Ion detection in complex systems is of significant importance for environmental monitoring, industrial process control, medical diagnostics, and scientific research [1,2]. Ion-selective electrodes (ISEs) have been widely used as potentiometric sensors in chemical analysis for decades [3,4]. Among them, solid-contact ion-selective electrode (SC-ISE) has been widely applied in environmental monitoring due to its series of advantages such as high sensitivity, portability, and rapid response [5-8]. The application of SC-ISEs is challenged by the growth of bacteria and other microorganisms in complex systems (such as seawater, urine) and the biofouling caused by biological substances adhering to the electrode surface [9,10]. "Biological fouling" refers to the undesirable attachment and colonization of organisms and their by-products on surfaces. This phenomenon arises when biomolecules, such as proteins, adhere to the surface, promoting attachment and facilitating the accumulation of bacteria, ultimately resulting in the formation of a distinct biofilm [11,12]. The attachment of biofouling to the surface of electrodes not only alters the interface morphology but also interferes with the interaction between the electrode and ions in the solution [13,14]. This interference can decrease the electrode's selectivity and responsiveness, significantly impacting the analytical accuracy and stability of sensors during long-term monitoring. Therefore, the exploration of various strategies to address sensor biofouling and develop antifouling materials continues to be a significant challenge.
Common surface-modified antifouling materials include hydrophilic materials such as polyethylene glycol-based polymers [15,16] and oligo ethylene glycol (OEG) [17]. While these materials exhibit excellent antifouling properties, drawbacks have been reported in the literature, such as susceptibility to oxidative damage, limiting their further applications. Furthermore, widely used antimicrobial materials, such as silver nanoparticles [18] and quaternary ammonium compounds [19], can potentially pose environmental issues in complex systems. Therefore, peptides with environmentally friendly attributes, ease of synthesis and storage, cost-effectiveness, and tunable structures, have recently been explored as potential antifouling materials [20-22]. Peptides, composed of amino acids, offer a diverse molecular structure that allows for the design of specific properties, such as resistance to biological adhesion [23,24] and antimicrobial performance [25]. For example, the peptide-modified sensor reported by our research group has demonstrated excellent resistance to nonspecific biofouling when detecting target analytes in sweat and blood [26-28]. The Bai's group has prepared a physically and biologically synergistic antifouling coating by grafting the antimicrobial peptide nisin onto a glass substrate. This coating enhances resistance against the attachment of algae and bacteria in seawater [29]. Overall, the reported peptides can generally be categorized into "anti-biofouling" peptides [30] that prevent the adhesion of organisms to surfaces and "bacteriostatic" peptides that kill accumulated bacteria [31].
In this work, we proposed a functional Y-type polypeptide (DOPA-K(RWRWRW-)EKEKEKEK)-modified potassium ion-selective electrode through a strategy of integrating anti-adhesion function and bactericidal function, as shown in Scheme S1 (Supporting information). It was worth noting that the material of the transducer layer significantly influences the ion-electron transfer rate of sensors. Conductive porous metal-organic frameworks (MOF) materials, owing to their large specific surface area, provide numerous active sites for ion transfer [32,33], facilitating the transport and conversion of ions. Consequently, they emerged as a competitive choice for transducer layer materials. In this study, the assembly of Ni3(HITP)2 material into an array on a gold electrode was chosen as the transducer layer. In addition, the antifouling layer was composed of Y-type peptides, comprising resistance chains, antibacterial chains, and adhesive amino acids. The role of the adhesive amino acids was to enhance the peptide's adhesion to the surface of the ion membrane, essentially acting as a bonding agent [34,35]. The prepared antifouling potassium ion sensor used an advanced microbial growth instrument to monitor the antifouling condition of the membrane surface in real time, and was applied in complex systems to characterize its detection performance. The experimental details are provided in Supporting information.
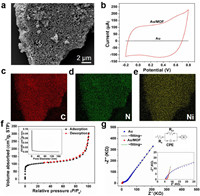
The scanning electron microscope (SEM) was utilized to identify the surface morphology of the solid transducer layer of MOF material and the images are shown in Fig. 1. The micrographs reveal the self-assembly of MOF on the surface of Au electrode, forming a micro-nano fiber array. The fibers exhibit lengths of approximately 1 µm, with diameters ranging from 80 nm to 110 nm. Energy dispersive X-ray spectroscopy (EDS) results (Figs. 1c–e) show the presence of C, N, and Ni elements, confirming the formation of a MOF comprising nickel and an organic linker. XPS was employed to further investigate the chemical states of surface elements in Ni₃(HITP)₂. The survey spectra in Fig. S1b (Supporting information) confirm the presence of Ni, N, C, and O resonance peaks originating from the material itself and water guest molecules. In the high-resolution XPS Ni 2p spectrum (Fig. S1c in Supporting information), the binding energy peaks at 873.1 and 855.5 eV correspond to the spin-orbitals of Ni 2p3/2 and 2p1/2, respectively, and are assigned to Ni-Nx. Additionally, the observed satellite peaks at 879.1 and 861.4 eV are likely attributed to the shakeup excitation of the high-spin Ni2+ states [36,37]. To further investigate the capacitive properties of Ni3(HITP)2, cyclic voltammetry (CV) test was also performed as displayed in Fig. 1b. Comparing to the bare gold electrode, the CV curve of the gold electrode post self-assembly of Ni₃(HITP)₂ exhibits a significantly larger and more rectangular-shaped current window, indicating a higher capacitance for Ni₃(HITP)₂. According to the formula C = Iavg/(s⋅ΔV′), the specific capacitance of the solid-state conductive layer is calculated to be 28 mF/cm2, where Iavg is the average current, s is the scan rate, and ΔV' is the potential window. This enhancement is attributed to the substantial specific surface area of Ni₃(HITP)₂, as evidenced by the N₂ adsorption-desorption isotherm presented in Fig. 1f, with its specific surface area calculated to be 325.6 m2/g. The inset in Fig. 1f further confirms the porous structure of Ni₃(HITP)₂, with the main micropore size about 1 nm. Moreover, the impedance spectrum of the solid-state transducing layer is illustrated in Fig. 1g, with a charge transfer resistance (Rct) of 5.36 kΩ cm2 for Ni₃(HITP)₂. The results affirm that Ni₃(HITP)₂ provides rapid electron transfer performance at the electrode-solution interface. CPE and W represent the constant phase element modeling the double-layer capacitance and the diffusion resistance, respectively (Table S1 in Supporting information).
|
Download:
|
| Fig. 1. (a) SEM images of MOF/Au, (b) Cyclic voltammetry curves for the gold and Au/MOF electrodes in 10–3 mol/L KCl solution at a scan rate of 100 mV/s. (c-e) EDS maps for MOF (c: carbon, d: nitrogen, e: nickel). (f) N2 adsorption-desorption isotherms and pore size distribution (inset) of the MOF. (g) Impedance spectra of the Au (blue) and Au/MOF (red) electrode recorded in 10–3 mol/L KCl solution at the open-circuit potential. The frequency range examined ranged from 100 kHz to 0.1 Hz with a potential amplitude of 10 mV. | |
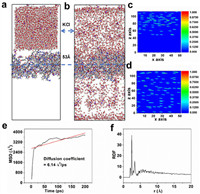
MD simulation was performed using large-scale parallel atomic/molecular simulation software (LAMMPS23) [38,39]. Antifouling peptides are used as a coating on the transfer interface in a 0.1 mol/L KCl solution to create an adsorption simulation model. The polypeptide is initially located at 53 Å in the model, as shown in Figs. 2a and b. Potassium ions are distributed at a longitudinal distance away from 53 Å. After molecular dynamics simulations, potassium ions successfully pass through the polypeptide and are dispersed on both sides of the polypeptide membrane. The variations in potassium ions and water molecules relative to the surface distance are depicted in Fig. S2 (Supporting information). To gain a more intuitive understanding of the potassium ion density distribution, the molecular distances in the 3D space were examined to validate the transport of ions (Figs. 2c and d). The diffusion of K+ ions is observed to be rapid at 22 ps in Fig. 2e, stabilizing thereafter. The calculated diffusion coefficient from the simulation is 6.14 Å2/ps, indicating a fast diffusion of potassium ions within the peptide coating. The radial distribution function (RDF) reveals that effective binding occurs between the nitrogen (N) in the peptide molecules and potassium ions at radial distances of 2–5 Å, indicating the existence of adsorption and desorption of potassium ions in the peptide layer (Fig. 2f). Collectively, the successful permeation of K+ through the polypeptide membrane can be attributed to three main reasons: (1) The Y-shaped polypeptide membrane has a porous structure, with pores acting as channels that facilitate ion transport, including adsorption, migration, and desorption processes. (2) The nitrogen in polypeptide amino groups has lone pair electrons, making it electrophilic and more likely to interact or bind with K+ ions. (3) The electrophilic nature of nitrogen may lead to interactions with K+ ions, involving mechanisms such as charge attraction, hydrogen bond formation, or other interaction modalities.
|
Download:
|
| Fig. 2. (a, b) Equilibrium configuration diagram and (c, d) 3D density distribution diagram of initial and final states of adsorption. (e) Molecular dynamics simulation K+ diffusion coefficient, and (f) K+-N radial distribution function. | |
Furthermore, optimization of the concentration of antimicrobial peptides against contamination was conducted based on the Nernstian response slope (Fig. S3 in Supporting information). A concentration of 0.1 mol/L was selected as the optimal experimental concentration for subsequent investigations.
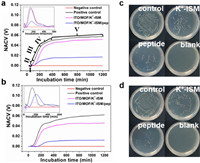
Herein, the antifouling property of the designed Y-shaped peptide modified electrode was evaluated. The bacterial growth kinetics were monitored in real-time using a 32-channel non-contact conductivity sensor [40,41]. As shown in Figs. 3a and b, the expected S-shaped curves were obtained for the diluted bacterial solution of the positive control (inoculation concentration of 106 CFU/mL), the electrode incubated with the peptide, and the electrode without the peptide incubated. Notably, whether in E. coli or S. aureus solutions, the microbial growth curve following the immersion of the electrode surface in bacterial solution after peptide incubation displayed a prolonged lag phase compared to electrodes without peptide incubation. Moreover, the onset of the exponential phase, representing the rapid growth rate stage, was delayed, as illustrated by the rate growth curves (inset in Figs. 3a and b). Following the exponential phase, a deceleration phase ensued until entering a stationary phase, with the normalized apparent conductivity values (NACV) of microbial growth curves on peptide-incubated electrodes being relatively smaller. The microbial growth kinetics data above suggest that peptide incubation effectively inhibits microbial adhesion on electrode surfaces and contributes to the delay in microbial growth. The plate counting method also verified the above conclusion (Figs. 3c and d). The number of colonies on the electrode surface was significantly reduced after incubating the peptide, indicating that the Y-type peptide can effectively inhibit the adhesion of microorganisms. It is noteworthy that replacing the potassium ion-selective membrane with a calcium ion-selective membrane yielded a similar antifouling effect (Fig. S4 in Supporting information). To validate the antibacterial activity of the Y-shaped peptide, fluorescence analysis was carried out on electrodes incubated with the peptide and electrodes without peptide incubation following immersion in bacterial liquid. As illustrated in Fig. S5 (Supporting information), a comparative analysis demonstrated a decrease in the number of viable bacteria after incubation with the peptide. Furthermore, an increased number of dead bacteria were observed on the surface of the electrode post peptide incubation, confirming the antibacterial efficacy of the Y-shaped peptide. Additionally, the antifouling results on the surfaces of electrodes treated with the test bacteria in a seawater environment are shown in Fig. S6 (Supporting information). The electrodes incubated with the peptide exhibited remarkable antifouling capabilities, leading to the inference that peptide incubation contributes to the prolonged operational stability of electrodes in real complex system.
|
Download:
|
| Fig. 3. Typical contactless conductometric sensor growth curves (NACV vs. incubation time) of (a) E. coli and (b) S. aureus with different electrodes. Illustrations in a and b represent microbial growth rate curves. Ⅰ: lag phase, Ⅱ: acceleration phase, Ⅲ: exponential phase, Ⅳ: deceleration phase, Ⅴ: stationary phase. The images of (c) E. coli and (d) S. aureus colonies plated on different coated surfaces. | |
The introduction of antifouling peptides should not come at the expense of compromising detection performance. In light of this, Nernstian potential response tests were conducted on Au/MOF/K+-ISM/pep, Au/MOF/K+-ISM, and Au/K+-ISM within the concentration range of 10–10.5–10–3 mol/L (Fig. 4a). The linear range of the sensor without self-assembled Ni₃(HITP)₂ was 10–6–10–3 mol/L with a Nernstian response slope of 55 ± 0.32 mV/dec. In comparison, the sensor treated with self-assembled Ni₃(HITP)₂ exhibits a broader linear range of 10–8–10–3 mol/L. This is attributed to the large specific surface area of the introduced porous MOF, which increased active sites, thereby enhancing the interfacial ion-electron transfer. Moreover, incubation with the peptide did not cause a significant change in the Nernstian response range of the sensor, with the detection limit slightly increasing from 1.26 nmol/L to 2.51 nmol/L. Experimental results indicate that the sensor maintains excellent detection performance after treatment with antifouling fouling peptides. As shown in Fig. 4b, the response time of Au/K+-ISM was 20–28 s, whereas the sensor prepared after self-assembly of porous Ni₃(HITP)₂ exhibited a reduced response time within 2–8 s to reach a stable response potential. Furthermore, the peptide layer features a broad ion transport channel, allowing ions to have a larger diffusion coefficient within the peptide layer (Fig. 2). Combined with the advantages of MOF, this enables a rapid response. Therefore, incubating the polypeptide does not affect the response time of the sensor.
|
Download:
|
| Fig. 4. (a) Potential response plots and (b) time-dependent potentiometric responses for Au/MOF/K+-ISM/pep, Au/MOF/K+-ISM and Au/K+-ISM in the concentration range of 10−10.5–10−3 mol/L. | |
Compared to previously reported all-solid-state K+-ISEs (Table S2 in Supporting information), the Au/MOF/K+-ISM/pep exhibits significant advantages in terms of detection limit, response time, and reduced chronopotentiometric potential drift, demonstrating its promising potential for practical application.
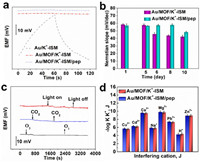
To investigate the short-term stability of the prepared sensors, a current of ±1 nA was applied to the electrode to obtain the chronopotentiometric test results (Fig. 5a). According to the calculation formula ΔE/Δt, the potential short-term stabilities were determined as 702.8, 2.8, and 2.5 µV/s for Au/K+-ISM, Au/MOF/K+-ISM/pep and Au/MOF/K+-ISM, respectively. The results indicate that using porous MOF as a transduction layer in constructing K+-ISE provides optimal short-term potential stability. This excellent capability may be attributed to the outstanding ion-to-electron transfer properties of porous MOF. Additionally, the introduction of a peptide antifouling layer does not compromise the short-term stability of the electrode.
|
Download:
|
| Fig. 5. (a) Chronopotentiograms for Au/MOF/K+-ISM/pep, Au/MOF/K+-ISM and Au /K+-ISM. (b) Long term stability of electrodes Au/MOF/K+-ISM/pep and Au/MOF/K+-ISM in bacterial solution environment. (c) Effects of O2, CO2 and light on the potential stability of Au/MOF/K+-ISM/pep. (d) Selectivity coefficients for Au/MOF/K+-ISM/pep and Au/MOF/K+-ISM. | |
Furthermore, the long-term stability of the prepared electrodes was explored by exposing them continuously to bacterial suspension for different durations, and the changes in Nernstian slope are illustrated in Fig. 5b. It can be observed that the K+-ISE with a peptide coating exhibits relatively stable potential response over an 8 days testing period. In contrast, the response slope of the control Au/MOF/K+-ISM remained stable for 5 days, but began to deviate from the theoretical value after 6 days of immersion, worsening over time. Moreover, the Au/MOF/K+-ISM/pep electrode maintained a stable standard electrode potential E0 of 676 ± 3.16 mV over 8 days. In contrast, the Au/MOF/K+-ISM electrode maintained a standard electrode potential E0 of 677 ± 1.41 mV for 5 days, but the potential dropped to 648 ± 2.36 mV on the sixth day (Fig. S7 in Supporting information). A thicker biofilm on the control electrode's surface impacted the all-solid-state ion-selective electrode's response. Additionally, the potential stability of Au/MOF/K+-ISM/pep was investigated under light, O2 and CO2. As shown in Fig. 5c, the prepared Au/MOF/K+-ISM/pep electrode is insensitive to light, O2, and CO2, and there is a slight potential fluctuation in the middle caused by external interferences such as airflow and external inductance. Moreover, the Au/MOF/K+-ISM/pep electrode demonstrates excellent reproducibility and repeatability (Fig. S8 in Supporting information). Additionally, in the water layer tests (Fig. S9 in Supporting information), Au/MOF/K+-ISM/pep and Au/MOF/K+-ISM exhibited smaller potential drifts, indicating that MOF, as a solid transduction layer material, enhanced the ion-electron transfer rate, thereby improving electrode stability. Similarly, the potential drift of Au/MOF/K+-ISM/pep over 10 days is 1.94 mV/day, while for Au/MOF/K+-ISM it is 2.02 mV/day, with little difference between the two (Fig. S10 in Supporting information).
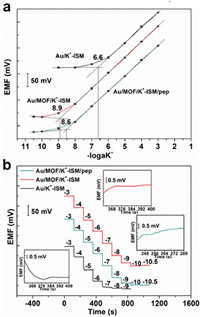
The selectivity of Au/MOF/K+-ISM/pep and Au/MOF/K+-ISM for K+ was examined using a separate solution method, testing in environments with a range of cations (Cu2+, Cd2+, H+, Na+, Mg2+, Ca2+, Pb2+, Zn2+) [42]. The potentiometric selectivity coefficients
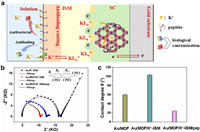
Reportedly, Ni3(HITP)2 represents a novel π-d conjugated MOF formed through robust metal–ligand orbital hybridization between the d orbitals of transition metal ions and the π orbitals of organic ligands, thereby generating π-d orbitals [43]. The presence of delocalized charges throughout the system contributes to its outstanding conductivity. Simultaneously, as an energy storage material with significant electric double-layer capacitance, its large specific surface area provides numerous active sites. This feature is beneficial for the efficient conversion and transmission of ions and electrons. As illustrated in Fig. 6a, the ion-electron process of the prepared sensor involves the complexation of potassium ions with the species within the ion-selective membrane. Subsequently, these ions pass through the ion membrane, and capacitive coupling occurs between the ions and the Ni3(HITP)2. At the interface, the charged ions KLn+ within the ion-selective membrane capacitively couple with certain electrons in the Ni3(HITP)2. The potential of the double electric layer undergoes modification by the potential compensation ions, thereby converting the ion signal into an electrical signal. Simultaneously, the excellent conductivity and large specific surface area of the Ni3(HITP)2 accelerates the ion-electron transfer rate (as revealed by EIS Fig. 6b), thereby enhancing the short-term stability of the electrode. By fitting the EIS data to the equivalent circuit (insert in Fig. 6b), the obtained fitting values are summarized in Table S4 (Supporting information). As shown in Fig. 6b, compared to Au/K+-ISM, Au/MOF/K+-ISM exhibits a smaller membrane resistance (Rb) (6.7 kΩ) and Rct (9.6 kΩ) in the high-frequency region. This is attributed to the porous Ni3(HITP)2, which possesses a larger specific surface area, providing more active sites for ions and facilitating ion-electron transfer. Additionally, the Rct of the sensor increases after incubation with the peptide, resulting from the non-conductive nature of the antifouling peptide membrane. Despite the increase in impedance, the impact on ion transport at the interface is minimized due to the presence of large ion transport channels in the peptide layer.
|
Download:
|
| Fig. 6. (a) Response mechanism of antifouling potassium ion selective electrode. (b) Impedance spectrum of Au/MOF/K+-ISM/pep, Au/MOF/K+-ISM and Au/K+-ISM. (c) Contact angle of Au/MOF, Au/MOF/K+-ISM and Au/MOF/K+-ISM/pep surfaces. | |
The designed Y-shaped antifouling peptide contains a bactericidal peptide chain and an antifouling peptide chain, which ensures that the bacteria in contact with the test system are killed while preventing them from adhering to the electrode surface to form biofouling (Fig. 6a). The antifouling mechanism of the designed peptide is to improve the hydrophobic potassium ion membrane interface (CA = 103° ± 0.12°) to a hydrophilic interface with a contact angle of 24° ± 0.08° (Fig. 6c and Fig. S12 in Supporting information). The water on the hydrophilic peptide interface competes with pollutants, easily forming a hydration layer on the peptide surface, reducing the non-specific adsorption of pollutants on the electrode interface, thereby achieving the purpose of anti-pollution. Moreover, peptides maintaining a near-neutral state in the testing environment can nullify the interaction of interface charges, effectively preventing the non-specific adsorption of pollutants (Fig. S13 in Supporting information).
To investigate the capability of Au/MOF/K+-ISM/pep in detecting potassium ions in real samples, the concentration of potassium ions in actual seawater samples and human urine was measured using both the direct measurement method with Au/MOF/K+-ISM/pep and Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES), with results presented in Table 1. The findings show that Au/MOF/K+-ISM/pep demonstrates excellent quantitative performance, consistent with the concentrations of potassium ions in complex systems as determined by ICP-AES. These results robustly validate that the sensor developed in this study can be effectively used for the accurate determination of potassium ion concentrations in actual complex systems.
|
|
Table 1 Determination of K+ concentration in authentic complex system samples by direct potentiometric and ICP-AES methods. |
A novel antifouling potassium SC-ISE was developed based on conductive MOF and designed Y-shaped antifouling peptides. The conductive MOF material provided a faster transmission interface for the SC-ISE, while the multifunctional Y-shaped peptide exhibited bactericidal and inhibition of biofouling, effectively suppressing the formation of biofouling on the electrode surface. Compared to SC-ISEs without peptide incubation, the fabricated antifouling potassium SC-ISE demonstrated excellent antifouling properties and maintained a good potential response during long-term testing. Importantly, the prepared electrode achieved precise detection of potassium ions in actual complex systems environments. The research on antifouling strategies based on conductive MOF and Y-shaped peptides provides a promising approach for constructing stably SC-ISEs operating in complex systems, particularly considering the long-term application of SC-ISEs in complex systems.
Ethical agreementThe urine samples from adult donors were provided from the Qingdao Central Hospital, as well as the informed consent for use of the human urine was obtained. All sample preparations were approved by the Institutional Review Committee of relevant hospital and carried out in accordance with institutional guidelines and conformed to the relevant regulatory standards Ethical.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementXianghua Zeng: Writing – original draft, Investigation, Data curation, Conceptualization. Weichen Meng: Formal analysis, Data curation. Xiaochun Han: Data curation. Jiachen Yang: Investigation, Formal analysis. Kaiqi Wu: Methodology, Data curation. Fengxian Gao: Writing – original draft. Xiliang Luo: Writing – review & editing, Resources, Conceptualization.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (Nos. 22174082, 22374085), the Key Research and Development Program of Shandong Province (No. 2021ZDSYS30) and Qingdao Postdoctoral Innovation Project Funding (No. QDBSH20220201038).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110564.
| [1] |
M. Li, Q. Shi, N. Song, et al., Chem. Soc. Rev. 52 (2023) 5807-5811. |
| [2] |
B. Kabagambe, A. Izadyar, S. Amemiya, Anal. Chem. 84 (2012) 7979-7986. DOI:10.1021/ac301773w |
| [3] |
C. Wardak, K. Pietrzak, K. Morawska, et al., Sensors 23 (2023) 5839. DOI:10.3390/s23135839 |
| [4] |
E. Zdrachek, E. Bakker, Anal. Chem. 93 (2020) 72-102. |
| [5] |
Y. Shao, Y. Ying, J. Ping, Chem. Soc. Rev. 49 (2020) 4405-4465. DOI:10.1039/c9cs00587k |
| [6] |
S. Yu, L. Ju, F. Li, et al., Chin. Chem. Lett. 23 (2012) 488-491. |
| [7] |
J. Bobacka, T. Lindfors, M. McCarrick, et al., Anal. Chem. 67 (1995) 3819-3823. DOI:10.1021/ac00116a034 |
| [8] |
S. Liu, L. Zhong, Y. Tang, et al., Anal. Chem. 96 (2024) 8594-8603. DOI:10.1021/acs.analchem.4c00609 |
| [9] |
S. Marcinek, A. Chapoulie, P. Salaün, et al., Talanta 226 (2021) 122170. |
| [10] |
Z. Liu, T. Jiang, W. Qin, Anal. Chem. 94 (2022) 11916-11924. DOI:10.1021/acs.analchem.2c02672 |
| [11] |
H. Jin, L. Tian, W. Bing, et al., Prog. Mater. Sci. 124 (2022) 100889. |
| [12] |
Y. Li, C. Ning, Bioact. Mater. 4 (2019) 189-195. |
| [13] |
B.T. Seaton, D.F. Hill, S.L. Cowen, et al., Anal. Chem. 92 (2020) 6334-6340. DOI:10.1021/acs.analchem.9b05194 |
| [14] |
M.J. Choi, K.J. Chae, F.F. Ajayi, et al., Bioresour. Technol. 102 (2011) 298-303. |
| [15] |
R. Wanka, F. Koschitzki, V. Puzovic, et al., ACS Appl. Mater. Interfaces 13 (2021) 6659-6669. DOI:10.1021/acsami.0c21212 |
| [16] |
Z. Xie, P. Zhang, Z. Zhang, et al., Chin. Chem. Lett. 35 (2024) 109768. |
| [17] |
W.J. Yang, K.G. Neoh, E.T. Kang, et al., Prog. Polym. Sci. 39 (2014) 1017-1042. |
| [18] |
L. Qi, T. Jiang, R. Liang, et al., Sens. Actuators B 328 (2021) 129014. |
| [19] |
C. Zhang, F. Cui, G.M. Zeng, et al., Sci. Total Environ. 518 (2015) 352-362. |
| [20] |
G.P. Sakala, M. Reches, Adv. Mater. Interfaces 5 (2018) 1800073. |
| [21] |
S. Zhao, Y. Zhang, Z. Xu, et al., Anal. Chim. Acta 1263 (2023) 341244. |
| [22] |
Z. Song, Y. Li, R. Li, ACS Sens. 9 (2024) 1525-1532. DOI:10.1021/acssensors.3c02706 |
| [23] |
Y. Li, R. Han, X. Yu, et al., Sens. Actuators B 373 (2022) 132723. |
| [24] |
Y. Zhang, Y. Ding, X. Li, et al., Chin. Chem. Lett. 32 (2021) 3636-3640. |
| [25] |
M.A. Fox, J.E. Thwaite, D.O. Ulaeto, et al., Peptides 33 (2012) 197-205. |
| [26] |
G. Wang, R. Han, Q. Li, et al., Anal. Chem. 92 (2020) 7186-7193. DOI:10.1021/acs.analchem.0c00738 |
| [27] |
M. Chen, R. Han, W. Wang, et al., Anal. Chem. 93 (2021) 13555-13563. DOI:10.1021/acs.analchem.1c02552 |
| [28] |
M. Chen, Z. Song, X. Yang, et al., Biosens. Bioelectron. 206 (2022) 114162. |
| [29] |
T. Lou, X. Bai, X. He, et al., Appl. Surf. Sci. 541 (2021) 148384. |
| [30] |
C. Jiang, G. Wang, R. Hein, et al., Chem. Rev. 120 (2020) 3852-3889. DOI:10.1021/acs.chemrev.9b00739 |
| [31] |
R. Saha, D. Bhattacharya, M. Mukhopadhyay, Colloids Surf. B 220 (2022) 112900. |
| [32] |
M.Y. Masoomi, A. Morsali, A. Dhakshinamoorthy, et al., Angew. Chem. 131 (2019) 15330-15347. DOI:10.1002/ange.201902229 |
| [33] |
Y. Zheng, N. Yang, R. Gao, et al., Adv. Mater. 34 (2022) 2203417. |
| [34] |
S.L. Gaw, G. Sakala, S. Nir, et al., Biomacromolecules 19 (2018) 3620-3627. DOI:10.1021/acs.biomac.8b00587 |
| [35] |
H. Lee, N.F. Scherer, P.B. Messersmith, Proc. Natl. Acad. Sci. U. S. A. 1035 (2006) 12999-13003. DOI:10.1073/pnas.0605552103 |
| [36] |
Y. Lian, W. Yang, C. Zhang, et al., Angew. Chem. Int. Ed. 59 (2020) 286-294. DOI:10.1002/anie.201910879 |
| [37] |
D. Sheberla, L. Sun, M.A. Blood-Forsythe, et al., J. Am. Chem. Soc. 136 (2014) 8859-8862. DOI:10.1021/ja502765n |
| [38] |
L. Martínez, R. Andrade, E.G. Birgin, et al., J. Comput. Chem. 30 (2009) 2157-2164. DOI:10.1002/jcc.21224 |
| [39] |
N. Nayir, A.C. Van Duin, S. Erkoc, J. Phys. Chem. C 123 (2018) 1208-1218. |
| [40] |
X. Zhang, X. Wang, H. Cheng, et al., J. Hazard. Mater. 413 (2021) 125320. |
| [41] |
X. Zhang, Q. Yang, L. Ma, et al., Biosens. Bioelectron. 239 (2023) 115626. |
| [42] |
X. Zeng, W. Jiang, X. Jiang, et al., Anal. Chim. Acta 1094 (2020) 26-33. |
| [43] |
W. Zhao, T. Chen, W.B. Wang, et al., Sci. Bull. 65 (2020) 1803-1811. |
 2025, Vol. 36
2025, Vol. 36 

