clade C) based on morphological differences and mtDNA sequence divergence. However, the species rank of C. cyclornata has been challenged by others (Artner, 2007, 2008; Fritz & Havas, 2007; Spinks & Shaffer, 2007; Spinks et al., 2009, 2012). Within Clade C (C. cyclornata), Blanck et al. (2006) could clearly differentiate between two divergent lineages both morphologically and by mtDNA, which they described as subspecies, namely C. cyclornata cyclornata and C. cyclornata meieri. The validity of C. cyclornata was genetically reverified by the recent studies of Tiedemann et al. (2014) and Li et al.(2015a, 2015b) where microsatellite markers and mt genome analysis clearly supported its genetic distinctiveness.
|
| Fig. 1 Individually based neighbor-joining tree of Nei's minimum genetic distance for the microsatellite data (Tiedemann et al., 2014) Each individual is represented by a symbol corresponding to its morphological species/subspecies assignment as shown in the legend. Letters & colors correspond to the letters & colors in Fig. 2. |
| |
|
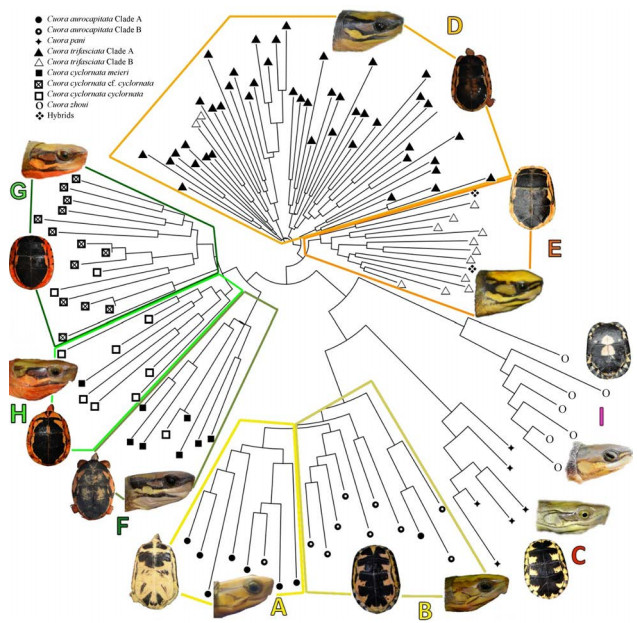
| Fig. 2 Coloration (left to right: plastron in adults, plastron in juveniles, head lateral), distribution, and sample origins of the analyzed Cuora taxa Letters in the map correspond to the letters to the left. |
| |
Furthermore, several specimens of unknown origin, belonging to yet another genetically divergent clade of C. trifasciata sensu lato were identified (i.e. C. trifasciata cf. trifasciata or Clade B, see Fig. 1). Mitochondrial data on this clade-however-may be obscured by the possibility of erroneously containing numts (nuclear-encoded mitochondrial DNA), rendering these data non-informative in this context (Spinks & Shaffer, 2007; Spinks et al., 2009). Even a very recent study (Spinks et al., 2012) that used nuclear markers could not resolve these issues, since the only potential C. cyclornata in their study was the holotype of C. cyclornata as designated by Blanck et al. (2006), for which Spinks et al. (2012) were unable to obtain genetic data. Specimens hypothesized by Spinks and Shaffer (2007), Spinks et al.(2009, 2012) to be of wild Hong Kong origin actually originated from urban areas (Crow, pers. obs.) and were thus very likely from captive origin. In addition, Spinks et al. (2012) hypothesized that C. mccordi might resemble a hybrid between (1) C. trifasciata and (2) C. aurocapitata or C. galbinifrons.
Yet another taxonomic uncertainty concerned the (morphologically) closely related species C. aurocapitata (Luo & Zong, 1988) and C. pani (Song, 1984), which are hypothesized to be morphologically weakly distinguishable (Artner, 2004; Blanck & Tang, 2005), extensively share mitochondrial haplotypes (Parham et al., 2004; Spinks et al., 2004), and might potentially interbreed in an intergradation zone in western Anhui or Hubei province (Blanck & Tang, 2005).
These many uncertainties were addressed with high resolution microsatellite markers by Tiedmann et al.(2014). That study showed that C. cyclornata is genetically distinct from C. trifasciata and that C. mccordi and C. zhoui are not of hybrid origin as speculated by Spinks et al. (2012) but are also genetically distinct. The genetic results of Tiedemann et al. (2014) and Li et al.(2015a, 2015b) agree perfectly with the available morphometric and colorimetric traits. In addition, that microsatellite study not only revalidated C. cyclornata but also showed that a potential third distinct group might exist. It clarified that specimens of C. trifasciata assigned to Clade B by Blanck et al. (2006) indeed represent a distinct genetic lineage clearly originating from Hainan Island. Given these date, it remains to be evaluated whether available mtDNA data are actually "numts" as hypothesized by Spinks and Shaffer (2007), Spinks et al.(2009, 2012) or whether they constitute authentic mitochondrial sequences. If the latter were true, this would further corroborate the distinctiveness of Clade B. Finally, the microsatellite study also revealed two genetically distinct populations of C. aurocapitata that are colorimetrically and geographically distinct (Blanck & Tang, 2005).
Blanck et al. (2006) also noticed additional variation within C. cyclornata, and considered what they called possible intergrades between C. c. cyclornata and C. c. meieri. During the past nine years since the description of C. cyclornata, the authors of this paper have studied hundreds of specimens representing all of the different forms of C. cyclornata and C. trifasciata. Despite the rarity of these turtles in nature, wild-caught specimens with data collected by locals together with the genetic studies of Tiedemann et al.(2014) indicate that this third variety of C. cyclornata is genetically more closely related to C. c. cyclornata than to C. c. meieri. It seems not to be a hybrid/intergrade, but rather its own ESU (evolutionary significant unit).
Blanck et al. (2006) reported that the Hainan population of C. trifasciata differed in certain morphological characters from the mainland populations, but they lacked mtDNA data from known locality Hainan specimens. Whereas Spinks and Shaffer (2007), Spinks et al.(2009, 2012) hypothesized that this clade was based on numts, the data provided by Tiedemann et al. (2014) clearly indicated that Hainan specimens are represented in Clade B and that they are divergent from Clade A on the mainland.
Using molecular techniques in the study of evolutionary histories has resulted in a gradual abandonment of morphological characters as the primary source of phylogenetic inference. However, morphological characters are valuable for phylogenetic reconstruction, especially for tracking adaptive changes across phylogeographic groups defined by genetic markers. With molecular techniques still evolving, critically endangered, morphologically identifiable ESU's could disappear before our techniques finally confirm them to be unique (as in the case with C. cyclornata). While current genetics indicate only a minor divergence between C. c. cyclornata and C. cf. cyclornata (Tiedemann et al., 2014), morphometric, colorimetric and geographic differentiation is evident. Thus, it is important, especially for conservation efforts, to verify if this variety of C. cyclornata warrants subspecific designation.
Mayr and Ashlock (1991) stated that a subspecies is an aggregate of phenotypically similar populations of a species inhabiting a geographic subdivision of the range of that species and differing taxonomically from other populations of that species. In the sense of Mayr (1963) reproductive isolation means isolation by intrinsic mechanisms and not just by geographical barriers. Cracraft (1989) proposed that even a single fixed character difference may define a geographic form as a separate species since he sees them as separately evolving metapopulation lineages according to the general lineage concept or minimum diagnosable units.
Vieites et al. (2009) came up with the concept of distinguishing between Unconfirmed Candidate Species (UCS), Confirmed Candidate Species (CCS) and Deep Conspecific Lineages (DCL). UCS lineages can be distinguished by molecular characters but cannot be confirmed by any other means. CCS lineages show detectable genetic differentiation plus distinctiveness characters based on Mayr (1963). DCL lineages show none or only slight differences in characters that mediate a reproductive barrier or species discrimination and/or indications of hybridisation with other species. Miralles et al. (2011) suggest using three different lines of evidence when following this concept. Following this approach, subspecies status applies if a candidate species qualifies for only one line of evidence and species status if a candidate species qualifies for two or all lines (i.e. 75%-100%) of evidence. Hawlitschek et al. (2012) discussed all these different views in detail and came to the conclusion that taxonomic inflation in species can be problematic. According to him, describing species purely based on a personal and subjective interpretation of "existence as a separately evolving metapopulation lineage", "minimum diagnosable unit" or lineage with its "own evolutionary tendencies and historical fate" can lead to very divergent species counts, the same problem for which the subspecies concept is now widely rejected. While species are given most attention in evolutionary studies, species lists and for conservation purposes (Phillimore & Owens, 2006), describing every minimum diagnosable unit as a species, or elevating such subspecies to species level, bears the risk that diagnosable units no longer representing separately evolving entities. Species, in our opinion, have to represent real evolutionary entities, though. In our view, subspecies are evolutionary significant units. Even though they may eventually interbreed with other subspecies of the same species, such gene flow is sufficiently small to let them follow different evolutionary trajectories, often associated with local adaptation. Therefore, subspecies (if identified) warrant conservation.
When following the concept of Miralles et al. (2011) and considering four important characters for turtles, i.e., geographic isolation, genetic divergence, morphometric and colorimetric differences for which a minimum of 75% have to be fulfilled for species level, we come to the following conclusion: C. trifasciata cf. trifasciata (Clade B) shows significant genetic divergence (Tiedemann et al., 2014), is geograpically well isolated, and morphologically distinct to a certain extend, which is however not sufficient to warrant species status in our opinion. Thus, in our view this Clade qualifies for subspecific status. For C. c. cf. cyclornata genetic divergence is sufficient for subspecific ranking but not near species level, colorimetric differences are significant but morphometric characters and geographic isolation are not as significant. Thus, this taxon also qualifies as a subspecies. C. aurocapitata Clade B yet shows sufficient genetic divergence for subspecific differentiation, and morphometric as well as colorimetric characters are significantly different (although its geographic status needs further evaluation) and it also qualifies as a subspecies.
Since the subspecies concept is still commonly and widely accepted in turtles (TTWG, 2014), we suggest that the three discussed taxa are best classified as subspecies.
While the concept of subspecies is controversial as can be seen above, and has been neglected in many species concepts other than the biological species concept, there is certainly utility in its application for conservation as reviewed by Haig et al. (2006). The only quantitative metric they found for defining a subspecies was the 75% rule (Amadon, 1949; Patten & Unitt, 2002); i.e., 75% of the individuals of one subspecies must be distinguishable from those from the other.
Our data suggest that the three taxa discussed above comply with this rule.This leads us to the conclusion that three new subspecies of three Cuora species are in need of description.
1 Materials & Methods 1.1 MaterialsAltogether 365 carapaces/323 plastra of adult primarily wild-caught specimens of seven Cuora species, namely the aquatic to semiaquatic species of this genus, were used in this study (15/31 C. aurocapitata Clade A, 11/23 C. aurocapitata Clade B, 8/8 C. aurocapitata intermediates, 40/43 C. c. cyclornata, 18/21 C. c. meieri, 23/24 C. c. cf. cyclornata, 45/51 C. mccordi, 26/25 C. pani, 77/31 C. trifasciata A, 55/22 C. trifasciata B, 23/22 C. yunnanensis, and 24/22 C. zhoui). Data from a carapace or plastron with abnormalities were discarded as were those where the plastron was in a closed condition. Nearly a quarter of the specimens were also genetically analyzed by the Agriculture, Fisheries and Conservation Department of Hong Kong (AFCD) Project at Hong Kong University and Tiedemann et al. (2014).
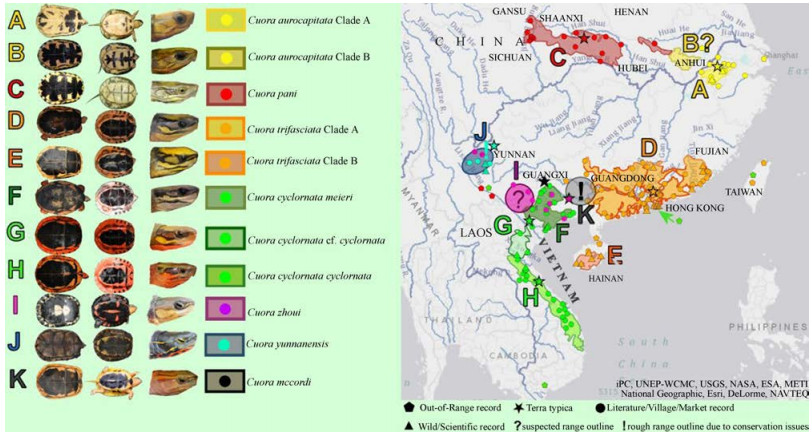
1.2 Geometric morphometric methodsMid-line straight carapace length (SCL in mm) was measured in all possible specimens with calipers to the nearest mm. Digital images of the carapace and the plastrons were obtained for each specimen. Following the classification of Bookstein (1997), 17 type 1 anatomical landmarks were established on the plastron and 25 type 1 and one of type 3 landmarks were established on the carapace (Fig. 3). These were recorded using TPSdig (Rohlf, 2008). Each set was then symmetrized across the axis of symmetry and one half was removed using BigFix6 (Sheets, 2003). Statistical examination was then performed on the remaining half of landmark sets. To remove the effects of position, orientation, and scale, using sets of X, Y coordinates of landmarks from each specimen, we employed the Procrustes superimposition method (Zelditch et al., 2004) performed in CoordGen6 (Sheets, 2003). To remove the effects of size (only in separate analysis of plastron and carapace shape in C. trifasciata Clades A and B, C. c. cyclornata, C. c. meieri and C. c. cf. cylornata) and sex (in all analysis) we used standardization on mean carapace length or sex (for each species and sex, separately) applied in the program Standard6 (Sheets, 2003). Partial warp scores for further statistical analysis were generated using PCAGen6 (Sheets, 2003). Differences in shell shape among all species were tested and visualized in the program Statistica 6 (Weiß, 2007).
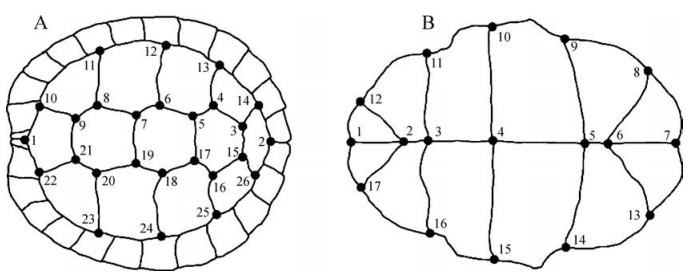
|
| Fig. 3 Dorsal (A) and ventral (B) view of a shell showing the landmarks placement on the carapace and plastron in this study |
| |
The microsatellite data of Tiedemann et al. (2014) were reanalyzed using the software PCAGEN 1.2, which performs principal component analysis (PCA) on gene frequency data.
1.4 Nomenclatural actsThe article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The LSID (Life Science Identifier) for this publication is: urn:lsid:zoobank.org:pub:EB370CB0-B26A-4579-89B8-72F1A56762E2.The electronic edition of this work was published in a journal with an ISSN, and has been archived.
2 ResultsDiscriminant analyses of the shell shape differences in the C. cyclornata and C. trifasciata complex (with both sex and size removed) were significant both for carapace (Wilks' Lambda=0.13, F(100, 720)=4.76, P < 0.000 1) and plastron (Wilks' Lambda=0.07, F(84, 421)=4.82, P < 0.000 1). We found significant differences in the shape of the carapace and plastron among all species and potential subspecies (Table 1). Discrimination was successful for 80.90% in carapace and 74.17% in plastron (Discrimination rate for each species in Table 2). Differences among species are demonstrated in the CVA for carapace (Fig. 4) and plastron (Fig. 5).
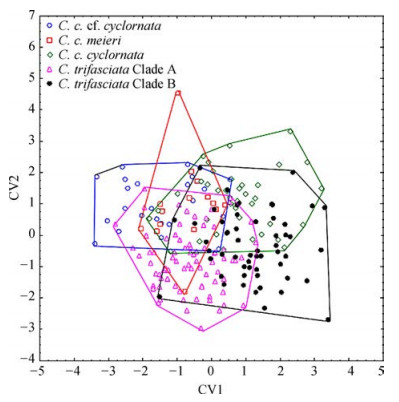
|
| Fig. 4 Canonical plot for carapace shape in Cuora cyclornata, C. trifasciata and their subspecies Each carapace is represented by a symbol as shown in the legend. Polygons enclose most divergent individuals of each species. X % of the total variation is accounted for by the first canonical variable; Y % by the second. Plastron (Fig. 5). First two axis account for 35.25% and 26.07% of the total variation. |
| |
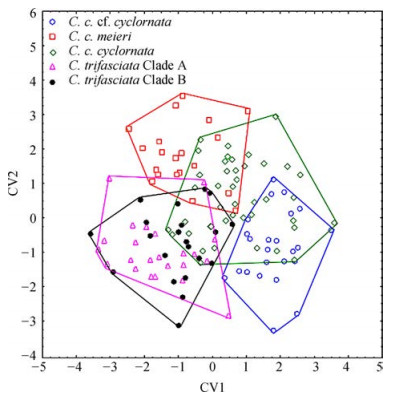
|
| Fig. 5 Canonical plot for plastron shape in Cuora cyclornata, C. trifasciata, their subspecies Each carapace is represented by a symbol as shown in the legend. Polygons enclose most divergent individuals of each species. X % of the total variation is accounted for by the first canonical variable; Y % by the second. First two axis account for 39.31% and 26.62% of the total variation. |
| |
| Carapace/plastron | C. c. meieri | C. c. cyclornata | C. trifasciata Clade A | C. trifasciata Clade B |
| C. c.cf. cyclornata | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 |
| C. c. meieri | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 | |
| C. c. cyclornata | < 0.000 1/ < 0.000 1 | < 0.000 1/ < 0.000 1 | ||
| C. trifasciata Clade A | < 0.000 1/0.006 7 |
| Carapace/plastron | Percent/% | C. c.cf. cyclornata | C. c. meieri | C. c. cyclornata | C. trifasciata Clade A | C. trifasciata Clade B |
| C. c.cf. cyclornata | 82.61/83.33 | 19/20 | 0/0 | 0/3 | 2/0 | 2/1 |
| C. c. meieri | 86.67/85.00 | 1/0 | 13/17 | 0/2 | 0/0 | 1/1 |
| C. c. cyclornata | 75.00/79.07 | 1/0 | 0/2 | 30/34 | 5/5 | 4/2 |
| C. trifasciata Clade A | 87.01/78.26 | 1/0 | 2/1 | 4/1 | 67/18 | 3/3 |
| C. trifasciata Clade B | 80.00/80.95 | 1/0 | 1/1 | 2/1 | 7/2 | 44/17 |
| Total | 82.38/80.92 | 23/20 | 16/21 | 36/41 | 81/25 | 54/24 |
| Note: Observed classifications are in rows, predicted classifications are in columns. | ||||||
Discriminant function analysis of the shell shape differences among all Cuora species (size was not removed) was significant both for carapace (Wilks' Lambda=0.006, F(150, 1887)=17.88, P < 0.000 1) and plastron (Wilks' Lambda=0.004, F(225, 2637)=10.93, P < 0.000 1). We found significant differences in the shape of the carapace and plastron among all taxa (P < 0.000 1; Table 1). Discrimination was successful for 85.25% of specimens based on carapace and for 86.83% based on plastron (discrimination rate for each species in Table 3). Differences among species are demonstrated in the Canonical plots for carapace (Fig. 6) and plastron (Fig. 7).

|
| Fig. 6 Canonical plot for carapace shape in all seven Cuora species Each carapace is represented by a symbol as shown in the legend.X% of the total variation is accounted for by the first canonical variable; Y% by the second. First two axis account for 49.82% and 26.72% of the total variation. First two axis account for 47.07% and 24.26% of the total variation. |
| |

|
| Fig. 7 Canonical plots for plastron shape in all seven Cuora species Each carapace is represented by a symbol as shown in the legend.X% of the total variation is accounted for by the first canonical variable; Y% by the second. |
| |
| Carapace/plastron | Percent/% | C. aurocapitata | C. cyclornata | C. mccordi | C. pani | C. trifasciata | C. yunnanensis | C. zhoui |
| C. aurocapitata | 94.12/85.48 | 32/53 | 0/0 | 0/2 | 2/7 | 0/0 | 0/0 | 0/0 |
| C. cyclornata | 77.94/83.33 | 0/1 | 53/70 | 0/0 | 0/0 | 15/13 | 0/0 | 0/0 |
| C. mccordi | 100/96.08 | 0/0 | 0/0 | 45/49 | 0/0 | 0/2 | 0/0 | 0/0 |
| C. pani | 92.31/88.00 | 0/2 | 0/1 | 0/0 | 24/22 | 1/0 | 1/0 | 0/0 |
| C. trifasciata | 88.64/79.25 | 0/0 | 12/10 | 2/0 | 1/0 | 117/42 | 0/1 | 0/0 |
| C. yunnanensis | 100/100 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 23/22 | 0/0 |
| C. zhoui | 100/86.36 | 0/0 | 0/0 | 0/2 | 0/0 | 0/0 | 0/1 | 24/19 |
| Total | 90.34/86.83 | 32/56 | 65/81 | 47/53 | 27/29 | 133/57 | 24/24 | 24/19 |
| Note: Observed classifications are in rows, predicted classifications are in columns. | ||||||||
Discriminant analysis of the shell shape differences in C. aurocapitata and C. pani was significant for both carapace (Wilks' Lambda=0. 043, F(50, 50)=3.80, P < 0.000 1) and plastron (Wilks' Lambda=0.21, F(42, 112)=3.15, P < 0.000 1). We found significant differences in both the shape of the carapace and plastron (Table 3) among all species and subspecies. Disc-rimination was successful on 96.15% in carapace and 82.28% in plastron. Differences among species are demonstrated in the Canonical plot for carapace (Fig. 8) and plastron (Fig. 9).

|
| Fig. 8 Canonical plot for carapace shape in Cuora aurocapitata and C. pani Each carapace is represented by a symbol as shown in the legend. X% of the total variation is accounted for by the first canonical variable; Y% by the second. First two axis account for 77.09% and 22.91% of the total variation. |
| |

|
| Fig. 9 Canonical plot for plastron shape in Cuora aurocapitata and C. pani Each plastron is represented by a symbol as shown in the legend. X% of the total variation is accounted for by the first canonical variable; Y% by the second. First two axis account for 74.5% and 25.5% of the total variation. |
| |
A principal component analysis (PCA) of the entire microsatellite data of Tiedemann et al. (2014) shows distinction in almost complete agreement with morphologica l assignments, pointing towards a strong genetic signal confirming the species delimitation (Fig. 10). If the putatively polytypic species are analyzed in separate PCAs, there is confirmation for two morphologically defined types in C. trifasciata (Fig. 11; with a single mismatching specimen), for three types in C. cyclornata (Fig. 12), and for two types in C. aurocapitata(Fig. 13). In essence, with the exception of a single specimen, the species-specific PCAs on microsatellite data provide a perfect match with the morphological assignments.

|
| Fig. 10 PCA of the specimens studied in Tiedemann et al., 2014 |
| |

|
| Fig. 11 PCA of the two Cuora trifasciata clades from Tiedemann et al., 2014 |
| |

|
| Fig. 12 PCA of the three Cuora cyclornata clades from Tiedemann et al., 2014 |
| |
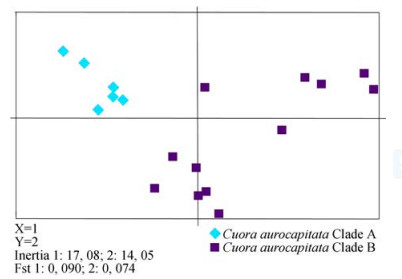
|
| Fig. 13 PCA of the two Cuora aurocapitata clades from Tiedemann et al., 2014 |
| |
The molecular data by Tiedemann et al. (2014, Fig. 1), the multivariate analysis of the morphometric data in this study, the PCA data, plus our colorimetric analyses (see below) clearly provide evidence that C. cyclornata is a distinct species and comprised of3 subspecies, while C. trifasciata and C. aurocapitata are each comprised of two subspecies, and that C. aurocapitata and C. pani are not as closely related as previously believed (Artner, 2004; Parham et al., 2004; Spinks et al., 2004; Blanck & Tang, 2005).
As it can be observed in Fig. 1, for some specimens morphological and genetic data do not match. This could be caused by multiple factors. First, Fig. 1 is based on a neighbor-joining (NJ) algorithm, rather than a model-based program such as multiple likelihood or Bayesian analysis. Second, intergradation zones frequently occur between turtle subspecies as goes introgression between species (Fritz et al., 2002). Based upon our morphological data the herein described subspecies show a convincing level of distinction, even though full reciprocal monophyly of the proposed subspecies is not evident in the NJ analysis.
While the subspecies of C. cyclornata and C. trifasciata are distinct clusters in the NJ, this is not the case for C. aurocapitata, in which Clade A is not confined to a single cluster, while Clade B is. Further phylogenetic analysis of more samples could resolve this issue more completely. A subspecies in our opinion (see also Phillimore & Owens, 2006) cannot necessarily be expected to comprise a genetically monophyletic clade, as different subspecies may overlap in their distribution, allowing for reciprocal introgression.
Monophyly with regard to microsatellite gene genealogies is generally difficult (or even impossible) to establish, for a combination of reasons inherent to this type of data:
●Under the infinite-allele model, monophyly at a microsatellite locus could only be assumed if (1) all individuals of any taxon share the same allele (i.e., they are fixed for this allele) and (2) this allele is only found in this taxon. This situation is rarely encountered within sexually reproducing species.
●Under the stepwise-mutation model, monophyly at a microsatellite locus could only be assumed if (1) all individuals of any taxon share the same allele range (i.e., all alleles are within this length range) and (2) this allele range is non-overlapping with the allele ranges of any other taxon.
●Both models assume that alleles of the same size are identical by descent, in other words, there is no homoplasy. This assumption is rarely met for real microsatellite loci (and not either under the stepwise-mutation model).
This is the reason why applications of microsatellites are (1) restricted to closely related species/subspecies and (2) microsatellite trees are typically based on distance measures (like NJ trees). Optimality-criterion based approaches (Maximum Likelihood, Maximum Parsimony) are-to our best knowledge-not directly applied to microsatellite data in the established and widespread phylogenetic software. In principle, one could specify a mutation model for the microsatellites (stepwise mutation model) and incorporate this into distance estimations (e.g., RST). In phylogenetic contexts, these measures have been found to perform worse than those based on the infinite allele model (see Richard & Thorpe, 2001). This is likely due to the invalid assumption of homoplasy-free data. In addition, this approach is also distance based.
In essence, our NJ tree on microsatellites appears to be the established approach to infer a signal of divergence from microsatellite data. This can be interpreted in the context of taxonomic hypotheses, but does not provide phylogenetical information about plesio-or apomorphy, due to the inherent limitation of distance-based trees and the mutation mode of microsatellites. The NJ tree is pretty well resolved and congruent with morphology, although, as correctly noted, it does not verify monophyly.
Besides the evidence presented in our NJ tree, we consider the congruence between genetic, morphological, and geographic data in the structure assignment included in Tiedemann et al. 2014 are particularly relevant. Indeed, this software uses a Maximum Likelihood approach to identify clusters, within which there is Hardy Weinberg equilibrium (HWE). HWE is indicative of random mating, while departure from HWE is indicative-among other reasons-of lack of interbreeding.
Note that our structure clusters are solely based on the genetic data, not a priori assignment to taxa or locations. Nonetheless, they perfectly match both phenotypes and locations. Note also that we had hardly any evidence for interbreeding among these clusters.
In fact, these structure clusters comprise genetically, phenotypically, and geographically distinct units, within which random mating is inferred, but among which genetic exchange is extremely limited or absent.
If one adopts the idea of defining genetically, phenotypically, and geographically distinct units as subspecies, we present evidence to do so in some species of Cuora.
In any case, the morphological characters are clearly sufficient to diagnose the subspecies at the 75% threshold.Given this 75% rule as stated above, it is not surprising that each subspecies is not perfectly reciprocally monophyletic in this tree. When combining the morphologic, structure, PCA and genetic data we provide above, subspecies are well supported. We herein describe a new subspecies in each of C. cyclornata, C. trifasciata and C. aurocapitata.
2.1 Cuora cyclornata annamitica ssp. nov.ZooBank LSID: urn:lsid:zoobank.org:act: E454BADB-4CA9-46F1-A0BF-6A58CD568BCA
Holotype: Vinh University Museum (VUM) R.Em.04 a sub-adult male (Fig. 14) from Vietnam, Nghe An province, Tan Ky district, near Ky Son village, 146 mm SCL, collected in July 1986 by Prof. Hoang Xuan Quang & Ngyen Tat So'n near a stream.

|
| Fig. 14 VUM R.Em.04, the holotype of Cuora cyclornata annamitica in lateral (A), dorsal (B) and ventral (C) view (Photos by Hoang Ngoc Thao) |
| |
Diagnosis: A large subspecies of C. cyclornata with a maximum SCL of 350 mm, distinguished from the other subspecies by the combination of the following characters: 1) chin amber orange, lateral head yellowish-orange (both orange in the nominate subspecies, and white in C. c. meieri, and yellow to cream in C. trifasciata); 2) predominantly to fully black plastral fore lobe, closely resembling the fully black one in C. c. meieri (never so in C. c. cyclornata where less than 40% of the humeral scute width is covered by black); 3) black pattern on gular scutes interconnected sometimes with a yellow spot along the intergular seam (black pattern always well separated in C. c. cyclornata; always fully connected without any yellow in C. c. meieri); 4) strongly reduced black pattern on the head (similar to C. c. cyclornata); 5) The black vertebral stripe often exhibiting arrow-like thickened areas (which are usually present in C. c. cyclornata too, but usually to an even stronger extent, always absent in C. c. meieri that also shows much finer stripes); and 6) a rather flat-domed, dorsally oval to rounded carapace (rather similar to that of C. c. cyclornata, less rounded in C. c. meieri).
Large males reach 18-26 cm SCL, females 22-35 cm SCL.C. c. cyclornata reaches similar sizes, while in C. c. meieri males are 17-24 cm and females 20-30 cm SCL.
Description of holotype:Semi-adult male specimen with a straight carapace length of 146.02 mm and a maximum carapace width of 104.38 mm at M6/7. The carapace color is light brown and seems slightly faded; the three black carapacial stripes have faded to dark brown but are well developed and the vertebral stripes show an arrow shape on the hind part of vertebrals three to five. No sign of dark spots or diagonal stripes are present on the lateral scutes. Plastron pattern is faded to dark brown. The fore lobe of the plastron is mainly black, the humerals are covered by about 95% black with a small yellow stripe along the front, and a small yellow blotch is present on the intergular seam. The head nearly completely lacks black pigmentation and the light brownish posterior head-stripes are connected to the dorsal olive-yellow head; chin color appears pinkish.
Derivatio nominis: The subspecies name annamitica (from Annam) refers to the distribution of this subspecies within the Annamite mountain range of central Vietnam and adjacent Laos.
Distribution: Cuora c. annamitica ssp. nov. is currently only known by specimens from Vietnam but almost certainly occurs in adjacent Laos (Bolikhamsai, Khammuan provinces) in the Annamite mountain range. In Vietnam it is known to occur in Thanh Hoa (photographic evidence), Nghe An (voucher specimens), Ha Tinh (photographic evidence, Fig. 15) and northern Quang Binh (photographic evidence) provinces. Current data indicate that it occurs northwards to the delta of the Red River. The greater Ca River basin combined with the high mountain ridge in Nghe An province seem to form the subspecies boundary between C. c. annamitica and C. c. cyclornata and likely prevents the species from extending into much of adjacent Laos. Further fieldwork is required to define the range boundaries of C. c. meieri, C. c. annamitica and C. c. cyclornata more precisely.

|
| Fig. 15 Adult female Cuora cyclornata annamitica from Huong Khe district, Ha Tinh province (Photo by Mai Van Que) |
| |
Remarks: Captive breeding has shown that the color characteristics are generally inherited (Blanck pers. obs., Meier pers. comm.) and stable in the offspring in all subspecies. In C. c. annamitica the amount of (black) pattern on the plastron fore lobe can be triggered with incubation temperature, similar to the head pattern on Graptemys sp. (Meier pers. comm., Vogt, 1978, 1993). Specimens incubated at temperatures above 29 ℃ showed a plastral pattern resembling that of C. c. cyclornata while specimens incubated at lower temperatures show the typical plastral pattern of C. c. annamitica as described above. The plastral forelobe in specimens with the C. c. cyclornata pattern darken with age and become predominantly black at an age of about five years. This variation caused by incubation temperature has not been observed in either C. c. cyclornata or C. c. meieri, and thus seems to be unique for C. c. annamitica. The chin coloration is not significantly affected by dietary components such as carotenoids and even remains unchanged in specimens that are in captivity since decades (Meier pers. comm., Blanck pers. obs.), the same is true for captive bred specimens, even if they are raised on a diet low in carotenoids. When hybridizing C. c. cyclornata andC. c. annamitica with each other, 50% of the offspring resemble C. c. cyclornata and 50% C. c. annamitica. The plastral pattern in these specimens stays constant and does not darken in the specimens resembling C. c. cyclornata.
2.2 Cuora trifasciata luteocephala ssp. nov.ZooBank LSID: urn:lsid:zoobank.org:act: 6252307C-37E7-4959-87C0-D69A81202612
Holotype: Chengdu Institute of Biology (CIB) 64III5279 a nearly adult female (Fig. 16) from China, Hainan province, Dan county, 300 m elevation, 152 mm SCL, collected 18 May 1964 by Prof. Ermi Zhao in a stream.
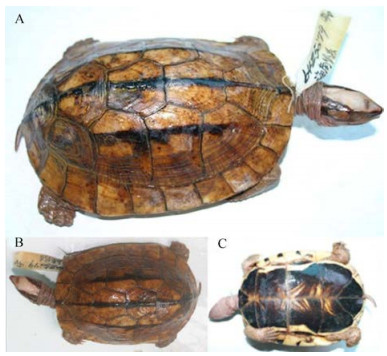
|
| Fig. 16 CIB64III5279, the holotype of Cuora trifasciata luteocephala in (A) dorsolateral, (B) dorsal and (C) ventral view (Photo A & B by Zhou Ting, photo C by Hou Mian) |
| |
Paratypes: American Museum of Natural History (AMNH) 30126-30153, all from Nodoa (Danzhou), Hainan collected by Pope 1922/1923; FMNH6614-6621 same as AMNH origin; Museum of Comparative Zoology Harvard (MCZ) 20689 same as AMNH; Museum of Vertebrate Zoology, Berkeley (MVZ) 23931 from Kachek (Qionghai), Hainan, collected by Gressit on 06 August 1935; MVZ23932 from Dwa Bi (Tai Pin), Hainan collected by Gressit on 24 July 1935; CIB64III6083 & CIB64III6845 from Lingshui Li aut. County, Hainan collected by Zhao in 1964; and CIB64III6532 & CIB64III6533 from the same locality, data and collector as the holotype.
Diagnosis: A large subspecies of C. trifasciata with a maximum SCL of 26 cm, distinguished from its congeners by the combination of the following characters: 1) a fine dark brown to grayish spotted pattern (usually forming fine darkish diagonal strips on the costal and vertebral scutes) is present on the otherwise brown carapace (also appearing in juvenile nominate specimens which apparently lose this pattern with age; never occurring in C. cyclornata ); 2) usually fine blackish, often slightly radiating black diagonal stripes extending from the dorso-posterior part of the costal scute, always connected to the dorsolateral longitudinal black stripe on each side, to the bottom front third of the costal scute (occasionally also occurs in mainland specimens, but these usually exhibit a much wider non-radiating stripe; C. cyclornata does not exhibit this pattern); 3) an intense yellow dorsal head pattern and a yellow chin [nominate specimens usually have a duller straw yellow pattern sometimes with an olive sheen and always with a darker (olive brownish) pattern at the tip of the nose; the chin in nominate specimens is cream-white to yellow, milder than in C. t. luteocephala; the dorsal head pattern of C. cyclornata is citrine-brownish and the chin is white (C. c. meieri) or orange]; 4) the amount of black pattern on the head is well developed, with most specimens exhibiting a pronounced V shaped dorsal yellow head pattern bordered by black (usually less developed in the nominate subspecies); 5) the limbs and soft parts are orange to pinkish with varying intensity in both subspecies, but in C. t. luteocephala they can occasionally also be yellow, a pattern uniquely occurring in this subspecies; 6) the humerals are mainly black with a yellow anterolateral border along the periphery, involving approximately 15% of the scute's lateral border forming a dent-like shape (nominate specimens can exhibit a rather similar pattern, where a fine yellow stripe is formed along the anterolateral area of the humeral scutes, but the dent-like shape is not formed, rather a longitudinal stripe; nominate specimens often also show an entirely black humeral scute); 7) males usually exhibit a darker brown ground color of the carapace than females (not yet observed in the nominate subspecies); 8) Maximum carapace width occurs at marginal 7/8-8 in females, at M8-M8/9 in males, and at M8 in juveniles (M7-7/8 in females and M7/8-8 in males in the nominate subspecies); 9) adult males tend to have a flared posterior carapace (not present in mainland specimens); 10) carapace dorsally more elongated (pear-like shape in the nominate subspecies and also more highly domed with a steeper decreasing angle laterally).
Large males are 18-23 cm SCL, females 20-26 cm SCL; in the nominate subspecies males are 15-21 cm SCL and females 16-23 cm SCL.
Description of holotype: CIB64III5279 is a moderately large female, 152 mm in straight carapace length. The maximum carapace width is 109 mm at marginal scute 7. Maximum carapace height 56 mm. Plastron length 153 mm. The carapace is light brown with the typical dark brownish fine spotted pattern on each carapace scute. The three black longitudinal stripes are fully developed. The two dorsolateral black stripes extend from the first to the fourth costal scutes. A finely radiated diagonal black stripe is present on costal one and two. The black pattern on the dorsolateral head is well developed and forms a V shape. The plastron shows some yellow radiating patches along the central seams. The humeral scutes are 98% black with a fine anterior yellow patch present.
Derivatio nominis: Derived from the latin word "luteus" meaning golden-yellow and the greek "κεφαλη (cephale)" meaning head; i.e., golden-yellow head, referring to the intensely golden-yellow head pattern.
Distribution:Cuora t. luteocephala is currently only known from the mountainous areas on Hainan Island, China. It might also (have) occur(ed) in southeastern Guangxi Zhuang Autonomous Region, China, but no specimen of C. trifasciata has been seen in the area since the early 1980's. Previously Mell (1938) mentioned that specimens from south of the West (Xi) River of the Pearl River system were different from specimens to the north. Two C. trifasciata (MNHN9102 & 9103) in the MNHN collected from Macao, China by Fontanier in 1850, presumably from a market and at the same time, represent both subspecies as was shown by Tiedemann et al. (2014). Since at that time the species was only traded from the mainland (Zhao, pers. comm.) it strongly indicates the occurrence of C. t. luteocephala south of the Xi River. The Hainan Turtle fauna is often regarded as comprising intermediate varieties of species/subspecies from Vietnam and southern China, as has been suggested for C. galbinifrons (Bourret, 1939) by Lehr et al. (1998), based on market specimens. According to Blanck (2013), C. galbinifrons on Hainan is very similar to mainland specimens occurring in North Vietnam, northern Laos and southwest Guangxi, China, and is by no means intermediate to C. bourreti (Obst & Reimann, 1994) as hypothesized by Lehr et al. (1998). This was also shown by genetic studies (Stuart & Parham, 2004). Shi et al. (2008) found a similar pattern with the genus Sacalia; specimens from Hainan were genetically similar to specimens from eastern Guangxi Zhuang Autonomous Region and southwestern Guangdong province. This is likely also true for Hainan C. trifasciata. In general the distributional pattern of the genus Sacalia and its genetic variation reflect the distribution pattern of C. trifasciata and C. cyclornata which are sympatric with Sacalia sp. across their ranges. The effects of the last ice age in Asia need to be evaluated further in order to understand the complex distributional patterns of East Asian turtle species. Hainan currently seems to harbor a general gene pool of several turtle species/subspecies that also occurred in the now heavily depleted areas of southern Guangdong province and Guangxi Zhuang Autonomous Region, China. Hainan was connected to the Chinese mainland during the last ice age until about 17 000 years ago (Voris, 2000). The connection to Vietnam was disconnected many times during the last ice age, generally ended earlier, and furthermore, it was intersected by the Red River paleo drainage basin, a potential barrier (Voris, 2000). Thus, it is far more plausible that most of the Hainan fauna dispersed to the island from the adjacent Chinese mainland.
Remarks: C. t. trifasciata and C. t. luteocephala are not as easy to differentiate on morphometric and colorimetric grounds as are the different subspecies of C. cyclornata, despite them being genetically further separated (making this new subspecies slightly cryptic). This has also been described in other species/subspecies. For example, Emys trinacris (Fritz et al., 2005, 2006) and E. orbicularis which are morphologically nearly indistinguishable, but are genetically distinct at the species level. While the pattern is very constant within C. t. luteocephala, the nominate subspecies can exhibit luteocephala-like traits in some specimens; however, the majority are easy to differentiate based upon the given diagnosis, especially when combining several of the diagnostic characters. It seems possible that the nominate subspecies still harbors a wide morphologic variability lacking in the Hainan population. This seems especially true in Hong Kong (compare Blanck et al., 2006) where specimens are more often quite similar to Hainan specimens in color (but not morphometrics and size) than on the remaining mainland. Still, Hong Kong animals are genetically clearly assignable to the nominate subspecies. In order to distinguish the subspecies at least three of the above mentioned diagnostic characteristics should be present to assign specimens morphologically.
2.3 Cuora aurocapitata dabieshani ssp. nov.ZooBank LSID: urn:lsid:zoobank.org:act: E4A9421F-475D-4C31-B9EA-B841897E987E
Holotype: Natural History Museum of Vienna (NMW) 32987: 2-a large male (Fig. 17) from Anhui province, China. 110.4 mm SCL, donated by Heinz Weissinger in 1993.

|
| Fig. 17 NMW32987: 2, the holotype of Cuora aurocapitata dabieshani in (A) lateral, (B) dorsal and (C) ventral view |
| |
Diagnosis: A large subspecies of C. aurocapitata with a maximum SCL of 195 mm, distinguished from the nominate subspecies by the combination of the following characters: 1) the carapace color pattern differs from the nominate subspecies in that vertebral and costal scutes 2 to 4 are dark brown to greyish black (olive-brown to orange-reddish, usually with a black line along the seams, in the nominate subspecies); carapace ground color generally darker than in the nominate subspecies; 2) the plastral pattern forms a interconnected black pattern closely resembling that of C. pani but differentiated from it by the general lack of the black intergular stripe always present in C. pani (Blanck & Tang, 2005), while the nominate subspecies exhibits a black pattern consisting of irregularly shaped and not fully interconnected black stripes and blotches commonly referred to by Chinese as a "bamboo pattern" (i.e., resembling bamboo leaves); 3) the carapace of C. a. dabieshani is slightly more domed than in the nominate subspecies; 4) the head pattern usually shows some brown areas and more greyish-black stripes than in the nominate subspecies, which can often exhibit a nearly completely golden yellow head; and 5) each ventral marginal scute with a triangular black spot often interconnecting to a black band on M2-M6/7 but with single triangles on all remaining scutes (in the nominate subspecies no triangles are present, some show a faint black stripe on some posterior marginals, but a black band extending from M2 to M6/7 is always present in this subspecies).
Large males are 11-13.5 cm SCL, females 14-19.5 cm SCL; males of the nominate subspecies are10-12.5 cm and females 13-16 cm SCL.
Description of holotype: NMW 32987:2; A large male with a SCL of 110.4 mm, a maximum carapace width of 74.5 mm and a plastron length of 102.1 mm. The carapace is dark greyish-black with dark reddish-brown spots on vertebrals 2 and 3 as well as costals 2. The horn-colored plastron shows a black bar along each scute seam except the intergular seam. The black pattern along the interhumeral seam is very thin compared to all other black bars. A black triangular spot is present on each marginal scute. Head yellowish with a darker blotch behind the eyes surrounded by fine black stripes.
Derivatio nominis: The subspecies name dabieshani (from Dabie Shan) refers to the distribution of this subspecies within the Dabie Shan Mountain range in Anhui province, China.
3 DistributionThis subspecies seems to be restricted to the Dabie Shan Mountain range extending north of the Yangtze River in Anhui province and into adjacent Henan and Hubei provinces. The adjacent Tongbai Shan in the north is inhabited by C. pani (Huang & Huang, 2011; Blanck pers. obs.). Detailed distribution records are absent so far and require further surveys. The nominate subspecies is confined to the Jiuhua Shan and Huang Shan Mountain ranges south of the Yangtze River in China's Anhui province.
Remarks: C. aurocapitata is functionally extinct in the wild despite estimates by Zhang & Wu(2005, 2006) that about 400 specimens could remain in the wild. Blanck and Zhang surveyed the last known habitats of this species in the Jiuhua Shan range in 2013 and were unable to find any natural habitat remaining. The rivers and streams have been heavily impacted by sand mining and dam building, etc. A few scattered individuals seem to remain but a viable population is no longer existent. The status in the Dabie Shan range has not really been assessed yet. As was reported recently, the morphometrically and colorimetrically closely related but genetically well-separated C. pani is not only restricted to the Qin Ling Mountain range as it was long believed (see Blanck & Tang, 2005), but also occurs in the Tongbai Shan range which borders directly to the Dabie Shan in the north (Huang & Huang, 2011; Blanck pers. obs.). Hence C. pani may possibly occur in northern Dabie Shan, and an intergradation zone with C. aurocapitata might occur. The plastral pattern differences between the two subspecies of C. aurocapitata are not temperature nor sex induced based on captive breeding efforts (Meier, pers. comm.), but the "bamboo" pattern of the nominate subspecies is colorimetrically dominant when the two subspecies are hybridized in captivity.
4 Final RemarksThe description of these three new subspecies has a critical impact on the conservation of these three already critically endangered species and should act as a basis for the management of studbooks for these taxa and potential future release projects. To our knowledge a large majority of specimens maintained in studbooks are already being maintained and bred following the patterns of color differentiation already recognized many years ago (Meier, 1997; Blanck & Tang, 2005; Blanck et al., 2006) and finally described herein. However many facilities and private keepers still mix specimens of the different subspecies producing offspring that are unsuitable for potential future release programs and also form a major danger to studbooks if such specimens are not correctly identified and thus included into such breeding programs. First steps are already in preparation for in-situ release projects and it is of highest importance that only 100% pure specimens are released into their native habitats where still a few lonely survivors might have escaped human collection. This subspecies description further clarifies the variability within the genus Cuora and provides a key for identifying specimens of unknown provenance, as it is very common with East Asian turtle species that, due to their rarity in the wild, originate from the pet or food trade without any further data.
Acknowledgements: We thank Zoo Blijdorp Rotterdam, Allwetterzoo Münster, Gerardo Garcia (Chester Zoo), William P. McCord, Herbert Becker, Elmar Meier (IZS), Martina Raffel, Thomas Ziegler & Bernd Marcordes (Zoo Köln), Roger Bour (MNHN), Richard Gemel (NMW), Wolfgang Böhme (ZFMK), Henri Cap (MNHT), Malgosia Nowak-Kemp (OUM), Peter Valentin, Minh Le (CRES), Tim McCormack, Thu Thuy, Pham van Thong and
| Amadon D. 1949. The seventy-five per cent rule for subspecies[J]. The Condor , 51 : 250–258. DOI:10.2307/1364805 |
| Artner HG. 2004. Haltung und Nachzucht von Pan's Scharnierschildkr te Cuora pani pani Song, 1984 und der Goldkopf-Scharnierschildkr te Cuora pani aurocapitata Luo & Zong, 1988[J]. Emys , 11(1) : 4–21. |
| Artner HG. 2007. Keeping and breeding of the Chinese three-striped box turtle Cuora trifasciata (Bell, 1825)[J]. Emys , 14(4) : 4–21. |
| Artner HG. 2008. The world's extant turtle species, Part 1[J]. Emys , 15(3) : 4–32. |
| Bell T. 1825. A monograph of the tortoises having a moveable sternum, with remarks on their arrangement and affinities[J]. Zoological Journal , 2 : 299–310. |
| Blanck T. 2013. Zu Besuch bei Cuora galbinifrons auf Hainan, China[J]. Reptilia (Münster) , 18(99) : 100–109. |
| Blanck T, Mccord WP, Le M. 2006. On the variability of Cuora trifasciata (B, 1825); the rediscovery of the type specimen, with descriptions of a new Cuora species and subspecies, and remarks on the distribution, habitat and vulnerability of these species (Reptilia: Testudines: Geoemydidae)[M]. Frankfurt, Edition Chimaira, Contributions to Natural History, (31): 155. |
| Blanck T, Tang M. 2005. Ein neuer Fundort von Cuora pani Song, 1984 mit Diskussion über den taxonomischen Status von Cuora pani und Cuora aurocapitata[J]. Sacalia , 7 : 16–37. |
| Bookstein FL. 1997. Morphometric tools for landmark data:geometry and biology[M]. New York: Cambridge University Press: 455 . |
| Bourret R. 1939. Notes herpétologiques sur l'Indochine française. XVⅢ. Reptiles et batraciens reçus au Laboratoire des Sciences Naturelle de l'Université au cours de l'année 1939. Descriptions de quatre espéces et d'une variété nouvelles[J]. Hanoi, Bulletin Général de l'Instruction Publique, 19(4. Décembre): 5-39. |
| Cracraft J. 1989. Speciation and its ontology: The empirical consequences of alternative species concepts for understanding patterns and processes of differentiation[M]//Otte D, Endler JA (Eds. ) Speciation and its Consequences. Sunderland, MA, Sinauer Associates: 28-59. |
| Fritz U, D'Angelo S, Pennisi MG, et al. 2006. Variation of Sicilian pond turtles, Emys trinacris What makes a species cryptic?[J]. Amphibia-Reptilia , 27(4) : 513–529. DOI:10.1163/156853806778877095 |
| Fritz U, Fattizzo T, Guicking D, et al. 2005. A new cryptic species of pond turtle from southern Italy, the hottest spot in the range of the genus Emys (Reptilia, Testudines, Emydidae)[J]. Zoologica Scripta , 34(4) : 351–371. DOI:10.1111/zsc.2005.34.issue-4 |
| Fritz U, Havas P. 2007. Checklist of chelonians of the world[J]. Vertebrate Zoology , 57(2) : 149–368. |
| Fritz U, Ziegler T, Herrmann HW, et al. 2002. Intergradation between subspecies of Cuora galbinifrons Bourret, 1939 and Pyxidea mouhotii (Gray, 1862) in southern north Vietnam (Reptilia:Testudines:Geoemydidae)[J]. Faunistische Abhandlungen, Staatliches Museum für Tierkunde, Dresden , 23 : 59–74. |
| Gray JE. 1855. Catalogue of shield reptiles in the collection of the British museum. Part Ⅰ. Testudinata (Tortoises)[M]. London: British Museum: 79. |
| Haig SM, Beever EA, Chambers SM, et al. 2006. Taxonomic considerations in listing subspecies under the U.S.[J]. Endangered Species Act:Conservation Biology , 20(6) : 1584–1594. |
| Hawlitschek O, Nagy ZT, Glaw F. 2012. Island evolution and systematic revision of comoran snakes:why and when subspecies still make sense[J]. Public Library of Science (PLoS) ONE , 7(8) : 1–19. |
| Huang B, Huang Y. 2011. A new record of distribution in Henan province:Cuora pani Song[J]. Journal of Xinyang Normal University (Natural Science Edition) , 1 : 71–72. |
| Lehr E, Fritz U, Obst FJ. 1998. Die Unterarten von Cuora galbinifrons Bourret, 1939[J]. Zoologische Abhandlungen Museum für Tierkunde Dresden , 50 : 77–97. |
| Li W, Zhang XC, Zhao J. 2015b. Complete mitochondrial genome of Cuora trifasciata (Chinese three-striped box turtle), and a comparative analysis with other box turtles[J]. Gene , 555(2) : 169–177. DOI:10.1016/j.gene.2014.10.060 |
| Li W, Zhao J, Shi Y. 2015a. The complete mitochondrial genome of the critically endangered Vietnamese three-striped box turtle (Testudines:Geoemydidae)[J]. Mitochondrial DNA , 26(6) : 925. DOI:10.3109/19401736.2013.863293 |
| Luo B, Zong Y. 1988. A new species of Cuora-Cuora aurocapitata[J]. Acta Herpetologica Sinica, Beijing , 3 : 13–16. |
| Mayr E. 1963. Animal species and evolution[M]. Cambridge, MA:Harvard University Press:273. |
| Mayr E, Ashlock PD. 1991. Principles of systematic zoology[M]. New York: McGraw-Hill: 416 . |
| Meier E. 1997. Eine Methode zur Zucht aggressiver und streßempfindlicher Wasserschildkr ten, exemplarisch dargestellt an der Moorschildkr te Clemmys muhlenbergii (Schoepff, 1801) und der Dreistreifen-Scharnierschildkr te Cuora trifasciata (Bell, 1825)[M]//Artner H, Meier E (Eds. ). Schildkr ten. Münster, Natur und Tier-Verlag: 184: 53-68. (English Title: A method to breed aggressive and easily stressed turtles species, examples used are the Bog turtle Clemmys muhlenbergii and the Chinese three-striped box turtle Cuora trifasciata) |
| Mell R. 1938. Beiträge zur Fauna Sinica. Ⅵ. Aus der Biologie chinesischer Schildkr ten[J]. Archiv für Naturgeschichte, N. F. , 7(3): 390-475. (English Title: Contributions to the Fauna Sinica. Ⅵ The biology of Chinese turtles) |
| Miralles A, Vasconcelos R, Perera A, et al. 2011. An integrative taxonomic revision of the Cape Verdean skinks (Squamata, Scincidae)[J]. Zoologica Scripta , 40(1) : 16–44. DOI:10.1111/zsc.2010.40.issue-1 |
| Obst FJ, Reimann M. 1994. Bemerkenswerte Variabilität bei Cuora galbinifrons Bourret, 1939, mit Beschreibung einer neuen geographischen Unterart: Cuora galbinifrons bourreti subsp. nov. (Reptilia: Testudines: Bataguridae)[J]. Zoologische Abhandlungen, Museum für Tierkunde, Dresden, 48(7): 125-138. (English Title: Notable variability within Cuora galbinifrons, with the description of a new geographic subspecies: Cuora galbinifrons bourreti) |
| Parham JF, Stuart BL, Bour R, et al. 2004. Evolutionary distinctiveness of the extinct Yunnan box turtle (Cuora yunnanensis) revealed by DNA from an old museum specimen[J]. Proceedings of the Royal Society of Biology (Supplement), Biological Letters, London , 271 : 391–394. DOI:10.1098/rsbl.2004.0217 |
| Patten MA, Unitt P. 2002. Diagnosability versus mean differences of sage sparrow subspecies[J]. The Auk , 119 : 26–35. DOI:10.1642/0004-8038(2002)119[0026:DVMDOS]2.0.CO;2 |
| Phillimore AB, Owens IPF. 2006. Are subspecies useful in evolutionary and conservation biology?[J]. Are subspecies useful in evolutionary and conservation biology Proceedings of the Royal Society B , 273 : 1049–1053. |
| Richard M, Thorpe RS. 2001. Can microsatellites be used to infer phylogenies? Evidence from population affinities of the western Canary Island lizard (Gallotia galloti)[J]. Molecular Phylogenetics and Evolution , 20 : 351–360. DOI:10.1006/mpev.2001.0981 |
| Rohlf RJ. 2008. tpsDig-Thin Plate Spline Digitizer, version 2. 12. Computer Program[P]. New York, Department of Ecology and Evolution, State University of New York, Stony Brook. |
| Sheets HD. 2003. IMP software, integrated morphometrics software package, version 6a[P]. Buffalo, NY, Department of Physics, Canisius College, Buffalo, NY and Department of Geology, SUNY at Buffalo, NY. http://www.canisius.edu/~sheets/morphsoft.html. |
| Shi H, Fong JJ, Parham JF, et al. 2008. Mitochondrial variation of the "eyed" turtles (Sacalia) based on known locality and trade specimens[J]. Molecular Phylogenetics and Evolution , 49 : 1025–1029. DOI:10.1016/j.ympev.2008.10.001 |
| Song M. 1984. A new species of the turtle genus Cuora (Testudoformes:Testudinidae)[J]. Acta Zootaxonomica Sinica, Xi'an , 9 : 330–332. |
| Spinks PQ, Shaffer HB. 2007. Conservation phylogenetics of the Asian box turtles (Geoemydidae, Cuora):mitochondrial introgression, numts, and inferences from multiple nuclear loci[J]. Conservation Genetics , 8 : 641–657. DOI:10.1007/s10592-006-9210-1 |
| Spinks PQ, Shaffer HB, Iverson JB, et al. 2004. Phylogenetic hypotheses for the turtle family Geoemydidae[J]. Molecular Phylogenetics and Evolution , 32(1) : 164–182. DOI:10.1016/j.ympev.2003.12.015 |
| Spinks PQ, Thomason RC, Shaffer HB. 2009. A reassessment of Cuora cyclornata Blanck, McCord and Le, 2006 (Testudines, Geoemydidae) and a plea for taxonomic stability[J]. Zootaxa , 2018 : 58–68. |
| Spinks PQ, Thomason RC, Zhang Y, et al. 2012. Species boundaries and phylogenetic relationships in the critically endangered Asian box turtle genus Cuora[J]. Molecular Phylogenetics and Evolution , 63 : 656–667. DOI:10.1016/j.ympev.2012.02.014 |
| Stuart BL, Parham JF. 2004. Molecular phylogeny of the critically endangered Indochinese box turtle (Cuora galbinifrons)[J]. Molecular Phylogenetics and Evolution , 31(1) : 164–177. DOI:10.1016/S1055-7903(03)00258-6 |
| Stuart BL, Parham JF. 2007. Recent hybrid origin of three rare Chinese turtles[J]. Conservation Genetics , 8 : 169–175. |
| TCC[Turtle Conservation Coalition] (Rhodin AGJ, Walde AD, Horne BD, et al. [eds. ]). 2011. Turtles in Trouble: The World's 25+ Most Endangered Tortoises and Freshwater Turtles-2011[M]. Lunenburg, MA: IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Turtle Conservation Fund, Turtle Survival Alliance, Turtle Conservancy, Chelonian Research Foundation, Conservation International, Wildlife Conservation Society, and San Diego Zoo Global: 54. |
| Tiedemann R, Schneider ARR, Havenstein K, et al. 2014. New microsatellite markers allow high-resolution taxon delimitation in critically endangered Asian box turtles, genus Cuora[J]. Salamandra , 50(3) : 139–146. |
| TTWG[Turtle Taxonomy Working Group] (Van Dijk PP, Iverson JB, Rhodin AGJ, et al. ). 2014. Turtles of the World, 7th Edition: Annotated Checklist of Taxonomy, Synonymy, Distribution with Maps, and Conservation Status. 000. v7[M]. Lunenburg, Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi: 10.3854/crm.5.000.checklist.v7.2014:329-479. |
| Vieites DR, Wollerberg KC, Andreone F, et al. 2009. Vast underestimation of Madagascar's biodiversity evidenced by an intergrative amphibian inventory[J]. Proceeding of the National Academy of Sciences of the United States of America , 106(20) : 8267–8272. DOI:10.1073/pnas.0810821106 |
| Vogt RC. 1978. Systematics and ecology of the false map turtle complex Graptemys pseudogeographica[D]. Madison, University of Wisconsin, USA: 750. |
| Vogt RC. 1993. Systematics of the false map turtles (Graptemys pseudogeographica complex:Reptilia, Testudines, Emydidae)[J]. Annals of Carnegie Museum , 62(1) : 1–46. |
| Voris HK. 2000. Maps of Pleistocene sea levels in south east Asia:shorelines, river systems, time durations[J]. Journal of Biogeography , 27 : 1153–1167. DOI:10.1046/j.1365-2699.2000.00489.x |
| WeißCH. 2007. StatSoft, Inc, Tulsa, OK. [KG-0. 5mm]: STATISTICA, Version 8[J]. AStA Advances in Statistical Analysis, 91(3): 339-341. |
| Zelditch ML, Swiderski DL, Sheets HD, et al. 2004. Geometric morphometrics for biologists: a primer[M]. London: Elsevier Academic Press: 443. |
| Zhang F, Wu XB. 2005. Investigation on the status of the wild golden-headed box turtle in China[J]. Chinese Wildlife , 26(5) : 51–54. |
| Zhang F, Wu XB. 2006. Current status of the wild golden headed box turtle (Cuora aurocapitata) in JingXian county, Anhui province[J]. Journal of Anhui Normal University (Natural Science) , 29(2) : 167–169. |
 2017, Vol. 36
2017, Vol. 36