文章信息
- 陈振明, 纪双斌, 史湘铃, 赵跃媛, 张雪峰, 金辉 .
- Chen Zhenming, Ji Shuangbin, Shi Xiangling, Zhao Yueyuan, Zhang Xuefeng, Jin Hui .
- Markov决策树模型在优化15~49岁女性戊型肝炎免疫接种策略中的应用
- Use the Markov-decision tree model to optimize vaccination strategies of hepatitis E among women aged 15 to 49
- 中华流行病学杂志, 2017, 38(2): 267-271
- CHINESE JOURNAL OF EPIDEMIOLOGY, 2017, 38(2): 267-271
- http://dx.doi.org/10.3760/cma.j.issn.0254-6450.2017.02.026
-
文章历史
收稿日期: 2016-11-01
2. 210009 南京, 江苏省疾病预防控制中心
2. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing 210009, China
戊型肝炎(戊肝)是由HEV感染引起的一种急性肠道传染病。世界上约1/3的人感染过HEV[1]。近年来戊肝在我国主要为散发流行,但发病率有上升趋势。戊肝在一般人群中的病死率为0.2%[2],而孕晚期女性感染后病死率高达21.0%[3]。疫苗接种是控制HEV感染的重要途径。目前我国自主研发的重组戊肝疫苗HEV239(商品名益可宁)已上市[4],将有助于控制HEV传播。目前Markov决策树模型已逐渐应用到医学决策与卫生经济学评价中[5],通过以Markov模型作为决策树结果节点,结合疾病的各种健康状态,最终计算过程中的成本和效用,以此帮助决策者做出科学决策。本研究以15~49岁女性作为研究人群,通过构建Markov决策树模型评价不同戊肝疫苗接种策略,从而为制定戊肝免疫接种策略提供科学依据。
对象与方法1. 模拟队列:模拟对象为女性群体,即从15岁模拟到49岁,此期间可能出现的孕妇人群。
2. Markov决策树模型:根据HEV感染后疾病的自然史,分成5个Markov状态:未感染(易感)、感染、自然免疫、疫苗免疫和死亡,其中死亡为吸收态。每个女性在某个时间点只处于这5个状态中的一个,建立的Markov状态模型见图 1。评价3种免疫策略:不接种策略;筛查后接种策略(对全人群进行血清学筛查,无免疫力者进行疫苗接种);100%接种策略(直接对全人群进行疫苗接种)。用货币单位“元”表示成本,用质量调整生命年(QALY)衡量健康效用,用增量成本效用比(ICUR)评价不同接种策略。以国内年人均GDP的3倍作为社会意愿支付,当策略的ICUR低于社会意愿支付时,说明该策略是可取的。
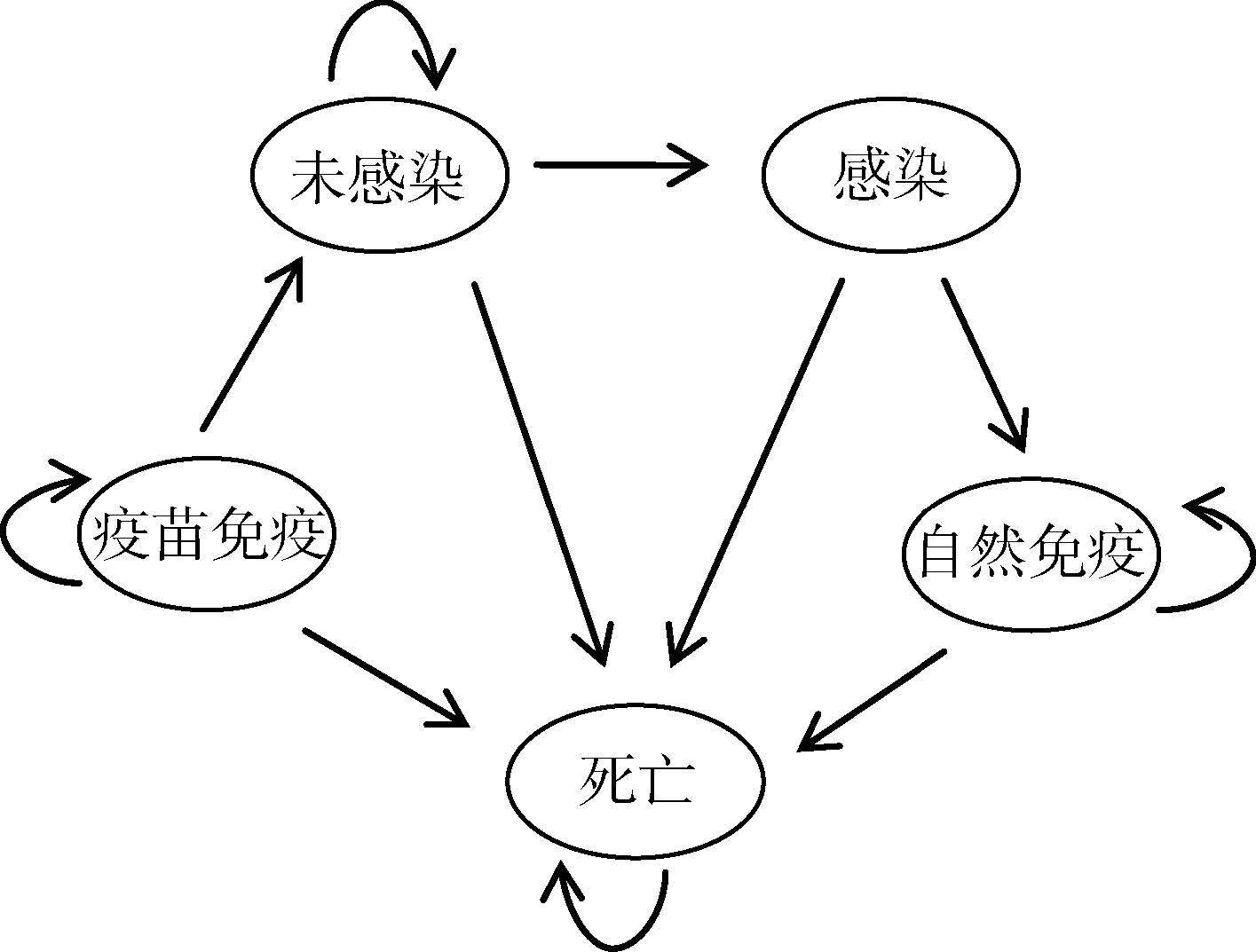
|
| 图 1 戊肝疫苗免疫策略的Markov决策树模型5个状态 |
3. 模型假设:对模型结构和参数进行以下假设:①假设人群疫苗接种均为3针,立即产生免疫力;②不考虑孕妇人群中与婴儿有关的健康生命年损失和产生的成本;③不考虑血清学检测假阳性和假阴性。
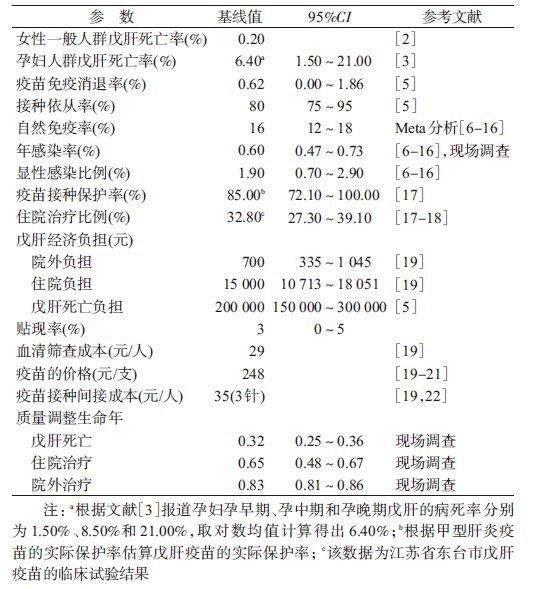
4. 模型的主要参数:模型中的参数主要通过文献检索、现场调查等途径获得,参数见表 1。
5. 统计学分析:应用决策树分析软件Tree Age Pro 2011建立Markov决策树模型,模拟戊肝的发生、发展过程,计算每个周期内不同状态的成本和效用,循环35个周期后,计算3种免疫策略的人均ICUR,综合比较各种方案,并对结果进行敏感性分析和阈值分析,戊肝Markov决策树模型见图 2。敏感性分析的因素包括孕妇戊肝的年感染率、孕妇的显性感染比例、疫苗价格、贴现率、自然免疫感染率、戊肝死亡的疾病负担、接种的依从率等变量。
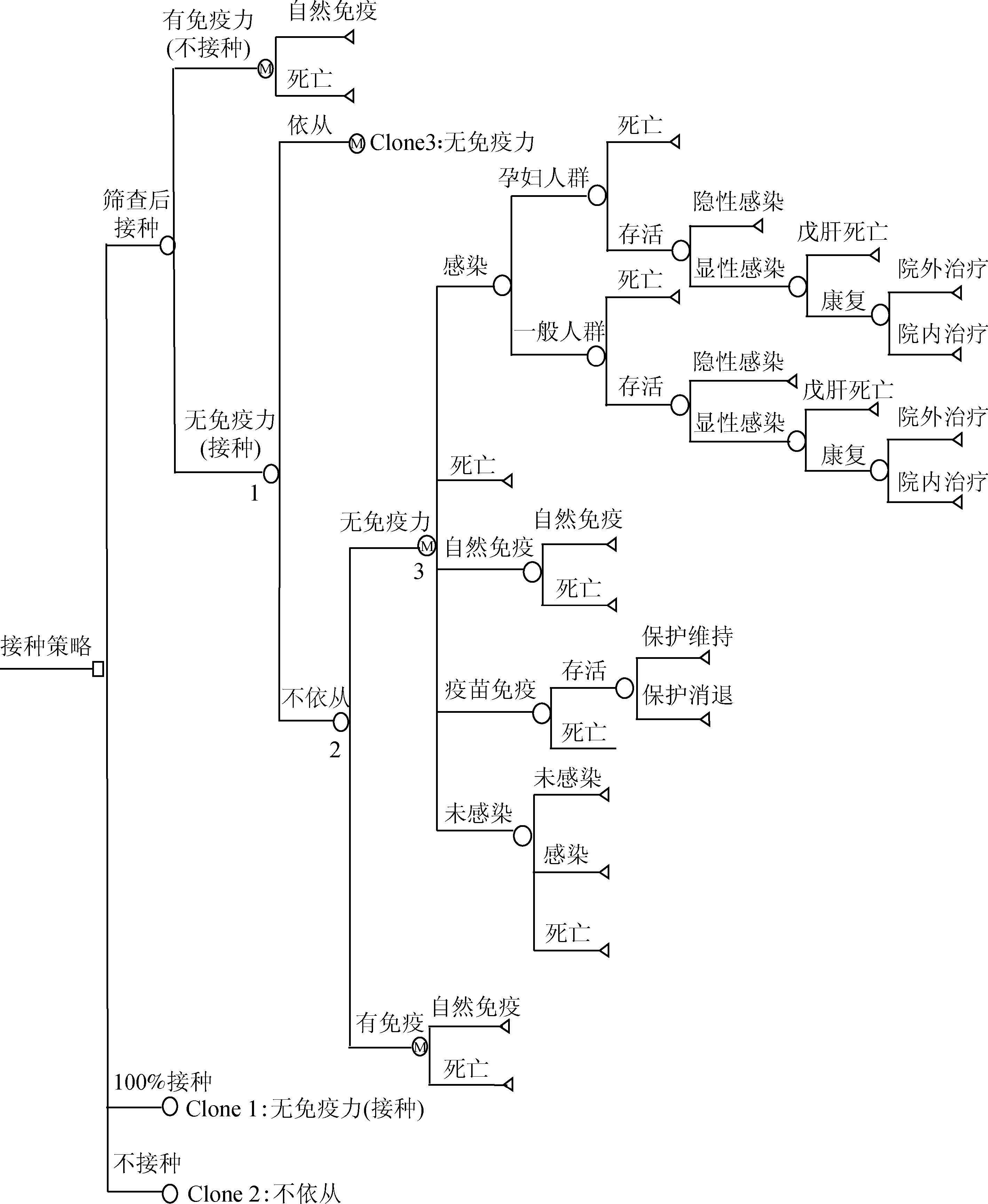
|
| 图 2 戊肝疫苗不同接种策略的Markov决策树模型 |
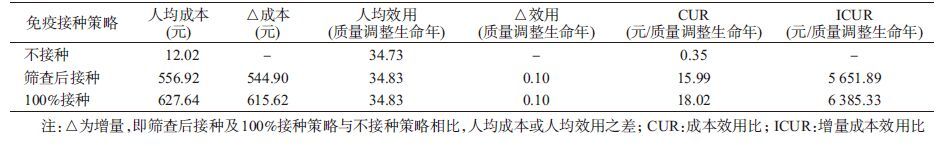
1. 不同接种策略的ICUR:相比于不接种策略,筛查后接种和100%接种策略均使健康效用人均增加0.10个QALY,ICUR分别是5 651.89元/QALY和6 385.33元/QALY。即人均增加1个QALY,筛查后接种和100%接种策略要分别增加成本5 651.89元和6 385.33元。见表 2。
假设样本10 000人模拟1 000次,以纵坐标为增量成本,横坐标为增量QALY作散点图(未显示)。以2014年国内人均GDP的3倍139 887元作为社会意愿支付,与不接种免疫策略相比,筛查后接种和100%接种策略中所有的点都落在位于社会意愿支付的下方,说明两种策略都有经济学效益。筛查后接种相对于100%接种策略虽然没有增量效用的增加,但人均成本更低,提示筛查后接种策略优于100%接种策略。
2. 敏感性分析:假定个人意愿支付为2 100元[19],单因素敏感性分析结果显示,ICUR值对疫苗价格变化最敏感,其次分别为易感者年感染率、戊肝显性感染比例、贴现率、戊肝死亡疾病负担、自然免疫率和接种依从率。见图 3。当接种依从率均<23%时,100%接种策略优于筛查后接种策略;而依从率均>23%时,筛查后接种策略优于100%接种策略。因为参数的改变可能会对接种策略选择产生影响,以下将探讨疫苗价格和易感者年感染率对结果的影响。
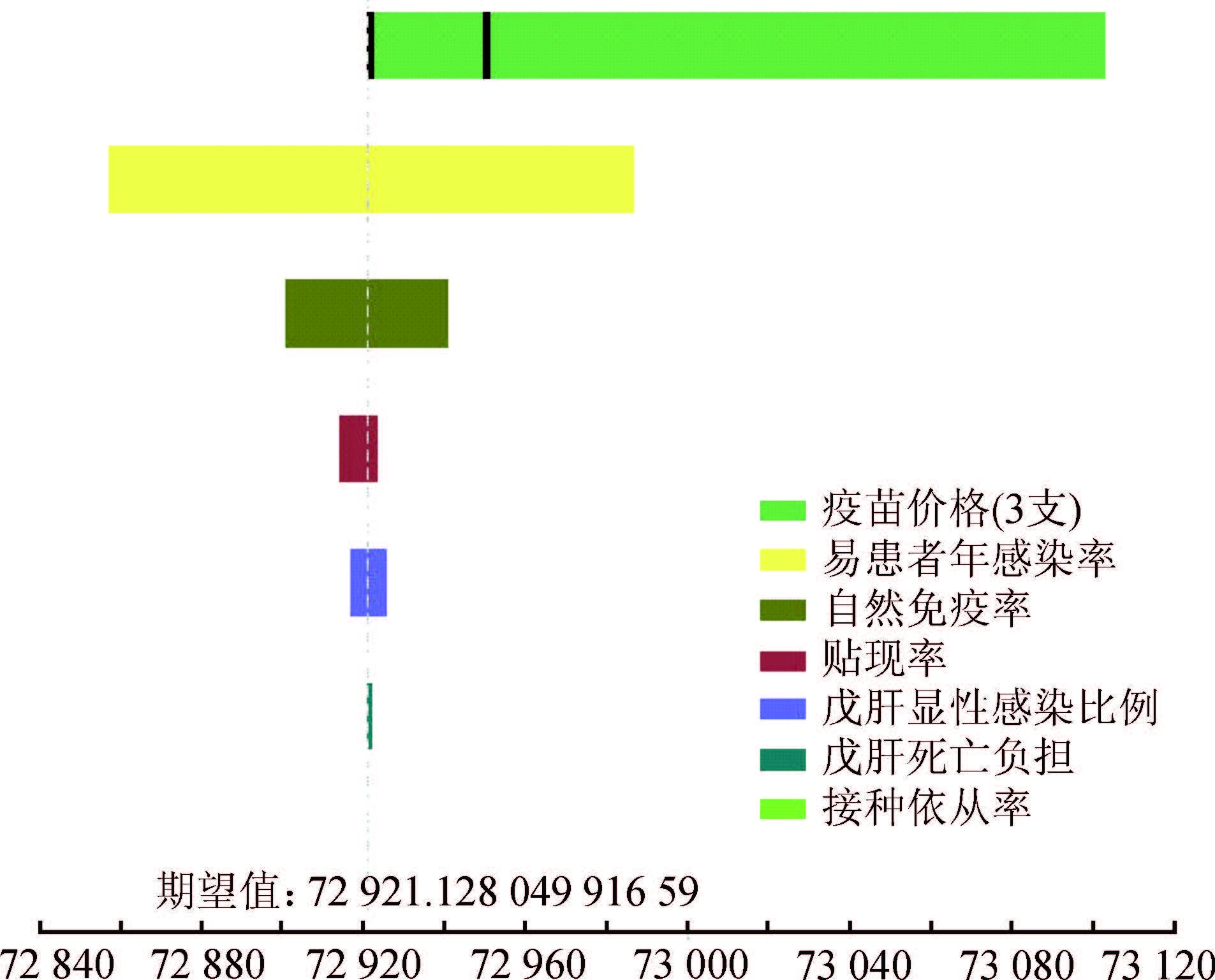
|
| 图 3 戊肝疫苗接种策略单因素敏感分析的Tornado图 |
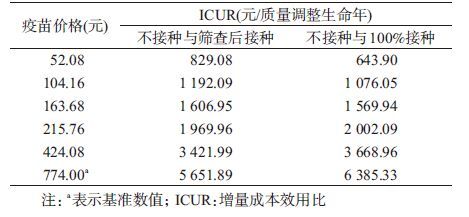
(1)戊肝疫苗价格对接种策略选择产生的影响:当戊肝疫苗价格发生变化时,相对于不接种策略,筛查后接种和100%接种策略的ICUR值在上升(表 3)。结合阈值分析,结果显示,当疫苗价格均<191.56元时,100%接种策略优于筛查后接种策略;当疫苗价格均>191.56元时,筛查后接种策略优于100%接种策略,提示戊肝疫苗价格会影响最优策略的选择。
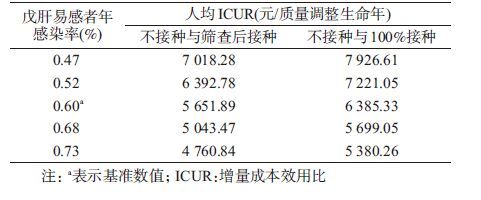
(2)戊肝易感者年感染率对接种策略选择产生的影响:根据模型假设,易感者年感染率为0.47%~0.73%,随着易感者年感染率的增加,相比不接种策略,筛查后接种和100%接种策略的ICUR值在减小,即实行筛查后接种策略和100%接种策略的经济效果在上升,但筛查后接种策略一直优于100%接种策略(表 4),提示年感染率不影响最优策略的选择。
戊肝是急性病毒性肝炎的一种,主要侵犯青壮年,隐性感染比例高,但是孕妇感染的预后差,即使患者存活下来也伴有流产和死胎的高风险[23],给孕妇及其家庭带来沉重的负担。为了降低孕妇感染戊肝风险,除了通过改善卫生条件来切断传播途径以外,接种疫苗是一种经济有效的措施。在我国有限的卫生资源条件下,科学合理的免疫接种策略将有助于决策者最大限度降低经济负担,同时能有效控制戊肝的蔓延。
本研究通过构建Markov决策树,采用不同的免疫接种策略,对15~49岁女性进行模拟接种,分析比较不同策略的成本效用。结果显示,筛查后接种策略和全人群接种策略与不接种策略相比,人均增加0.10个和0.10个健康生命年,ICUR为5 651.89元/QALY和6 385.33元/QALY。在社会意愿支付为139 887元的背景下,采取筛查后接种策略具有经济学效果,但疫苗价格和接种依从性会影响最优策略的选择。
本研究存在局限性。由于数据主要通过文献查询获得,文献来源会影响研究结果的质量。文献中存在的发表偏倚和选择偏倚、文献之间的异质性会给研究结果带来影响。对于戊肝疾病死亡负担的数据,由于国内缺乏相关的研究报道,在不违背科学性的前提下本研究借鉴了甲型肝炎的数据,这在一定程度上影响了结果的推广和应用。此外,戊肝疫苗接种时,随着孕妇人群依从性的增加,疫苗接种效果也在提高,所以在实践中应当把戊肝疫苗接种以及相关筛检等问题考虑在内,不同地区应根据当地戊肝的流行病学特征、社会经济和文化差异,因地制宜制定免疫策略,以使投入的卫生资源取得最佳的效果。
利益冲突: 无
| [1] | Purcell RH, Emerson SU.Prevention//Thomas HC,Lemon S,Zuckerman AJ. Viral Hepatitis[M].3rd ed. Malden: MA Blackwell Publishing, 2005: 635–645. |
| [2] |
吴伟慎, 赵莹, 陈静, 等.
天津市2004-2013年戊型肝炎流行趋势和特征分析[J]. 中国病毒病杂志, 2015, 5(1): 45–49.
Wu WS, Zhao Y, Chen J, et al. Epidemiologic analysis of hepatitis E in Tianjin,China[J]. Chin J Viral Dis, 2015, 5(1): 45–49. DOI:10.16505/j.2095-0136.2015.01.008 |
| [3] | Zhuang H, Cao XY, Liu CB, et al. Epidemiology of hepatitis E in China[J]. Gastroenterol Jpn, 1991, 26(Suppl 3): S135–138. DOI:10.1007/BF02779283 |
| [4] |
张少然.
中国研制成功并上市世界首个戊型肝炎疫苗[J]. 健康向导, 2012(2): 35.
Zhang SR. The world's first vaccine of hepatitis E developed in China[J]. Health Guide, 2012(2): 35. |
| [5] |
潘新娟, 冯艳铭, 庄贵华.
Markov-决策树模型在甲肝免疫策略优化中应用[J]. 中国公共卫生, 2013, 29(4): 590–592.
Pan XJ, Feng YM, Zhuang GH. Use Markov-decision tree model to optimize hepatitis A vaccination strategies[J]. Chin J Public Health, 2013, 29(4): 590–592. |
| [6] |
蒋理, 谢而付, 杨瑞霞, 等.
中孕孕妇血清中戊型肝炎血清标志物的分析[J]. 南京医科大学学报:自然科学版, 2009, 29(12): 1721–1723.
Jiang L, Xie EF, Yang RX, et al. Analysis of hepatitis E virus makers in the serum of second trimester women[J]. Acta Univ Med Nanjing:Nat Sci, 2009, 29(12): 1721–1723. |
| [7] |
李丽, 马天武, 曾韦锟, 等.
昆明市孕妇人群戊型肝炎病毒血清学调查[J]. 医学研究杂志, 2012, 41(11): 65–67.
Li L, Ma TW, Zeng WK, et al. Seroepidemiological survey of hepatitis E virus in pregnant women in Kunming city[J]. J Med Res, 2012, 41(11): 65–67. DOI:10.3969/j.issn.1673-548X.2012.11.020 |
| [8] |
杨瑞霞, 蒋理, 凌芸.
围生期妇女血清肝炎标志物检测结果分析[J]. 中国基层医药, 2013, 20(15): 2278–2280.
Yang RX, Jiang L, Ling Y. Analysis of the various types of hepatitis markers in perinatal women[J]. Chin J Prim Med Pharm, 2013, 20(15): 2278–2280. DOI:10.3760/cma.j.issn.1008-6706.2013.15.016 |
| [9] |
马天武. 昆明市戊型肝炎病毒流行病学调查及ORF2蛋白的原核表达研究[D]. 昆明:昆明理工大学,2013:89.
Ma TW. Studies on epidemic investigation of hepatitis E virus in Kunming and prokaryotic expression of virus ORF2 gene[D]. Kunming University of Science and Technology,2013:89. http://cdmd.cnki.com.cn/Article/CDMD-10674-1013346415.htm |
| [10] | Huang F, Ma TW, Li L, et al. Low seroprevalence of hepatitis E virus infection in pregnant women in Yunnan,China[J]. Braz J Infect Dis, 2013, 17(6): 716–717. DOI:10.1016/j.bjid.2013.02.006 |
| [11] |
王强, 夏洁, 谢跃文.
武汉市青山地区2812例孕晚期妇女肝炎病毒感染现状调查[J]. 检验医学, 2014, 29(2): 106–109.
Wang Q, Xia J, Xie YW. Study on hepatitis virus infections of 2812 pregnant women during late pregnancy in Qingshan area of Wuhan city[J]. Lab Med, 2014, 29(2): 106–109. DOI:10.3969/j.issn.1673-8640.2014.02.004 |
| [12] |
杨春蓉.
某市2500例孕晚期妇女肝炎病毒感染现状的调查[J]. 中国医药指南, 2014, 12(34): 237–238.
Yang CR. A survey of 2500 cases of late pregnancy women hepatitis virus infection status[J]. Guide China Med, 2014, 12(34): 237–238. DOI:10.15912/j.cnki.gocm.2014.34.182 |
| [13] |
周璇, 黄红玉, 段红蕾, 等.
南京地区中孕孕妇戊型肝炎病毒感染血清流行病学调查[J]. 中国产前诊断杂志, 2015, 7(1): 26–30.
Zhou X, Huang HY, Duan HL, et al. Seroepidemiological survey of pregnant women of hepatitis E infection in Nanjing[J]. Chin J Pren Diagn, 2015, 7(1): 26–30. DOI:10.13470/j.cnki.cjpd.2015.01.006 |
| [14] |
周璇, 徐飚, 徐陈瑜, 等.
分娩及产后7个月~12个月母婴戊型肝炎病毒抗体的动态观察[J]. 中国卫生检验杂志, 2015, 25(16): 2656–2659.
Zhou X, Xu B, Xu CY, et al. Dynamic monitoring of anti-HEV in mothers and their newborns from delivery to postpartum 7-12 months[J]. Chin J Health Lab Tecehnol, 2015, 25(16): 2656–2659. |
| [15] | Cong W, Sui JC, Zhang XY, et al. Seroprevalence of hepatitis E virus among pregnant women and control subjects in China[J]. J Med Virol, 2015, 87(3): 446–450. DOI:10.1002/jmv.24058 |
| [16] |
张乐, 黄红玉, 顾光煜, 等.
江苏地区孕中期孕妇抗-HEV阳性率及分娩后6年随访观察[J]. 中国病毒病杂志, 2015, 5(2): 118–124.
Zhang L, Huang HY, Gu GY, et al. Prevalence of anti-HEV in the second trimester pregnant women in Jiangsu province of China and a 6-year follow-up[J]. Chin J Viral Dis, 2015, 5(2): 118–124. DOI:10.16505/j.2095-0136.2015.02.011 |
| [17] | Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults:a large-scale,randomised,double-blind placebo-controlled,phase 3 trial[J]. Lancet, 2010, 376(9744): 895–902. DOI:10.1016/S0140-6736(10)61030-6 |
| [18] | Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine[J]. N Engl J Med, 2015, 372(10): 914–922. DOI:10.1056/NEJMoa1406011 |
| [19] |
修仕信. 东台地区戊型肝炎的疾病负担及免疫干预的卫生经济学评价[D]. 南京:东南大学,2011.
Xiu SX. Disease burden of hepatitis E and health economic evaluation of vaccine interventions in Dongtai Area[D]. Nanjing:Southeast University,2011. |
| [20] | Das A, Sinha M. A cost-effectiveness analysis of a candidate vaccine against hepatitis E virus using the institute of medicine model[J]. Am J Gastroenterol, 2001, 96(9 Suppl 1): S263. DOI:10.1016/S0002-9270(01)03619-X |
| [21] | Riedmann EM. Chinese biotech partnership brings first hepatitis E vaccine to the market[J]. Hum Vaccin Immunother, 2012, 8(12): 1743–1744. |
| [22] | Rein DB, Hicks KA, Wirth KE, et al. Cost-effectiveness of routine childhood vaccination for hepatitis A in the United States[J]. Pediatrics, 2007, 119(1): e12–21. DOI:10.1542/peds.2006-1573 |
| [23] | Khuroo MS, Kamili S. Aetiology,clinical course and outcome of sporadic acute viral hepatitis in pregnancy[J]. J Viral Hepat, 2003, 10(1): 61–69. DOI:10.1046/j.1365-2893.2003.00398.x |
 2017, Vol. 38
2017, Vol. 38