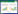扩展功能
文章信息
- 张锋, 高志忠, 陈会明
- Feng ZHANG, Zhizhong GAO, Huiming CHEN
- 多腺巨伪蝎(伪蝎目:苦伪蝎科)的雌性新发现
- First Descriptionon on the Female of Megachernes glandulosus Mahnert, 2009 (Pseudoscorpiones, Chernetidae)
- 四川动物, 2016, 35(5): 723-727
- Sichuan Journal of Zoology, 2016, 35(5): 723-727
- 10.11984/j.issn.1000-7083.20160096
-
文章历史
- 收稿日期: 2016-04-22
- 接受日期: 2016-06-15
2. 贵州科学院生物研究所, 贵阳 550009
2. Institute of Biology, Guizhou Academy of Sciences, Guiyang 550009, China
The genus Megachernes was erected by Beier (1932). Species of Megachernes occur widely throughout the eastern region of the Old World, with the 22 known species ranging from the Black Sea in the west to Japan in the northeast and as far south as eastern Australia (Harvey et al., 2012), and to date, five Megachernes species have been reported in China (Schawaller, 1995; Harvey, 2013).
The genus Magachernes can be distinguished from other genera by the following combination of characters: carapace finely granulated, about as long as broad or somewhat shorter; both transverse furrows clear; eyespot absent; tergites divided; setae of body and palpi toothed, not clavate; rallum with 3 setae; palpi robust, finely granulated; the tactile hair isb, ib, ist and it distributed over the total length of finger, not concentrate to one group; est distant from esb, and situated somewhat in the middle portion between esb and et; legs slender, the tactile hair on tarsus of the 4th pairs lies somewhat distal of the middle; coxa of the 4th legs with convex angle expanded posterio-laterally (Morikawa, 1960).
Megachernes glandulosus Mahnert, 2009 was originally described from a cave in Hubei province based on one male specimen. While examining the pseudoscorpion specimens collected from a cave in Kuankuoshui National Nature Reserve, Guizhou province, we found an adult male, an adult female and three juvenile specimens belonging to Megachernes. The male was identified to M. glandulosus. The female specimen has habitus and outstanding characters similar to the male, and conforms to the characters of M. glandulosus (Mahnert, 2009). Therefore, we come to the conclusion that both male and female are conspecific, and here the female of M. glandulosus was described for the first time.
The material was preserved in 80% alcohol. Photographs were taken by a Leica M205a stereomicroscope equipped with a Leica DFC550 camera and LAS (Ver. 4.6), which was also used for the microscopic examination and measurements. Detailed examination was carried out with an Olympus BX53 compound microscope. Temporary slide mounts were made in glycerol. Terminology and mensuration mostly follow Chamberlin (1931), Harvey (1992), Judson (2007) and Harvey et al. (2012). The following abbreviations are used in the text. Trichobothria: b=basal; sb=sub-basal; st=sub-terminal; t=terminal; ib=interior basal; isb=interior sub-basal; ist=interior sub-terminal; it=interior terminal; eb=exterior basal; esb=exterior sub-basal; est=exterior sub-terminal; et=exterior terminal; MHBU=Museum of Hebei University, Baoding city, China. All measurements are given in millimetres (mm).
Megachernes glandulosus Mahnert, 2009 (Figs. 1~3)

|
| Fig. 1 Megachernes glandulosus Mahnert, 2009, female, unless stated otherwise a. habitus of male, dorsal; b. habitus of female, dorsal. |
| |
|
| Fig. 2 Megachernes glandulosus Mahnert, 2009, female a. carapace, dorsal; b. left chela, lateral; c. left pedipalp, dorsal; d. coxae, ventral; e. chelal fingers, lateral; f. left chelicera, dorsal; g. genital area, ventral. |
| |
|
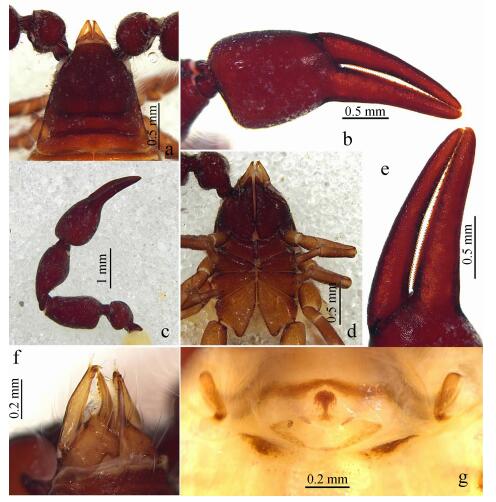
| Fig. 3 Megachernes glandulosus Mahnert, 2009, female a. brood-sac, ventral; b. left leg Ⅰ, lateral; c. left leg Ⅳ, lateral; d. femur and patella of leg Ⅳ, lateral; e. tarsus Ⅳ, lateral; f.rallum, lateral; g. galea, dorsal. |
| |
Megachernes glandulosus Mahnert, 2009: 192~195, Figs. 13~19 (♂)
Material examined: One male and one female (with attached brood-sac) (MHBU), three juveniles: Kuankuoshui National Nature Reserve (28°14.017′N, 107°09.145′E), Zunyi city, Guizhou province, China, elevation 1 513 m, 3 June 2010, Chen H.M., Li Z.X. & Wang L.Y. leg.
Diagnosis: This species differs from the other members of the genus Megachernes by the following combination of characters: pedipalps very stout (Fig. 1: a), palpal patella with a prominent median hump, femur and patella with numerous, densely-set, fur-like setae (Fig. 2: c), cephalothorax with normal setae, coxae, half-sternites Ⅳ~Ⅸ and on basal lateral face of fixed chelal finger with numerous glandular pores (Fig. 2: d), numerous glandular microsetae present on ventral side of femur and patella of legs Ⅲ and Ⅳ (Fig. 3: d). This new species is closely related to M. barbatus from Vietnam, but it differs from M. glandulosus by its more slender palpal segments, the absence of a protuberance on the male patella, the absence of glandular structures and the presence of a “fur-like” seta of the cephalothorax in both sexes.
Description: Female : Carapace and palps dark-reddish, remaining parts (legs, sternites and pleural membranes) yellowish-brown (Fig. 1: b). All setae pointed and simple.
Carapace (Fig. 2: a): Slightly shorter than broad (0.96×), eyespots absent, with two distinct deep transverse furrows. The distance between the subbasal transverse furrow and the posterior margin somewhat as same as that to median furrow, with lots of setae, including 10 on anterior margin and 18 on posterior margin.
Abdomen: All tergites narrow divided (including the last one). Each half-tergites with about 11~16 setae and with a dark spot in interior 1/3 side, the last tergite with 4 long tactile setae, the last sternite with 4 long tactile setae and 2 lyrifissures, anus with 2 simple and acuminate setae. Manducatory process with 4 normal setae. All sternites narrow divided, setae short and acuminate, each half-sternite with 13~15 setae. Coxae Ⅰ to Ⅳ with numerous simple and acute setae.
Chelicera: Weakly scale-shaped sculpture. Seven setae in hand (Fig. 2: f), all of them simple and acuminate; with one lyrifissure on palm; fixed fingers with 5 different size teeth in the end. Movable finger with a seta in subdistal finger and a big tooth; serrula exterior with 28 lamellae; rallum with 3 blades (Fig. 3: f); galea with some small branches in terminal (Fig. 3: g).
Pedipalps: Palp short and strong (Fig. 2: c), most segments with numerous fur-like setae, include the chelal fingers (Fig. 2: b), which with normal fine granulate; setae acuminate and long; without tactile setae on femur, patella or hand; trochanter with high and broad rounded dorsal hump; proportions: trochanter 1.37 times as long as broad; femur 1.92 times as long as broad; patella 2.13 times as long as broad; chela with pedicel 3.36 times as long as broad, chela without pedicel 3.13 times as long as broad, hand with pedicel 1.55 times, without pedicel 1.30 times, as long as broad. Movable finger 1.27 times as long as hand with pedicel, and 1.52 times without pedicel. Fixed finger with about 68 teeth, 12 accessory teeth on retrolateral side, 13 accessory teeth on prolateral side; movable finger with about 72 teeth, 10 accessory teeth on retrolateral side, 13 accessory teeth on prolateral side (Fig. 2: e); trichobothrial pattern similar to that of male.
Coxae (Fig. 2: d) with numerous small setae over entire ventral surface, with longer setae on posterior margin of coxae Ⅳ; posterior corners of coxae Ⅳ rounded and slightly enlarged.
Venom apparatus only present in moveable chela fingers, venom duct long and slender, extend over trichobothrium t in movable fingers; nodus ramosus closer to t than to st.
Genital area (Fig. 2: g), anterior genital operculum with some setae, most of them short; spermathecae T-shaped; brood-sac (Fig. 3: a) with 34 underdeveloped embryos.
Leg Ⅰ (Fig. 3: b): Facies with granulate, with numerous simple acuminate setae, tactile setae absent, claws simple; proportions: trochanter 1.13 times; femur 1.47 times; patella 2.86 times; tibia 3.32 times; tarsus 3.50 times as long as deep; arolium shorter than claws.
Leg Ⅳ (Fig. 3: c): Surface weakly granulate; femur and patella with larger field of glandular microsetae (Fig. 3: d); tibia and tarsus with numerous simple acuminate setae, trochanter 2.06 times; femur+patella 4.16 times; tibia 5.00 times; tarsus 4.50 times as long as deep. Tactile seta in middle tarsus (0.40) (Fig. 3: e); arolium undivided and shorter than the simple and large claws, subterminal seta smooth and curved.
Measurements(length/width): Total length 4.80; carapace 1.75/1.83. Palp: trochanter 0.92/0.67; femur 1.59/0.83; patella 1.62/0.76; chela with pedicel 2.92; length of chela without pedicel 2.72; length of hand with pedicel 1.35, without pedicel 1.13; length of movable finger 1.72. Leg Ⅰ(length/depth): trochanter 0.36/0.32; femur 0.50/0.34; patella 0.83/0.29; tibia 0.73/0.22; tarsus 0.56/0.16. Leg Ⅳ (length/depth): trochanter 0.67/0.46; femur+patella 1.64/0.47; tibia 1.34/0.29; tarsus 0.72/0.21.
Distribution: Southwest China (Hubei, Guizhou)
Remarks: Species of Megachernes are closely associated with other animals, either in the nests or fur of small mammals, nests of birds and bumblebees, or in bat guano within caves. The specimens that used in this study were collected from a rat nests (not Niviventer) in the cave.
Acknowledgements: Our thanks are greatly indebted to Mr. Wang Luyu (Southwest University, Chongqing, China) and Mr. Li Zongxu (Kunming Institute of Zoology, CAS, Kunming, China) for kindly assistant in the field work.| Beier M. 1932. Pseudoscorpionidea Ⅱ. Subord. C. Cheliferinea[M]. Tierreich, 58: i-xxi, 1-294. |
| Chamberlin JC. 1931. The arachnid order Chelonethida[M]. Stanford University Publications. Biological Sciences, 7: 1-284. http://cn.bing.com/academic/profile?id=564661401&encoded=0&v=paper_preview&mkt=zh-cn |
| Harvey MS, Ratnaweera PB, Udagama PV, et al. 2012. A new species of the pseudoscorpion genus Megachernes (Pseudoscorpiones: Chernetidae) associated with a threatened Sri Lankan rainforest rodent, with a review of host associations of Megachernes[J]. Journal of Natural History , 46 : 2519–2535. DOI:10.1080/00222933.2012.707251 |
| Harvey MS. 1992. The phylogeny and classification of the Pseudoscorpionida (Chelicerata: Arachnida)[J]. Invertebrate Taxonomy , 6 : 1373–1435. DOI:10.1071/IT9921373 |
| Harvey MS. 2013. Pseudoscorpions of the world, version 3.0. Western Australian Museum, Perth[DB/OL]. [2016-04-07]. http://museum.wa.gov.au/catalogues-beta/pseudoscorpions. |
| Judson MLI. 2007. A new and endangered species of the pseudoscorpion genus Lagynochthonius from a cave in Vietnam, with notes on chelal morphology and the composition of the Tyrannochthoniini (Arachnida, Chelonethi, Chthoniidae)[J]. Zootaxa , 1627 : 53–68. |
| Mahnert V. 2009. New species of pseudoscorpions (Arachnida, Pseudoscorpiones, Chthoniidae, Chernetidae) from caves in China[J]. Revue Suisse de Zoologie , 116(2) : 185–201. |
| Morikawa K. 1960. Systematic studies of Japanese pseudoscorpions[J]. Memoirs of Ehime University (2B) , 4 : 85–172. |
| Schawaller W. 1995. Review of the pseudoscorpion fauna of China (Arachnida: Pseudoscorpionida)[J]. Revue Suisse de Zoologie , 102 : 1045–1064. DOI:10.5962/bhl.part.80489 |
 2016, Vol. 35
2016, Vol. 35