b Department of Pharmacy, Peking University First Hospital, Beijing 100034, China
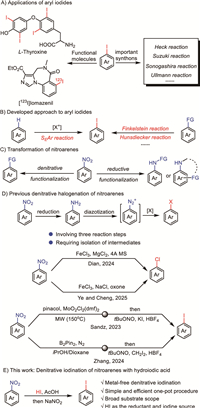
Owing to the distinctive functional reactivity of aromatic C-I bond, aryl iodides are widely served as pharmaceuticals [1], imaging agents [2,3], natural products [4], and other functional molecules (Fig. 1A) [5]. Meanwhile, the strong polarity of C-I bond enables aryl iodides to be ideal partners in many cross-coupling reactions to prepare aryl derivatives (Fig. 1A) [6–10]. Although traditional electrophilic aromatic substitution reactions [11–24] provide very convenient approach to aryl iodides from aromatics, the regioselectivity and limited substrate scope restrict their potential applications. Besides electrophilic aromatic substitution reactions, the preparation of aryl iodides via functional group conversion is also very popular. Many well-known reactions including Finkelstein reaction [25–29], Hunsdiecker reaction [30,31], and others [32,33] have been developed for synthesizing aryl iodides from aromatic derivatives via one-pot process (Fig. 1B). Despite the significance of these methods, the pre-installation of functional groups restricts their potential application. Hence, exploring efficient protocols to aryl iodides from commercially available aromatics under simple, practical, and low-cost conditions is highly desirable and will offer promising avenues for further applications.
|
Download:
|
| Fig. 1. (A) Applications of aryl iodides. (B) Developed approach to aryl iodides. (C) Transformation of nitroarenes. (D) Previous denitrative halogenation of nitroarenes. (E) This work: Denitrative iodination of nitroarenes with hydroiodic acid. | |
Nitroarenes, which could be easily prepared by the nitration of various aromatics [34–40], are widely employed as inexpensive and commercially available substrates in organic synthesis. The nitro group on nitroarenes could be substituted by other groups via denitrative progress [41–49]. Besides the reduction of nitroarenes to anilines [50], the reductive functionalization of nitroarenes provides efficient strategies to functionalized aniline derivatives (Fig. 1C) [51–61]. In the past decades, nitroarenes have functioned as oxygen-source reagent [62–66], nitrene precursor [67–72], and hydrogen atom transfer reagent [73,74] under photochemical process. These pioneering researches have widely broadened the application of nitroarenes. Although nitro group on nitroarenes could be substituted by many groups [41], the conversion of nitroarenes to aryl halides generally required a tedious three-step pathway which involved reduction, diazotization, and halogenation, suffering from isolation of intermediates and laborious synthetic procedure (Fig. 1D) [75–83]. Simplified denitrative halogenation reactions via one-step have not been reported until very recently. In 2024, Dian et al. [84] reported a photo-induced iron-catalyzed denitrative chlorination of nitroarenes under mild conditions. Within the same year, Ye and Cheng et al. [85] also reported a light-promoted aromatic denitrative chlorination, which broadened the substrate range to both nitroarenes and nitroalkenes. However, both of the two methods could not be applied in the denitrative iodination. In order to achieve denitrative iodination of nitroarenes, Sandz et al. [86] employed microwave-assisted high-temperature conditions in a complex reaction system. Recently, Zhang et al. [87] reported a visible light-induced denitrative iodination method, which followed a complex pathway involving the addition of B2Pin2 as the reductant, CH2I2 as the iodine source and HBF4 as an additional acid to stabilize the intermediates (Fig. 1D). All of these denitrative halogenation strategies relied on the microwave or visible light-induced excitation of the nitro group to generate radicals. Thus, a simplified strategy for the denitrative iodination of nitroarenes still holds significant potential.
The well-known Sandmeyer reaction has been firmly established as a reliable and mature strategy for the preparation of aryl iodides [75–83]. What excited us more is that our preliminary exploration revealed the capability of hydroiodic acid (HI) to reduce nitro group, which has also been documented in previous reports [88–90]. This notable property of HI sparked our interest to sequentially connect the reductive reaction with a Sandmeyer-type step. Building upon our previous research on preparation of aryl halides [91–93], we herein present the HI-mediated denitrative iodination of nitroarenes via a streamlined one-pot protocol integrating reduction, diazotization, and iodination, employing the iodide ion dually as the reductant and iodine source (Fig. 1E). Cost-effective and commercially available reagents, simple and sustainable reaction system, practical and easily scalable process, and the broad substrate scope make this method a highly practical approach for preparing aryl iodides with potential future applications.
Initially, commercially available 4-nitroacetophenone 1 was chosen as the model substrate. After careful optimization of reaction conditions, substrate 1 was successfully converted to 4-iodoacetophenone 2 with 85% isolated yield in the presence of aqueous hydroiodic acid (HI, 10 equiv.) and NaNO2 (2.0 equiv.) with acetic acid (AcOH) as the solvent via the sequenced one-step (Table 1, entry 1). The absence of HI prevented the denitrative reaction, confirming its irreplaceable function in this transformation (entry 2), while a solvent-free condition leaded to a significant decrease in yield (entry 3). Halving the usage of HI also caused a substantial drop of yield to 50% (entry 4). Attempts to employ alternative halogen source including hydrochloric acid (HCl) or sodium iodide (NaI) proved to be ineffective in facilitating the reaction (entries 5 and 6). A combination of HCl and NaI could promote the iodination with lower yield, revealing the importance of both proton and iodide (entry 7). When the AcOH solvent was replaced by trifluoroacetic acid or hexanoic acid, the yield of 2 was reduced (entries 8 and 9). Other solvents such as i-PrOH, dioxane, or xylene were proven to be inferior to AcOH (entries 10–12). Lowering the reaction temperature to 90 ℃ hindered the progress (entry 13).
|
|
Table 1 Initial optimization of the reaction conditions.a |
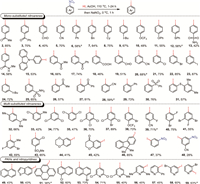
Under optimal reaction conditions, a detailed study on the substrate scope was investigated (Scheme 1). It was delightful to find that the denitrative iodination proceeded efficiently regardless of the electronic effects and positional substituents on the aromatic ring. A wide variety of mono-substituted nitrobenzenes were successfully converted to the corresponding aryl iodides 2–31 in moderate to good yields with the tolerance of alkyl, halogen, aldehyde, ketone, carboxyl, trifluoromethoxy, diaryl(thio)ethers, amides, and sulfonyl motifs. Our method also demonstrated high efficiency with multi-substituted nitroarenes bearing either identical substituents (32, 34–37) or diverse functional groups with distinct electronic effects (33, 38–43). Furthermore, substrates containing benzo-fused saturated (hetero)cycles were converted to corresponding products 44–46 under the optimized conditions. To our delight, dinitrobenzenes were proved to be suitable substrates under standard condition, affording mono-iodinated products 47 and 48 in lower yield with selective denitrative iodination of one nitro group. It was also worth emphasizing that this approach was compatible with polycyclic aromatic hydrocarbons (PAHs) as we successfully obtained the homologous products of nitro-substituted naphthalenes (49, 50), pyrene (51), and benzothiophene (52, 53).
|
Download:
|
| Scheme 1. Scope of nitroarenes. Reaction conditions: nitroarene (0.5 mmol), HI (55% aqueous, 5.0 mmol) and AcOH (1.0 mL) were stirred for 1–24 h at 110 ℃ under reflux. Then the reaction mixture was cooled to 0 ℃, and NaNO2 (1.0 mol/L aqueous, 1.0 mmol) was added. After that the mixture was stirred at 0 ℃ for 1 h. Isolated yield. a 90 ℃. | |
The iodination of pyridines is very challenging due to the inert property of pyridines. Many significant strategies have been developed to achieve the regioselective aromatic iodination of pyridines [94–97]. To our delight, the denitrative iodination of substituted nitropyridines (54–59) also proceeded smoothly under standard conditions. In contrast to traditional iodination methods, the regioselectivity of this method is governed solely by the position of the nitro group on pyridine ring, and C-2, C-3 and C-4 iodinated pyridines were all obtained via this denitrative iodination strategy.
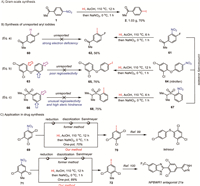
To further evaluate the synthetic applicability of this method, gram-scale experiment of 1 was conducted to afford corresponding aryl iodide 2 with good yield (Fig. 2A), demonstrating the potential practical application of this methodology. In order to explore the prospect of our strategy in uncharted chemical space, the synthesis of unreported aryl iodides was explored (Fig. 2B). Due to its high electron deficiency of the aromatic ring, the iodination reaction of substrate 60 remained undocumented. Through our established method, its nitro precursor 61 was efficiently transformed to the corresponding iodinated product 62 (Fig. 2B, Eq. a). Theoretically, aryl iodide 65 could be prepared by the iodination of substrate 63. However, this reaction also has not been reported yet perhaps because of the poor regioselectivity. Employing its commercially available nitro derivative 64, which is also known as herbicide nitrofen [98], aryl iodide 65 was synthesized with high efficiency by this denitrative iodination process (Fig. 2B, Eq. b). The electrophilic of sulfone 66 usually occurred at meta-C-H bond or isopropyl C—H bond. With this developed chemistry, the ortho-iodinated sulfone 68 could be easily prepared from nitroarene 67, overcoming the traditional regioselectivity and steric hindrance from the sulfonyl group (Fig. 2B, Eq. c).
|
Download:
|
| Fig. 2. Synthetic application. | |
Ultimately, driven by the recognized value of aryl iodides in synthetic applications, our method was applied to the synthesis of key intermediates for bioactive molecules (Fig. 2C). Iodobenzene 70, an important intermediate of insecticide tetrasul [99], was efficiently prepared from substrate 69. Meanwhile, the transformation from nitrobenzene 71 to compound 72, a building block of a newly reported small molecule antagonist of NPBWR1 [100], was simply accomplished. These shown approaches represent a marked improvement of our practical and efficient one-pot method over the three-step process commonly employed.
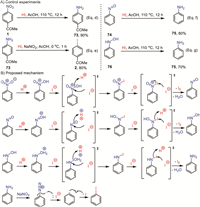
To understand the mechanism of the denitrative progress, some control experiments were carried out (Fig. 3A). 4-Aminoacetophenone 73 was obtained in 90% yield in the presence of HI and AcOH at 110 ℃, revealing the aniline was the intermediate (Fig. 3A, Eq. d). Exposure of 71 in HI and NaNO2 delivered aryl iodide 2 in 80% yield (Fig. 3A, Eq. e). These experiments disclosed that present denitrative iodination proceed via the reduction and downstream Sandmeyer mechanism. According to previous literatures [101–103], we hypothesized that nitrosobenzene and phenylhydroxylamine may serve as intermediates in the iodide-induced reduction of nitro groups. Conforming to our expectations, nitrosobenzene 74 and phenyl hydroxylamine 76 were both smoothly converted to aniline 75 in high yields under the HI-mediated reduction condition (Fig. 3A, Eqs. f-g). These findings pointed to the possibility that the reduction process might operate through both two intermediates.
|
Download:
|
| Fig. 3. Mechanistic investigations. | |
Based on the aforementioned experimental observations, a possible mechanism was proposed in Fig. 3B. The nitro group was initially activated by the high concentration of protons in the system to generate a highly electrophilic active species, which subsequently underwent nucleophilic attack by iodide ions and deoxygenation to form a N-I bonded species. Then the subsequent I2 elimination progress took place, yielding the nitrosoarene intermediate 74. The generated nitroso group is further activated in the acidic medium, undergoing a similar deoxygenation transformation to form the phenylhydroxylamine 76, which was further activated and reduced to form the aniline 75. Upon the addition of sodium nitrite, the aniline was oxidized to the corresponding diazonium salt, which subsequently experienced single-electron transfer (SET) reduction by the remaining iodide ions. This process released nitrogen and formed aryl radicals, which were immediately captured by the simultaneously generated iodine atoms to yield the target aryl iodide product.
In conclusion, we have established a simple and efficient denitrative iodination of nitroarenes driven by hydroiodic acid via a sustainable and easy-handled one-pot progress. As opposed to the prior reports, this methodology provided a short and high atom-economic strategy to convert nitroarenes into corresponding aryl iodides, and has been successfully applied in the concise synthesis of functional molecules. Mechanistic studies reveal that this transformation follows a continuous pathway involving reduction, diazotization, and iodination. The exclusive addition but dual role of hydroiodic acid, simple and practical reaction conditions, utilization of economical and sustainable reagents, and good functional group tolerance make this chemistry a distinctive and practical strategy for the preparation of aryl iodides, offering substantial prospects for widespread utilization in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementQingxuan Kong: Writing – original draft, Investigation. Changwei Jiang: Validation, Formal analysis. Bin Lyu: Formal analysis, Data curation. Zhaoting Li: Formal analysis, Data curation. Ning Jiao: Writing – review & editing. Song Song: Writing – review & editing, Project administration, Funding acquisition.
AcknowledgmentsWe acknowledge the National Key Research and Development Project (No. 2023YFF1205103), the National Natural Science Foundation of China (No. 22371007), and the Peking University Medicine plus X Pilot Program-Key Technologies R&D Project (No. 2024YXXLHGG001) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2025.111444.
| [1] |
B.R. Smith, C.M. Eastman, J.T. Njardarson, J. Med. Chem. 57 (2014) 9764-9773. DOI:10.1021/jm501105n |
| [2] |
Y. Kondo, H. Kimura, M. Sasaki, et al., ACS Omega 8 (2023) 24418-24425. DOI:10.1021/acsomega.3c01974 |
| [3] |
H. McErlain, M.J. Andrews, A.J.B. Watson, S.L. Pimlott, A. Sutherland, Org. Lett. 26 (2024) 1528-1532. DOI:10.1021/acs.orglett.4c00356 |
| [4] |
M. Diop, A. Samb, V. Costantino, E. Fattorusso, A. Mangoni, J. Nat. Prod. 59 (1996) 271-272. DOI:10.1021/np960053n |
| [5] |
A. Yoshimura, V.V. Zhdankin, Chem. Rev. 116 (2016) 3328-3435. DOI:10.1021/acs.chemrev.5b00547 |
| [6] |
A.B. Dounay, L.E. Overman, Chem. Rev. 103 (2003) 2945-2964. DOI:10.1021/cr020039h |
| [7] |
R. Chinchilla, C. Nájera, Chem. Soc. Rev. 40 (2011) 5084-5121. DOI:10.1039/c1cs15071e |
| [8] |
D. Tilly, F. Chevallier, F. Mongin, P.C. Gros, Chem. Rev. 114 (2014) 1207-1257. DOI:10.1021/cr400367p |
| [9] |
F. Khan, M. Dlugosch, X. Liu, M.G. Banwell, Acc. Chem. Res. 51 (2018) 1784-1795. DOI:10.1021/acs.accounts.8b00169 |
| [10] |
B. Wei, Y.-H. Chen, P. Knochel, Acc. Chem. Res. 57 (2024) 1951-1963. DOI:10.1021/acs.accounts.4c00242 |
| [11] |
G.K.S. Prakash, T. Mathew, D. Hoole, et al., J. Am. Chem. Soc. 126 (2004) 15770-15776. DOI:10.1021/ja0465247 |
| [12] |
C.N. Kona, R. Oku, S. Nakamura, et al., Chem 10 (2024) 402-413. DOI:10.1016/j.chempr.2023.10.006 |
| [13] |
A. Podgoršek, M. Zupan, J. Iskra, Angew. Chem. Int. Ed. 48 (2009) 8424-8450. DOI:10.1002/anie.200901223 |
| [14] |
F. Mo, J.M. Yan, D. Qiu, et al., Angew. Chem. Int. Ed. 49 (2010) 2028-2032. DOI:10.1002/anie.200906699 |
| [15] |
Q. Shi, S. Zhang, J. Zhang, et al., J. Am. Chem. Soc. 138 (2016) 3946-3949. DOI:10.1021/jacs.5b12259 |
| [16] |
X. He, X. Wang, Y.L. Tse, Z. Ke, Y.Y. Yeung, Angew. Chem. Int. Ed. 57 (2018) 12869-12873. DOI:10.1002/anie.201806965 |
| [17] |
S.C. Fosu, C.M. Hambira, A.D. Chen, J.R. Fuchs, D.A. Nagib, Chem. 5 (2019) 417-428. DOI:10.1016/j.chempr.2018.11.007 |
| [18] |
Y. Nishii, M. Ikeda, Y. Hayashi, S. Kawauchi, M. Miura, J. Am. Chem. Soc. 142 (2020) 1621-1629. DOI:10.1021/jacs.9b12672 |
| [19] |
M. Liu, L.J. Li, J. Zhang, H. Xu, H.X. Dai, Chin. Chem. Lett. 31 (2020) 1301-1304. DOI:10.1016/j.cclet.2019.09.057 |
| [20] |
W. Wang, X. Yang, R. Dai, et al., J. Am. Chem. Soc. 144 (2022) 13415-13425. DOI:10.1021/jacs.2c06440 |
| [21] |
H. Mondal, S. Patra, S. Saha, et al., Angew. Chem. Int. Ed. 62 (2023) e202312597. DOI:10.1002/anie.202312597 |
| [22] |
W. Wang, S. Song, N. Jiao, Acc. Chem. Res. 57 (2024) 3161-3181. DOI:10.1021/acs.accounts.4c00501 |
| [23] |
Y. Wang, C. Bi, Y. Kawamata, et al., Nat. Chem. 16 (2024) 1539-1545. DOI:10.1038/s41557-024-01539-4 |
| [24] |
S. Torabi, M. Jamshidi, G. Hilt, Angew. Chem. Int. Ed. 64 (2025) e202422442. DOI:10.1002/anie.202422442 |
| [25] |
H. Finkelstein, Ber. Dtsch. Chem. Ges. 43 (1910) 1528-1532. DOI:10.1002/cber.19100430257 |
| [26] |
L. Li, W. Liu, H. Zeng, et al., J. Am. Chem. Soc. 137 (2015) 8328-8331. DOI:10.1021/jacs.5b03220 |
| [27] |
W. Liu, X. Yang, Y. Gao, C.J. Li, J. Am. Chem. Soc. 139 (2017) 8621-8627. DOI:10.1021/jacs.7b03538 |
| [28] |
T. Morack, T.E. Myers, L.J. Karas, et al., J. Am. Chem. Soc. 145 (2023) 22322-22328. DOI:10.1021/jacs.3c08727 |
| [29] |
A. Varenikov, E. Shapiro, M. Gandelman, Chem. Rev. 121 (2021) 412-484. DOI:10.1021/acs.chemrev.0c00813 |
| [30] |
H. Hunsdiecker, C. Hunsdiecker, Ber. Dtsch. Chem. Ges. 75 (1942) 291-297. DOI:10.1002/cber.19420750309 |
| [31] |
Y.H. Lee, B. Morandi, Nat. Chem. 10 (2018) 1016-1022. DOI:10.1038/s41557-018-0078-8 |
| [32] |
J.M. Murphy, X. Liao, J.F. Hartwig, J. Am. Chem. Soc. 129 (2007) 15434-15435. DOI:10.1021/ja076498n |
| [33] |
C. Cheng, J.F. Hartwig, Science 343 (2014) 853-857. DOI:10.1126/science.1248042 |
| [34] |
S. Rozen, M. Carmeli, J. Am. Chem. Soc. 125 (2003) 8118-8119. DOI:10.1021/ja035616d |
| [35] |
R. Calvo, K. Zhang, A. Passera, D. Katayev, Nat. Commun. 10 (2019) 3410. DOI:10.1038/s41467-019-11419-y |
| [36] |
S. Majedi, S. Majedi, F. Behmagham, Chem. Rev. Lett. 2 (2019) 187-192. |
| [37] |
T. Long, L. Liu, Y. Tao, et al., Angew. Chem. Int. Ed. 60 (2021) 13414-13422. DOI:10.1002/anie.202102287 |
| [38] |
I. Mosiagin, A.J. Fernandes, A. Budinská, et al., Angew. Chem. Int. Ed. 62 (2023) e202310851. DOI:10.1002/anie.202310851 |
| [39] |
S. Patra, I. Mosiagin, R. Giri, T. Nauser, D. Katayev, Angew. Chem. Int. Ed. 62 (2023) e202300533. DOI:10.1002/anie.202300533 |
| [40] |
Y. Zheng, Q.Q. Hu, Q. Huang, Y. Xie, Org. Lett. 26 (2024) 3316-3320. DOI:10.1021/acs.orglett.4c01006 |
| [41] |
K. Muto, T. Okita, J. Yamaguchi, ACS Catal. 10 (2020) 9856-9871. DOI:10.1021/acscatal.0c02990 |
| [42] |
H. Sun, S.G. DiMagno, Angew. Chem. Int. Ed. 45 (2006) 2720-2725. DOI:10.1002/anie.200504555 |
| [43] |
F. Inoue, M. Kashihara, M.R. Yadav, Y. Nakao, Angew. Chem. Int. Ed. 56 (2017) 13307-13309. DOI:10.1002/anie.201706982 |
| [44] |
M.R. Yadav, M. Nagaoka, M. Kashihara, et al., J. Am. Chem. Soc. 139 (2017) 9423-9426. DOI:10.1021/jacs.7b03159 |
| [45] |
W. Chen, K. Chen, W. Chen, M. Liu, H. Wu, ACS Catal. 9 (2019) 8110-8115. DOI:10.1021/acscatal.9b02760 |
| [46] |
P. Ryabchuk, T. Leischner, C. Kreyenschulte, et al., Angew. Chem. Int. Ed. 59 (2020) 18679-18685. DOI:10.1002/anie.202007613 |
| [47] |
J. Liu, Y. Yang, K. Ouyang, W.X. Zhang, Green Synth. Catal. 2 (2021) 87-122. DOI:10.1117/12.2604794 |
| [48] |
M. Kashihara, Y. Nakao, Acc. Chem. Res. 54 (2021) 2928-2935. DOI:10.1021/acs.accounts.1c00220 |
| [49] |
Z. Lei, J. Yao, Y. Xiao, et al., Chem. Sci. 15 (2024) 3552-3561. DOI:10.1039/d3sc06618e |
| [50] |
R.V. Jagadeesh, A.E. Surkus, H. Junge, et al., Science 342 (2013) 1073-1076. DOI:10.1126/science.1242005 |
| [51] |
X. Liu, H.Q. Li, S. Ye, et al., Angew. Chem. Int. Ed. 53 (2014) 7624-7628. DOI:10.1002/anie.201404543 |
| [52] |
C.W. Cheung, M.L. Ploeger, X. Hu, Nat. Commun. 8 (2017) 14878. DOI:10.1038/ncomms14878 |
| [53] |
M. Rauser, C. Ascheberg, M. Niggemann, Angew. Chem. Int. Ed. 56 (2017) 11570-11574. DOI:10.1002/anie.201705356 |
| [54] |
C.W. Cheung, J.A. Ma, X. Hu, J. Am. Chem. Soc. 140 (2018) 6789-6792. DOI:10.1021/jacs.8b03739 |
| [55] |
T.V. Nykaza, J.C. Cooper, G. Li, et al., J. Am. Chem. Soc. 140 (2018) 15200-15205. DOI:10.1021/jacs.8b10769 |
| [56] |
J. Xiao, Y. He, F. Ye, S. Zhu, Chem 4 (2018) 1645-1657. DOI:10.1016/j.chempr.2018.04.008 |
| [57] |
L. Wang, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 58 (2019) 5417-5421. DOI:10.1002/anie.201814146 |
| [58] |
G. Li, T.V. Nykaza, J.C. Cooper, et al., J. Am. Chem. Soc. 142 (2020) 6786-6799. DOI:10.1021/jacs.0c01666 |
| [59] |
H. Song, Z. Yang, C.H. Tung, W. Wang, ACS Catal. 10 (2020) 276-281. DOI:10.1021/acscatal.9b03604 |
| [60] |
G. Li, S.P. Miller, A.T. Radosevich, J. Am. Chem. Soc. 143 (2021) 14464-14469. DOI:10.1021/jacs.1c07272 |
| [61] |
B.D. Akana-Schneider, D.J. Weix, J. Am. Chem. Soc. 145 (2023) 16150-16159. DOI:10.1021/jacs.3c04647 |
| [62] |
A. Bhunia, K. Bergander, C.G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 60 (2021) 8313. DOI:10.1002/anie.202015740 |
| [63] |
T. Patra, T. Wirth, Angew. Chem. Int. Ed. 61 (2022) e202213772. DOI:10.1002/anie.202213772 |
| [64] |
A. Ruffoni, C. Hampton, M. Simonetti, D. Leonori, Nature 610 (2022) 81-86. DOI:10.1038/s41586-022-05211-0 |
| [65] |
D.E. Wise, E.S. Gogarnoiu, A.D. Duke, et al., J. Am. Chem. Soc. 144 (2022) 15437-15442. DOI:10.1021/jacs.2c05648 |
| [66] |
H. Qin, R. Liu, Z. Wang, et al., Angew. Chem. Int. Ed. 64 (2025) e202416923. DOI:10.1002/anie.202416923 |
| [67] |
L.W. Ye, X.Q. Zhu, R.L. Sahani, et al., Chem. Rev. 121 (2021) 9039-9112. DOI:10.1021/acs.chemrev.0c00348 |
| [68] |
B. Li, A. Ruffoni, D. Leonori, Angew. Chem. Int. Ed. 62 (2023) e202310540. DOI:10.1002/anie.202310540 |
| [69] |
G. Li, M.N. Lavagnino, S.Z. Ali, S. Hu, A.T. Radosevich, J. Am. Chem. Soc. 145 (2023) 41-46. DOI:10.1021/jacs.2c12450 |
| [70] |
E. Matador, M.J. Tilby, I. Saridakis, et al., J. Am. Chem. Soc. 145 (2023) 27810-27820. DOI:10.1021/jacs.3c10863 |
| [71] |
R. Sánchez-Bento, B. Roure, J. Llaveria, A. Ruffoni, D. Leonori, Chem 9 (2023) 3685-3695. DOI:10.1016/j.chempr.2023.10.008 |
| [72] |
R. Mykura, R. Sánchez-Bento, E. Matador, et al., Nat. Chem. 16 (2024) 771-779. DOI:10.1038/s41557-023-01429-1 |
| [73] |
J. Elfert, A. Bhunia, C.G. Daniliuc, A. Studer, ACS Catal. 13 (2023) 6704-6709. DOI:10.1021/acscatal.3c00958 |
| [74] |
J.M. Paolillo, A.D. Duke, E.S. Gogarnoiu, D.E. Wise, M. Parasram, J. Am. Chem. Soc. 145 (2023) 2794-2799. DOI:10.1021/jacs.2c13502 |
| [75] |
T. Sandmeyer, Ber. Dtsch. Chem. Ges. 17 (1884) 1633-1635. DOI:10.1002/cber.18840170219 |
| [76] |
F. Kaufler, Ber. Dtsch. Chem. Ges. 37 (1904) 59-66. DOI:10.1002/cber.19040370113 |
| [77] |
H.H. Hodgson, Chem. Rev. 40 (1947) 251-277. DOI:10.1021/cr60126a003 |
| [78] |
F. Mo, G. Dong, Y. Zhang, J. Wang, Org. Biomol. Chem. 11 (2013) 1582-1593. DOI:10.1039/c3ob27366k |
| [79] |
Q. Liu, B. Sun, Z. Liu, et al., Chem. Sci. 9 (2018) 8731-8737. DOI:10.1039/c8sc03346c |
| [80] |
F. Mo, D. Qiu, Y. Zhang, J. Wang, Acc. Chem. Res. 51 (2018) 496-506. DOI:10.1021/acs.accounts.7b00566 |
| [81] |
R. Akhtar, A.F. Zahoor, N. Rasool, M. Ahmad, K.G. Ali, Mol. Divers. 26 (2022) 1837-1873. DOI:10.1007/s11030-021-10295-3 |
| [82] |
N. Sivendran, F. Belitz, D. Sowa Prendes, et al., Chem. Eur. J. 28 (2022) e202103669. DOI:10.1002/chem.202103669 |
| [83] |
J. Mateos, T. Schulte, D. Behera, et al., Science 384 (2024) 446-452. DOI:10.1126/science.adn7006 |
| [84] |
M. Deng, K. Liu, Z. Ma, G. Luo, L. Dian, Green Chem. 26 (2024) 11556-11562. DOI:10.1039/d4gc04210g |
| [85] |
T. Liang, Z. Lyu, Y. Wang, et al., Nat. Chem. 17 (2025) 598-605. DOI:10.1038/s41557-024-01728-1 |
| [86] |
R. Hernández-Ruiz, S. Gómez-Gil, M.R. Pedrosa, S. Suárez-Pantiga, R. Sanz, Org. Biomol. Chem. 21 (2023) 7791-7798. DOI:10.1039/d3ob01187a |
| [87] |
J.W. Li, T.S. Duan, B. Sun, F.L. Zhang, Org. Biomol. Chem. 22 (2024) 2819-2823. DOI:10.1039/d4ob00309h |
| [88] |
A. Goto, A. Ohtsuki, H. Ohfuji, M. Tanishima, H. Kaji, J. Am. Chem. Soc. 135 (2013) 11131-11139. DOI:10.1021/ja4036016 |
| [89] |
J.A. Luján-Montelongo, J.B. Mateus-Ruiz, R.M. Valdez-García, Eur. J. Org. Chem. 26 (2023) e202201156. DOI:10.1002/ejoc.202201156 |
| [90] |
M. Lemmerer, V. Tona, D. Just, et al., Angew. Chem. Int. Ed. 64 (2025) e202409688. DOI:10.1002/anie.202409688 |
| [91] |
S. Song, X. Li, J. Wei, et al., Nat. Catal. 3 (2020) 107-115. DOI:10.17245/jdapm.2020.20.3.107 |
| [92] |
W. Wang, X. Li, X. Yang, et al., Nat. Commun. 12 (2021) 3873. DOI:10.1038/s41467-021-24174-w |
| [93] |
W. Wang, T. Huo, X. Zhao, et al., CCS Chem. 2 (2020) 566-575. DOI:10.31635/ccschem.020.202000172 |
| [94] |
B.T. Boyle, J.N. Levy, L. de Lescure, R.S. Paton, A. McNally, Science 378 (2022) 773-779. DOI:10.1126/science.add8980 |
| [95] |
J.N. Levy, J.V. Alegre-Requena, R. Liu, R.S. Paton, A. McNally, J. Am. Chem. Soc. 142 (2020) 11295-11305. DOI:10.1021/jacs.0c04674 |
| [96] |
H. Cao, Q. Cheng, A. Studer, Science 378 (2022) 779-785. DOI:10.1126/science.ade6029 |
| [97] |
C. Li, Z. Yan, B. Wang, et al., Chem. 10 (2024) 628-643. DOI:10.1016/j.chempr.2023.10.015 |
| [98] |
J.M. Jacobs, P.R. Sinclair, N. Gorman, et al., J. Biochem. Mol. Toxicol. 7 (1992) 87-95. DOI:10.1002/jbt.2570070206 |
| [99] |
I. Ghosh, N. Shlapakov, T.A. Karl, et al., Nature 619 (2023) 87-93. |
| [100] |
R. Moningka, F.A. Romero, N.B. Hastings, et al., Bioorg. Med. Chem. Lett. 30 (2020) 127510. DOI:10.1016/j.bmcl.2020.127510 |
| [101] |
G. Wienhöfer, I. Sorribes, A. Boddien, et al., J. Am. Chem. Soc. 133 (2011) 12875-12879. DOI:10.1021/ja2061038 |
| [102] |
Q. Xiao, S. Sarina, E.R. Waclawik, et al., ACS Catal. 6 (2016) 1744-1753. DOI:10.1021/acscatal.5b02643 |
| [103] |
J. Strachan, C. Barnett, A.F. Masters, T. Maschmeyer, ACS Catal. 10 (2020) 5516-5521. DOI:10.1021/acscatal.0c00725 |
 2025, Vol. 36
2025, Vol. 36 


