b Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, Guangzhou 510080, China;
c Center for Nanomedicine and Department of Anesthesiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, United States;
d Department of Hematology, the Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen 518107, China
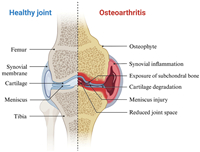
Osteoarthritis (OA) is a prevalent joint disease, particularly among older individuals, and affects millions worldwide [1]. It is characterized by the progressive loss of hyaline articular cartilage, thickening of the subchondral bone, osteophyte formation, synovial inflammation, and changes to ligaments and menisci (Fig. 1) [2–4], which leads to joint pain, stiffness, and reduced mobility [5,6]. The incidence of OA is expected to increase significantly in the coming decades due to factors such as aging populations, obesity, and joint injuries [7,8].
|
Download:
|
| Fig. 1. Comparison of healthy and osteoarthritic cartilage (created with BioRender.com). | |
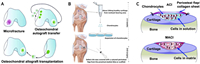
Current treatment options for OA aim to relieve symptoms but are limited in their ability to address the underlying cartilage damage. Non-surgical approaches such as exercise and pharmacological treatments are often insufficient for long-term relief, while surgical interventions, including joint replacement, are typically reserved for advanced cases [6,7]. Regenerative techniques, such as microfracture and osteochondral autograft transplantation (OAT), have been developed to repair cartilage defects, but these procedures often result in mechanically inferior fibrocartilage or are limited by donor site morbidity (Fig. 2A) [9–12]. More advanced techniques, such as autologous chondrocyte implantation (ACI) and matrix-induced autologous chondrocyte implantation (MACI), provide better outcomes but remain complex and costly (Fig. 2, Fig. 2) [13–16].
|
Download:
|
| Fig. 2. Conventional cartilage repair techniques. Copied with permission [16]. Copyright 2020, Wiley Publishing Group. (A) Microfractures, osteochondral autografts and allografts. (B) Schematic diagram of the process of autologous chondrocyte implantation (ACI). (C) The difference between ACI and MACI. | |
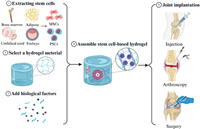
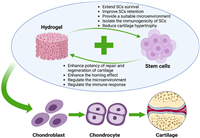
Given the limitations of these existing therapies, there has been growing interest in stem cell-based hydrogel for cartilage regeneration (Fig. 3). In particular, the combination of stem cells with hydrogels offers a promising avenue for improving the repair of cartilage defects by providing a supportive microenvironment that mimics the extracellular matrix (ECM) [17,18]. Hydrogels can help enhance stem cell survival and differentiation, making them an ideal candidate for future cartilage repair therapies.
|
Download:
|
| Fig. 3. Schematic Illustration of stem cell-based hydrogel for cartilage repair and regeneration (created with BioRender.com). Firstly, stem cells with differentiation potential need to be extracted as well as selecting the most paired hydrogel carriers. Then they are assembled with the addition of biofactors. Finally, the appropriate method of cartilage implantation is selected, including surgery, arthroscopy, and injections. | |
The primary functions of articular cartilage are to absorb impact, prevent friction between adjacent joints, and maintain low-friction surfaces [19,20]. Comprising up to 80% water, articular cartilage lacks blood vessels, nerves, and lymphatic vessels, consisting solely of chondrocytes and ECM [2,19,21]. Chondrocytes, the specialized cells of mesenchymal origin, make up only 2% of the tissue's volume and exhibit minimal proliferation [19,20]. These cells are responsible for producing and maintaining the ECM by secreting enzymes, growth factors, and inflammatory mediators [20,22]. As shown in Fig. 4, the ECM is predominantly composed of type Ⅱ collagen (Col Ⅱ), proteoglycans, and hyaluronic acid (HA), providing cartilage with its structure and biomechanical properties, such as compressive stiffness, elasticity, and shear resistance (Fig. 4B) [19,21,23]. Cartilage tissue is classified into three types: elastic cartilage, fibrocartilage, and hyaline cartilage, the latter being the primary component of articular cartilage. Normal articular cartilage is divided into four distinct zones: superficial, middle, deep, and calcified, with tide mark separating it from the subchondral bone (Fig. 4A) [2,20]. Cartilage defects were classified into 4 grades according to the depth of the cartilage defect (Fig. 4C) [24].

|
Download:
|
| Fig. 4. The structure, composition and damage grading of articular cartilage. (A) The articular cartilage structure was divided into superficial, middle, deep and calcified areas. Tidal marks separate articular cartilage from subchondral bone. (B) Cartilage is composed of a type Ⅱ collagen network (blue), interwoven with long hyaluronan chains (green) and their associated proteoglycan complexes. (C) ICRS classification. This figure is created with BioRender.com. | |
Cartilage tissue regeneration is a complex process. When cartilage is damaged, chondrocytes are activated by growth factors and cytokines, prompting them to synthesize new ECM, including collagen and proteoglycans. This process is accompanied by the reconstruction of blood vessels and restoration of nutrient supply. While articular cartilage may appear simple and homogeneous, it is actually composed of highly organized, heterogeneous structures with biomechanical properties that have proven difficult to replicate or repair with high fidelity [21]. The limited blood supply in the joint, the low number of chondrocytes, and the dense cartilaginous matrix, which restricts chondrocyte migration to the injury site, severely limit the cartilage's self-healing capacity [19]. As a result, cartilage repair often leads to the formation of fibrocartilage, which is mechanically weak and lacks the necessary elasticity for proper joint function [25]. Even minor injuries can accelerate the progression of OA, potentially leading to complete joint destruction and the need for arthroplasty.
Given the limitations of current cartilage tissue engineering approaches, several key factors must be considered when designing materials for cartilage regeneration [26,27]. These include the material's ability to withstand mechanical stress, promote microcirculation regeneration, carry a sufficient number of regenerative cells, and facilitate cell migration within the scaffold.
3. Stem cellsCartilage tissue engineering relies on three key components: cells, scaffolds, and cytokines. The choice of cells is critical for successful cartilage regeneration. While autologous chondrocytes and stem cells are commonly used, autologous chondrocytes present significant limitations such as limited availability, poor differentiation ability, and loss of functionality over time [28]. These challenges have shifted research focus towards the use of stem cells, which offer the advantage of being able to differentiate into chondrocytes, potentially overcoming issues related to tissue harvesting and donor site morbidity. Two primary stem cell types are commonly utilized in cartilage tissue engineering: mesenchymal stem cells (MSCs) and pluripotent stem cells (PSCs), with MSCs being particularly promising for their cartilage regeneration potential (Fig. S1 and Table S1 in Supporting information) [29].
MSCs, adult stem cells of mesodermal origin, can be sourced from various tissues such as bone marrow, adipose tissue, synovium, and even umbilical cord blood [30,31]. MSCs hold great promise in cartilage regeneration for several reasons: they can proliferate extensively in an undifferentiated state [29], are easily harvested through minimally invasive methods [30], and possess a strong capacity for cartilage differentiation. Both autologous and allogeneic MSCs have shown safety and effectiveness in clinical trials for OA treatment, demonstrating an ability to alleviate symptoms without significant adverse effects [32]. However, challenges persist, such as the invasive procedures required to harvest sufficient MSCs and the high costs associated with ensuring optimal viability during storage and transport [33]. Moreover, MSCs tend to lose their self-renewal capacity and proliferative potential with age, limiting their effectiveness in older patients [34,35].
In response to these challenges, researchers have explored alternatives like PSCs, which include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [36–39]. PSCs have the ability to self-renew and differentiate into a variety of tissue types, offering a potential source for generating large numbers of MSCs. This could address issues related to cell senescence and variability in donor tissues [40]. While PSCs show promise in chondrocyte differentiation for cartilage regeneration, obstacles remain, particularly with respect to hypertrophic differentiation and ossification during the development of chondrocytes from PSCs [41]. Although clinical trials for PSC-derived therapies are ongoing, further research is needed to refine these methods for safe and effective cartilage repair.
3.1. MSCs 3.1.1. Bone marrow mesenchymal stem cells (BMSCs)BMSCs are among the most commonly used stem cells in cartilage tissue engineering due to their ease of collection [42,43] and well-established safety profile [44,45]. BMSCs can be expanded in vitro to significant numbers, up to 30–40 population doublings [46,47], while maintaining strong differentiation potential. Under appropriate culture conditions, BMSCs can differentiate into chondrocytes, osteoblasts, and other connective tissue cells. In practice, BMSCs not only counteract the inhibitory effects of oxidative stress on chondrocyte proliferation and migration but also significantly upregulate the expression of COL2A1, which promotes ECM synthesis and aids in the repair of osteoarthritic cartilage [48–51].
BMSCs are widely used in research and clinical practice for cartilage injury and OA. A Canadian phase Ⅰ/Ⅱa clinical study [52] showed that autologous BMSCs significantly improved patient symptoms and quality of life, with no serious adverse events. The cohort experienced improvements in patient-reported outcome metrics (PROM), knee injury and osteoarthritis outcome scores (KOOS), and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 12 months. Additionally, BMSCs reduced synovial inflammation, a key factor in OA progression. Despite these benefits, BMSCs have limitations such as donor site morbidity [53], limited cell yield [54], and declining differentiation potential with donor age [55–57], posing challenges for their use in older patients. Early clinical treatments have enhanced cartilage volume and quality, but significant improvements in cartilage morphology were not observed in more advanced OA cases, limiting their broader clinical use [58,59].
3.1.2. Adipose-derived stem cells (ADSCs)ADSCs are the second most commonly used type of MSCs after BMSCs, largely due to their simple isolation procedures and high yields [60]. ADSCs can be obtained via liposuction, followed by digestion and culture of the adipose tissue [61]. Compared to BMSCs, ADSCs are more accessible and abundant since adipose tissue is widely available throughout the body [62]. ADSCs also demonstrate a stronger homing effect [63,64], leading to better efficacy when administered via intra-articular injections, while minimizing the need for additional surgeries to expose cartilage lesions [61]. Additionally, ADSCs rely less on mitochondrial respiration, making them better suited for survival in the hypoxic environment of joint cavities. Their superior immunosuppressive capacity also makes ADSCs a more controllable source of stem cells for cartilage repair [65]. ADSCs are the best choice for pain relief and favorable outcomes [66,67].
A phase Ⅱb clinical trial demonstrated that intra-articular injection of ADSCs provided superior pain relief and quality of life improvement compared to HA for knee osteoarthritis (KOA) patients [68]. The trial also showed a significant increase in cartilage volume after 12 months. Similar results were observed in another phase Ⅱb trial [69]. A phase Ⅱ clinical trial of allogeneic ADSCs further confirmed significant reductions in WOMAC scores and improvements in KOOS scores across multiple domains, including pain and daily functioning, compared to a control group [70].
Although ADSCs possess the necessary surface markers for chondrogenic differentiation, their capacity remains weaker compared to BMSCs [71,72]. Factors such as donor variability and differences in adipose tissue density can affect ADSC quality [73]. The extraction and preparation process also plays a significant role in determining ADSC effectiveness [54]. Obesity, commonly associated with osteoarthritis, may offer an abundant source of ADSCs, but it also introduces complications due to systemic inflammation, which can impair cell quality. Studies show that ADSCs from obese patients tend to have lower proliferative capacity, increased senescence, and higher levels of pro-inflammatory cytokines [74–76].
3.1.3. Umbilical cord-derived mesenchymal stem cells (UC-MSCs)UC-MSCs have recently gained significant attention as a potential cell source for tissue engineering and regenerative medicine [77]. UC-MSCs offer several advantages: They are easily collected from umbilical cord blood banks using painless, non-invasive methods [78,79], and their higher proliferation rate and greater migration potential make them a reliable source for generating sufficient cells for treatment [80]. Moreover, UC-MSCs maintain their stemness after multiple passages and expansion [81], and their embryonic origin gives them stronger chondrogenic potential compared to BMSCs [81–83]. Additionally, UC-MSCs enhance the secretion of chondrogenic factors, further supporting their stem cell potential [84,85]. Their low levels of surface antigens also make them ideal candidates for allogeneic therapies with minimal risk of graft rejection.
Wharton's jelly (WJ) in umbilical cords is a promising source of MSCs due to its high yield, painless collection, and lack of ethical concerns [86]. WJ-derived stem cells (WJSCs) have a high capacity for proliferation and differentiation, with low immunogenicity and no tumorigenic risk [86]. A phase Ⅰ/Ⅱ clinical study showed that intra-articular injections of UC-MSCs significantly improved WOMAC pain and disability scores at 12 months compared to HA [87]. A 7-year follow-up study further demonstrated stable clinical outcomes, with regenerated hyaline-like cartilage and no evidence of osteogenesis or tumor formation [88].
3.1.4. Amniotic fluid stem cells (AFSCs)AFSCs are isolated from amniotic fluid through the immunoselection of c-Kit-positive cells. Since amniotic fluid is routinely collected during prenatal diagnosis using minimally invasive procedures, AFSCs present a promising and ethically non-controversial source of stem cells [89]. These cells have the potential to differentiate into various lineages, including adipocytes, chondrocytes, and osteoblasts [90]. Moreover, c-Kit-positive populations exhibit enhanced chondrogenic potential [91]. AFSCs also possess low immunogenicity [92,93] and secrete immunomodulatory factors, which may be beneficial in treating autoimmune diseases or for use in allogeneic implants [94].
AFSCs, isolated through immunoselection of c-Kit-positive cells, present a promising, ethically non-controversial source of stem cells due to their routine collection during prenatal diagnosis [89]. These cells can differentiate into various lineages, including chondrocytes, and exhibit low immunogenicity and high chondrogenic potential [90,91]. AFSCs also secrete immunomodulatory factors, which could be beneficial in treating autoimmune conditions and allogeneic implants [92,93]. Compared to ESCs, AFSCs have a lower risk of tumor development, require no epigenetic reprogramming, and display fewer somatic mutations due to their early developmental origin [23,93,95]. However, their clinical use is limited by significant senescence after the fifth passage [96].
3.2. PSCs 3.2.1. ESCsESCs are the most primitive and totipotent cells in the human body, capable of differentiating into almost any cell type and proliferating rapidly to generate large quantities of cells [97,98]. Experimental studies have demonstrated their potential to differentiate into chondrocytes and repair cartilage defects [99].
The use of ESCs in clinical applications remains highly controversial due to ethical concerns surrounding their derivation from fertilized eggs [100]. Additionally, ESCs present risks of tumor formation and immune rejection [101,102], limiting their clinical potential despite their advantages over adult stem cells. Consequently, most studies now focus on differentiating ESCs into MSCs through methods such as co-culture, mechanical stress, and small molecules to harness their regenerative capabilities in a more clinically acceptable manner [35].
3.2.2. iPSCsiPSCs offer a promising alternative to address the limitations of MSCs and the ethical concerns surrounding ESCs. Unlike MSCs, whose proliferative capacity declines with age, iPSCs retain excellent differentiation potential and proliferative abilities comparable to ESCs. Importantly, iPSCs avoid ethical controversies, as they are created by reprogramming somatic cells with transcription factors like Oct4, Sox2, c-Myc, and Klf4, thus bypassing the need for embryonic cells or oocytes [103,104]. This patient-specific approach also reduces the risk of immune rejection, making iPSCs an ideal source for cell-based therapies [105,106]. However, the use of viral vectors in iPSC generation raises concerns about tumor formation due to the risk of insertional mutagenesis and reactivation of transgenes, as the viral genome can integrate randomly into the host cell's genome [107]. To mitigate these risks, safer, integration-free technologies have been developed, including plasmid vectors, non-integrating viral vectors, piggyBac transposons, and methods based on microRNAs or synthetic mRNAs [108].
While in vitro and in vivo experiments have shown the effectiveness of iPSC-based therapies [109–111], differentiating iPSCs into stable chondrocytes that avoid tumorigenicity remains a challenge. Current processes for reprogramming and differentiation are complex, time-consuming, and costly, limiting clinical application [109,110]. No clinical studies have been published on iPSCs for chondrocyte therapy, and future research needs to focus on developing standardized, scalable methods for their preparation.
4. Stem cell-based hydrogel for cartilage repairIn cartilage tissue engineering, hydrogels play a critical role by creating a three-dimensional structure that supports the attachment and growth of inoculated cells, while also providing mechanical support. Hydrogels mimic the ECM, creating a microenvironment conducive to cartilage regeneration. For effective application, hydrogels must meet several key requirements, they should: (1) Support cell adhesion, proliferation, and differentiation; (2) provide sufficient structural strength and elasticity to sustain mechanical loads; (3) be adjustable in size and composition for various biomedical applications; (4) function as an active matrix that can regulate cell morphology, growth, and differentiation; (5) promote the chondrogenic differentiation of stem cells while maintaining cellular phenotypes; and (6) exhibit non-toxicity, low immunogenicity, and degradability, as they are foreign materials introduced into the body.
Despite their advantages, some hydrogels face limitations, such as insufficient mechanical stiffness, poor osteoconductivity, and challenges in injectability or printability. Over the past decade, researchers have developed various hydrogel systems tailored for cartilage tissue engineering, achieving remarkable success. Hydrogels can be classified based on their material composition [10], which includes natural polymers (e.g., proteins, carbohydrates, peptides), synthetic polymers (e.g., polyethylene glycol (PEG), polyvinyl alcohol (PVA), polylactic acid (PLA)), and composite polymers (Fig. S2 and Table S2 in Supporting information). Natural polymers offer advantages such as good biocompatibility, degradability, and favorable interactions with cells, but they often lack stability. In contrast, synthetic polymers provide superior mechanical properties and controlled degradation but are limited by their non-natural origin and weaker biological properties. Recent advances have focused on combining stem cells with these hydrogels to enhance cartilage regeneration, offering promising results for future clinical applications.
4.1. Polysaccharides 4.1.1. Hyaluronic acid (HA)HA is a hydrophilic polysaccharide composed of repeating units of glucuronic acid and N-acetylglucosamine, making it the most abundant component of the ECM [112]. HA provides a microenvironment similar to natural cartilage, with excellent biocompatibility and antioxidant properties [113]. Additionally, HA interacts with cell surface receptors, promoting stem cell adhesion, proliferation, and differentiation [114,115]. However, its clinical application is limited by its long gelation time and rapid degradation by hyaluronidase in vivo. Interestingly, lower levels of hyaluronidase 2 and 3 have been observed in MSC-laden HA hydrogels cultured in cartilage medium compared to growth medium [116].
Currently, HA hydrogels support the early differentiation of MSCs into a chondrogenic lineage. A phase Ⅰ/Ⅱ clinical trial [88] of a hydrogel complex based on HA and allogeneic human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) demonstrated an improvement in visual analog scale (VAS) score and International Knee Documentation Committee (IKDC) subjective score in OA patients at 7 weeks. Additionally, the trial showed the presence of hyaline cartilage with high GAG content for up to 7 years of follow-up, which is more sustainable and durable than fibrocartilage. Furthermore, the use of MSCs-laden HA hydrogel was found to be superior to intra-articular injections of HA alone [117,118] and treatment with MSCs alone, in terms of both cartilage regeneration and clinical remission [118]. As HA is a naturally occurring component of cartilage, it can enhance adhesion and facilitate the role of MSCs by interacting with stem cell surface receptors. Studies have shown that HA hydrogel carrying MSCs had 43 times the chondrogenic capacity of PEG [116]. According to a study, HA hydrogel loaded with BMSCs demonstrated superior therapeutic levels compared to other cells. Additionally, it exhibited nearly 600 times the level of type Ⅱ collagen when compared to ADSCs and chondrocytes [119].
Difficulties still exist in the clinical application of HA hydrogel for transforming into hMSCs due to significant differences in the chondrogenic capacity of hMSCs in HA hydrogels among different individuals [120]. Increasing cell density can improve mechanical properties, but it may also cause shrinkage of the hydrogel complex. Similarly, increasing the density of macromolecular monomers can enhance ECM production, but it may also limit the centrality of the forming matrix and reduce mechanical properties. When treating with stem cell-laden HA hydrogel, it is important to individually adjust the cell density and mechanical properties of the hydrogel to improve articular cartilage formation. Additionally, modifying HA hydrogels can help address some of their disadvantages. Sulphated HA can address the clinical susceptibility to degradation of HA. Additionally, sulphated HA has a higher affinity for proteins, enabling it to retain more transforming growth factor (TGF), which inhibits MSCs differentiation into hypertrophic phenotypes and promotes chondrogenesis [121]. The mechanical strength of HA hydrogels can be improved by modifying methacrylic anhydride (MA). Studies have shown that HA-MA not only enhances mechanical strength but also promotes the proliferation of BM-MSCs and induces chondrogenic differentiation [122].
4.1.2. ChitosanChitosan, the second most abundant natural polymer after cellulose, is derived from chitin and found primarily in the exoskeletons of crustaceans and insects [123]. Its structure resembles that of cartilage glycosaminoglycans (GAGs), making it highly biocompatible and biodegradable. Chitosan is also known for its antimicrobial, antioxidant, and adhesive properties, which make it an attractive material for cartilage tissue engineering [124]. Its cationic amino groups enable electrostatic interactions with anionic GAGs and other negatively charged substances, forming hydrogels that facilitate scaffold attachment [124]. While chitosan is more affordable and contains a higher concentration of amino groups than hydrogels like collagen or HA, it faces challenges such as poor mechanical properties, limited cell adhesion, low water solubility under physiological conditions, and the potential for allergic reactions [125–128].
Despite these challenges, there have been numerous studies on chitosan hydrogels for piggybacking stem cells. When comparing the chondrogenic capacity of hydrogels from various sources, it was found that chitosan produced the highest levels of GAG and collagen in both in vivo and in vitro settings, as opposed to alginate and fibronectin [129]. Compared to hydrogels made from other materials, chitosan has the advantage of facilitating the production of cartilage matrix while preserving its phenotype and appearance [130]. Therefore, chitosan is an excellent material for cartilage tissue engineering [129]. Additionally, chitosan is an excellent scaffolding material for stem cells, as it enhances cell-matrix interactions and promotes the performance of MSCs, as confirmed in several studies [131–133]. Furthermore, the use of chitosan enhances the cytocompatibility of the scaffolds and promotes a more uniform distribution of MSCs. However, this modification does not significantly improve the physical elasticity properties of the hydrogel. Instead, it leads to the formation of higher quality cartilage tissues [134]. In comparison to cells alone, therapies based on chitosan hydrogel not only prevented the inflammatory state of chondrocytes and hypertrophic differentiation of ADSCs, but also increased the production of type Ⅱ collagen and proteoglycans. These components contribute to the repair of cartilage in knee OA [135]. The quality of the cartilage was also influenced by the structure of chitosan hydrogels.
The performance of chitosan is influenced by its structure. Chitosan-based fibrous scaffolds enhance the cartilage potential of MSCs more than sponge scaffolds, resulting in better matrix production and improved type Ⅱ collagen expression [136]. The combined application of chitosan also yields benefits. Chitosan hydrogels are frequently used in combination with HA. Chitosan/HA complexes have better solubility than chitosan alone, creating a comfortable environment for stem cell differentiation into articular cartilage [137]. A study discovered that the combination of chitosan-gelatin (C-GF) enhances the adhesion, viability, and proliferative capacity of stem cells. It is reasonable to assume that C-GF scaffolds can stimulate chondrogenic differentiation of MSCs based on environmental conditions [138].
4.1.3. AlginateAlginate is a naturally occurring polysaccharide primarily found in the cell walls of brown algae and some bacteria [139]. It is abundant, inexpensive, and non-toxic to cells compared to other natural polymers [140,141]. Alginate cross-links efficiently with divalent cations (e.g., Ca²⁺) at room temperature, allowing for rapid gel formation. Alginate hydrogels can prolong stem cell viability, enhance immunomodulation, and support the sustained release of bioactive factors [142]. However, they have limitations, including weak mechanical properties, poor cell adhesion, and limited stability during long-term applications [143–145]. As a result, alginate is often combined with other materials to enhance its mechanical strength and biological performance.
Several studies have demonstrated that stem cell-based hydrogel complexes can aid in cartilage formation. in vitro and in vivo experiments have shown that MSC-loaded alginate hydrogel is effective in cartilage tissue engineering, resulting in improved cartilage repair and regeneration [146]. Additionally, co-culturing OA explants with alginate hydrogel has been found to increase total sulfated glycosaminoglycan (sGAG) content, DNA content, and Ki67 cells, indicating cell proliferation, compared to OA cartilage explants cultured alone [142]. Although it is a common belief that locally injected MSCs disappear after a few weeks, a study has found that encapsulation in high guluronic acid (high G) alginate can prolong the lifespan of allogeneic MSCs and maintain metabolic activity for at least 8 weeks [147]. Hydrogel scaffolds alone cannot maintain the environment necessary for long-term cartilage regeneration. Scientists have found that cells implanted in alginate scaffolds barely adhere to the scaffold, instead forming loose clusters of cells. Therefore, the use of alginate hydrogels typically requires a mixture of other materials [148]. The addition of CS can increase the negative charge of the scaffolds and improve the poor adhesion of alginate [149,150]. It can also compensate for the mechanical properties of alginate hydrogels [151]. On the other hand, the addition of fibronectin can provide a suitable biomimetic environment for hyaline cartilage differentiation of MSCs in vitro [148], taking advantage of its high biostability. Fibronectin can create a suitable environment for in vitro hyaline cartilage differentiation of MSCs [148,152].
The form of stem cells can affect the results after implantation, in addition to the hydrogel composite itself. Obtaining the large numbers required for cartilage tissue engineering is difficult with conventional cell culture methods, which has led to the emergence of bioreactor systems. A study [153] compared the efficacy of two-dimensional (2D) cultured ADSCs and three-dimensional (3D) cultured ADSCs spheres in calcium alginate scaffolds. The results showed that 3D culture improves biological properties such as cell viability, proliferative capacity, morphologic stability, and metabolic function. Additionally, it alleviates arthritic degeneration and stimulates ECM secretion.
4.1.4. AgaroseAgarose is a polysaccharide extracted from red algae, composed of alternating sequences of β-D-galactanoyl and 3,6-anhydrous-α-L-galactanoyl [154]. Agarose hydrogels are biocompatible, biodegradable, and exhibit good mechanical strength and stiffness. Additionally, they promote high cell adhesion [155–157]. Due to its similarity to natural cartilage, agarose is widely used in cartilage tissue engineering. Agarose hydrogels are stable between 17℃ and 40℃ and become soluble above 65℃. This temperature-responsive gelation capability makes agarose hydrogels easy to form and use in various applications [158]. Additionally, agarose has a tunable water adsorption capacity, providing cells with a microenvironment suitable for cellular activity [159]. Tensile properties are an important indicator for cartilage tissue engineering. The tensile properties of cell-encapsulated agarose hydrogels can increase with increasing incubation time. Additionally, the tensile properties of MSC-based agarose hydrogel are similar to those of chondrocyte complexes, which confirms the potential of combining MSCs with agarose hydrogels [160,161]. While agarose and alginate hydrogels exhibit similar mechanical and rheological properties, agarose hydrogels demonstrate better cell viability and matrix production over a 28-day culture period [156]. Carboxy agarose-derived hydrogels are considered more suitable as stem cell scaffolds compared to natural agarose due to the significantly higher survival rate of human MSCs [162]. Furthermore, pressure loading can promote chondrogenic differentiation of SMSCs and maintain their phenotype [163].
However, it was found that agarose composites based on MSCs had lower performance than chondrocytes under the same conditions [164]. They exhibited weaker chondrogenic capacity and poorer mechanical properties of the resulting cartilage. Therefore, further optimization is required for MSCs-based agarose hydrogels to produce properties similar to those of chondrocytes. The mechanical properties of agarose can be improved by integrating hydrogels made of different materials. The mechanical properties of agarose and PEG hydrogels inoculated with MSCs were significantly enhanced and superior to either component. This creates a microenvironment that supports cell viability and function, and further promotes stem cell chondrogenesis [165]. An additional hydrogel containing alginate-agarose-incorporated human dental pulp stem cells (hDPSCs) was used in this study. Although it did not reach the differentiation capacity of chondrocytes, it allowed hDPSCs to form large cell aggregates. The size of these aggregates was proportional to the degree of differentiation of the cells, and they expressed significant amounts of type Ⅱ collagen and proteoglycans [166]. To enhance the correlation with hydrogel complexes, the density of inoculated cells can be increased, and they can be briefly exposed to TGF-β3. In this study, 60 million MSCs per milliliter of agarose were inoculated with TGF-β3 and exposed to it for three weeks. As a result, the hydrogels exhibited strong anabolism and reached an equilibrium modulus of 200 kPa [167]. However, chondrocytes induced to produce using TGF-β tend to hypertrophy. Co-culturing chondrocytes with MSCs in a three-dimensional agarose system can drive chondrogenic differentiation of the MSCs. This method has a reduced tendency to hypertrophy and provides a clinical improvement for agarose application. It offers a strategy for clinical improvement of agarose [168].
4.2. Proteins 4.2.1. CollagenCollagen is a major structural protein found in skin, bone, cartilage, and tendons [169]. Type Ⅱ collagen makes up about 90% of the dry weight of articular cartilage, providing tensile strength and maintaining the shape and function of the tissue [170]. Collagen hydrogels mimic the natural environment of cartilage, offering excellent biocompatibility, biodegradability, and cellular adaptability. They create a three-dimensional scaffold that supports cell attachment, migration, and the formation of the ECM, while also reducing the risk of immune rejection [171,172]. However, due to its weak mechanical properties and batch-to-batch variability, collagen is often combined with other materials to improve its stability and performance in cartilage tissue engineering.
Several experiments have demonstrated the ability of type Ⅱ collagen hydrogels to provide a suitable environment for MSCs and induce their differentiation into cartilage. In vivo, BMSCs encapsulated in type Ⅱ collagen differentiated into evenly distributed chondrocytes without requiring TGF-β1. They expressed proteoglycans, type Ⅱ and type X collagens, which are essential components of cartilage ECM. Overall, their performance was superior to that of the collagen-alginate hydrogel. This may be attributed to the aggregation of signaling molecules for chondrogenesis in vivo. In vitro, type Ⅱ collagen effectively differentiated MSCs by recruiting chondrogenic transcription factors, promoting GAG synthesis, major matrix components, MMPs/TIMPs enzyme action, and activating kinase-dependent signaling cascades to promote chondrogenic differentiation of WJSCs and pulp-derived MSCs. This brings the treatment closer to the clinic [173]. Collagen type Ⅰ hydrogels possess the same potential for cartilage regeneration. After exposing WJSCs to type Ⅰ collagen hydrogels and TGF, the expression of collagen Ⅱ, proteoglycan, COMP (cartilage oligomeric matrix protein), and sox9 all significantly increased and remained viable [174]. A suitable hydrogel for cartilage tissue engineering should be stiffer than collagen, and the ECM formed should persist long enough to be replaced. A study was conducted on a silanized collagen hydrogel that was stiffer than collagen but lower than normal cartilage. The study achieved survival of embedded MSCs for >21 days and upregulation of chondrocyte markers [175].
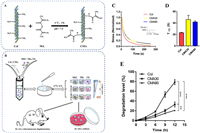
Collagen is advantageous due to its ease of modulation compared to other material hydrogels. The degradation rate and stiffness of collagen hydrogels can be adjusted to modulate cell spreading and proliferation. In one study, collagen hydrogels were obtained with modifiable degradation rates and mechanical properties by adjusting the degree of substitution of the methacrylate ester of collagen (Fig. 5) [176]. Additionally, in an in vitro study, researchers discovered that the contraction and degradation of collagen hydrogels could stimulate chondrogenic potential. Contraction favored an increase in local cell density and interactions, while degradation promoted the aggregation and condensation of BMSCs [177]. The morphologies of collagen hydrogels have an impact on the microenvironment of stem cell chondrogenic differentiation. An experiment was conducted to compare the effects of collagen hydrogel blocks (CHBs) and collagen hydrogel microspheres (CHMs) on MSCs. The results showed that CHMs were superior to CHBs in terms of cell number and volume. However, it could be superior to CHB and exhibit more GAG staining, chondrogenic genes (AGG, COL2A1, SOX9), and lower hypertrophic genes (COL10A1). This suggests that CHM has the potential to stably produce cartilage [178].
|
Download:
|
| Fig. 5. (A) Illustration of synthesis of methacrylated collagen. (B) Fabrication of cell/hydrogel constructs and the chondrogenesis of constructs in vitro and in vivo culture. (C) Modifiable mechanical properties: the stress relaxation profiles of Col, CMA30 and CMA80 hydrogels. (D) Timescale of stress relaxation, τ1/2, at which the stress is relaxed to half of its original value during stress relaxation tests in (C). (E) Modifiable degradation rates: The degradation curves of hydrogels measured in collagenase I solution (30 µg/mL) at 37℃. Copied with permission [176]. Copyright 2022, Elsevier. | |
Gelatin, a derivative of collagen, is a key component of connective tissues like bone and cartilage [18]. It contains an arginine-glycine-aspartate (RGD) sequence that promotes cell adhesion and a matrix metalloproteinase (MMP) target sequence that supports cellular remodeling [18]. Gelatin hydrogels are highly bioactive, with excellent cell affinity due to their interaction with RGD sequences [179,180]. Compared to collagen, gelatin offers lower immunogenicity, better biocompatibility, and superior degradability [181–184]. Additionally, gelatin hydrogels have unique low-temperature gelation properties, forming physically crosslinked hydrogels at temperatures below 30℃ [185]. However, their mechanical properties and thermal stability are often insufficient for cartilage repair, necessitating further modifications or cross-linking for clinical use [186,187].
While MSCs-laden gelatin hydrogel has been shown to have superior joint repair capabilities compared to gelatin hydrogel alone [188], its instability at certain temperatures necessitates additional modification or cross-linking for clinical applications. Gelatin methacryloyl gelatin (GelMA) hydrogel is a low-cost bio-ink that meets the mechanical stability requirements of regenerative cartilage. This is achieved through methacryloyl modification, and it also has a photo crosslinked structure that promotes cell survival and monitoring [189,190]. Furthermore, GelMA effectively repairs mitochondrial dysfunction in cartilage and thereby reduces the overall inflammatory response [191].
Altering the morphology of the hydrogel can ameliorate the significant limitation of nutrients and oxygen required by stem cells in commonly used block gelatin. In comparison to block hydrogel systems, gelatin microspheres are superior stem cell carriers due to their spherical shape and high surface area-to-volume ratio. This results in a faster mass transfer rate and more opportunities for cell-material interactions. Additionally, their wide range of porosities promotes cell adhesion and interactions, ultimately leading to the formation of a larger, more homogeneous matrix of neoplastic cartilage [192,193]. Furthermore, honeycomb gelatin hydrogels demonstrate up to five times higher swelling ratios and greater porosity compared to conventional block gelatin. This scaffold has also been proven to promote the differentiation of UC-MSCs into chondrocytes in vitro [194]. A microbanded macroporous structure based on gelatin has been found to promote neoarchitectonic cartilage deposition by eliminating physical constraints. This structure also significantly improves the in vitro culturing of MSCs in terms of compression modulus and total amount of newborn cartilage [195].
4.2.3. Silk fibroin (SF)SF, a primary component of silk, has been widely used in biomedical applications, particularly as sutures, due to its ability to mimic the collagen structure of natural cartilage [196]. SF is abundant, easy to process, and has excellent biocompatibility, making it a popular choice for cartilage tissue engineering [197,198]. Research shows that SF scaffolds with optimal porosity (e.g., 12 µm) can promote cell infiltration and chondrogenic differentiation [199].
Filipin proteins have a disadvantage due to a defect in the cell adhesion sequence, which results in insufficient specific binding sites between stem cells and hydrogels, leading to poor adhesion [200]. To modulate cell adhesion to hydrogels, the RGD amino acid sequence, which is present in a variety of proteins, is commonly used as a ligand for integrins. The RGD amino acid sequence is a ligand for integrins and is commonly used to modulate cell adhesion to hydrogels. The injection of RGDS into the light chains of filipin proteins resulted in an increase in the mRNA expression of integrins α5 and β1. This led to an increase in cell adhesion to filipin proteins, which promoted cartilage formation and facilitated the maintenance of cartilage phenotypes [201]. Arachnid proteins contain RGD sequences. In an in vitro study, arachnid proteins were incorporated into filipin proteins. The improved filipin hydrogels provided better cell adhesion and improved cellular adhesion. They could also provide better cell attachment and significantly support chondrogenic differentiation of WJSCs. Additionally, they could improve the mechanical properties of the hydrogel [202].
Silk proteins can serve as a single material for creating various types of scaffolds or as composites with other biomaterials. Silk protein/chitosan hybrid scaffolds promote cell attachment and proliferation, while silk protein/chitosan scaffolds offer a suitable 3D environment for cell proliferation, intercellular contact, and differentiation in both in vivo and in vitro settings [203]. The attachment of silk protein/chitosan scaffolds promotes chondrogenic differentiation and maintenance of the cartilaginous phenotype in MSCs compared to the culture of MSCs alone. This significantly increases the expression of GAGs and collagen [204]. In addition, SF can compensate for the disadvantages of HA's instability and susceptibility to degradation. Therefore, they are often combined, resulting in a hydrogel that exhibits a regular porous structure with good swelling and water absorption. This hydrogel significantly increases the expression of chondrogenic markers, such as Col2a, Agg, and Sox9, in cultures of UC-MSCs inoculated with HUMSCs [205]. Filipin protein/gelatin-chondroitin sulphate-HA (SF-GCH) scaffolds promoted the proliferation of BMSCs more effectively than SF scaffolds. SF serves as the primary structure for the mechanical stability of the hydrogel, while GCH provides an ECM-like biomimetic surface for the proliferation and chondrogenic differentiation of MSCs [206].
4.3. Synthetic polymers 4.3.1. PEGPEG is widely researched for cartilage regeneration due to the Food and Drug Administration (FDA) approval for medical use and proven safety, even when ingested [207]. PEG is highly modifiable, offering excellent water absorption, nutrient transport, and better mechanical properties than natural polymers [208]. Its concentration can influence ECM secretion, with lower concentrations maximizing free space for cell growth while maintaining stiffness. For example, PDLLA-PEG 1000 strikes a balance between mechanical strength and chondrogenic capacity [209].
Although PEG has some biological drawbacks, such as biological inertness, poor cell adhesion, and poor degradation properties, its functionality can be enhanced by incorporating biologically active components, such as peptides or ligands. This results in a hydrogel system that combines both mechanical and biological properties [210]. For instance, the inclusion of RGDs improved the adhesion capacity of the PEG hydrogels. This, in turn, enhanced cell viability and proliferation, and resulted in higher GAG and chondrogenic gene expression [211]. Additionally, PEG hydrogels modified with glucosamine (GA) promoted chondrogenesis in hBMSCs. The expression of protein fibrosis and hypertrophic chondrogenic markers could be reduced with an increase in the GA moiety [212]. The integration of HA with PEG maintained the long-term release of the drug and significantly inhibited the progression of OA in mice. The efficacy of the combined treatment was superior to that of PEG or HA hydrogels used alone [213].
4.3.2. PVAPVA is a water-soluble polymer with a wide range of clinical applications, including wound healing, artificial lenses, and uterine artery embolization [214]. PVA hydrogels possess excellent properties such as high strength, porosity, and stability, making them suitable for tissue engineering [215,216]. In animal studies, PVA scaffolds containing growth factors have promoted cell migration and osteochondral regeneration, resulting in hyaline-like cartilage formation [214]. However, PVA's hydrophilic nature impairs cell adhesion, limiting its effectiveness in tissue repair [216,217]. To overcome this, PVA is often combined with other materials, such as chitosan, to improve cell attachment and biological performance [217].
Although PVA has excellent mechanical and biochemical properties, it is challenging to apply it alone due to its weak biological properties. However, PVA has multiple attachment sites that can be used for material modification, making it possible to form multifunctional hydrogels [218]. Combining PVA with chitosan significantly promotes the cartilage repair function of MSCs at a similar level of performance as alginate, while exhibiting optimal mechanical properties [219,220]. Hydrogels combining PVA with collagen also exhibit better chondrogenic capacity. Another PVA combined with gelatin, chondroitin sulphate, and HA formed an ECM-mimetic hydrogel with improved flexibility and viscoelasticity based on good mechanical stability [221].
5. ConclusionStem cell-based therapies hold great promise for cartilage regeneration, but current clinical research has yet to achieve full repair of articular cartilage defects. Key challenges include short retention times for stem cells and limited differentiation potential. Hydrogels, with their biocompatibility and ability to support stem cell viability, offer a promising scaffold for improving cartilage repair.
The fate of stem cells is influenced by biological and physical factors in their environment, and hydrogels offer several benefits as scaffolds for cartilage repair (Fig. 6). (1) Hydrogels extend the survival and metabolic activity of stem cells in vivo [142,147]. (2) They improve stem cell retention: (a) Hydrogels interact with cell surface receptors, enhancing adhesion [114,115,131–133] (b) Hydrogels with RGD sequences, such as gelatin, provide adhesion sites and show good affinity for stem cells [179,180]; (c) Gelatin microspheres, with their high surface area to volume ratio, promote cell-material interactions and support stem cell adhesion [192,193,222]. (3) Hydrogels create a favorable microenvironment for cartilage regeneration: (a) They provide an aqueous environment for cell proliferation and ECM formation [159]; (b) They facilitate stem cell migration, aiding in tissue regeneration [199,223]; (c) Hydrogels enhance stem cell differentiation, improving therapeutic outcomes compared to stem cell therapy alone [83]; (d) Their physical properties improve the quality of the newly formed cartilage [134,160,161]. (4) Hydrogels reduce immunogenicity by promoting ECM formation and controlling inflammation [171,172,191]. (5) Co-culturing hydrogels with stem cells minimizes hypertrophic differentiation [168].
|
Download:
|
| Fig. 6. Hydrogel can provide a suitable environment for the proliferation and differentiation of stem cells. In turn, stem cells enhance the microenvironment of hydrogel, creating a virtuous circle that promotes cartilage repair and regeneration (created with BioRender.com). | |
Despite the promising potential of stem cell-based hydrogels, several technical and regulatory challenges remain before they can be widely applied in clinical settings. Technically, most current research focuses on full-thickness cartilage defects, while solutions for the more common partial-thickness defects are lacking. Advances in 3D and four-dimensional (4D) bioprinting offer the possibility of personalized therapies, but further research is needed to tailor treatment strategies for different types of cartilage damage [224,225]. The production of stem cells and hydrogels also presents challenges due to complex and costly procedures. Simplified, standardized, and reproducible manufacturing processes are essential to ensure a stable supply of high-quality cells and materials. Incorporating digital modeling and machine learning can help reduce variability and enhance consistency. Once incorporated into hydrogels, maintaining the normal differentiation and functional behavior of stem cells over time remains difficult, as prolonged in vitro culture can lead to phenotypic changes. Combining gene editing or delivery methods with growth factors and biomolecules may help regulate stem cell differentiation and prevent hypertrophic cartilage formation. Designing specific microenvironments within hydrogels to support stem cell behavior is also crucial for tissue healing. The rate of hydrogel degradation must be carefully controlled to match the pace of cartilage growth, which can be achieved through optimized gelation methods or materials with tunable biodegradability. Additionally, enhancing the mechanical properties of hydrogels by combining them with other biomaterials or tissues can improve their structural integrity and functionality. Comparative studies among different stem cell types are still needed to evaluate their accessibility, differentiation potential, and immunogenicity. From a regulatory perspective, stem cell therapies often face strict oversight due to the unique nature of the cells. On one hand, stem cells must undergo rigorous safety testing to ensure they do not form tumors or elicit immune responses. On the other hand, ethical issues surrounding stem cells complicate public acceptance and regulatory approval. Therefore, regulatory approval, long-term safety and efficacy assessments, and the translation between research and commercialization are essential steps. To achieve clinical translation, extensive preclinical and clinical validation is still required. Advanced animal models can help study the long-term development of hydrogels under real physiological conditions, thereby enabling large-scale clinical trials—an endeavor that presents significant challenges.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementZeyang Yao: Writing – original draft, Project administration, Investigation, Conceptualization. Xinru You: Writing – original draft, Project administration, Investigation, Conceptualization. Xudong Wang: Writing – original draft, Project administration, Investigation, Conceptualization. Yunze Kang: Writing – original draft, Project administration, Investigation, Conceptualization. Liying Wang: Writing – review & editing, Supervision. Ziji Zhang: Writing – review & editing, Supervision.
AcknowledgmentsThis work was supported by the Natural Science Foundation of Guangdong Province (No. 2022A1515012279), the Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515110126).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110607.
| [1] |
S. Glyn-Jones, A.J.R. Palmer, R. Agricola, et al., Lancet 386 (2015) 376-387. |
| [2] |
B. Johnstone, M. Alini, M. Cucchiarini, et al., Eur. Cell Mater. 25 (2013) 248-267. DOI:10.22203/eCM.v025a18 |
| [3] |
Q. Yao, X. Wu, C. Tao, et al., Signal Transduct. Target Ther. 8 (2023) 56. |
| [4] |
J. Martel-Pelletier, A.J. Barr, F.M. Cicuttini, et al., Nat. Rev. Dis. Prim. 2 (2016) 16072. |
| [5] |
D. Chen, J. Shen, W. Zhao, et al., Bone Res. 5 (2017) 16044. |
| [6] |
L. Sharma, N. Engl. J. Med. 384 (2021) 51-59. DOI:10.1056/nejmcp1903768 |
| [7] |
S. Safiri, A.A. Kolahi, E. Smith, et al., Ann. Rheum. Dis. 79 (2020) 819-828. DOI:10.1136/annrheumdis-2019-216515 |
| [8] |
D.J. Hunter, D. Schofield, E. Callander, Nat. Rev. Rheumatol. 10 (2014) 437-441. DOI:10.1038/nrrheum.2014.44 |
| [9] |
A. Galperin, R.A. Oldinski, S.J. Florczyk, et al., Adv. Healthc. Mater. 2 (2013) 872-883. DOI:10.1002/adhm.201200345 |
| [10] |
R.S. Tuan, A.F. Chen, B.A. Klatt, J. Am. Acad. Orthop. Surg. 21 (2013) 303-311. |
| [11] |
P.N. Chalmers, H. Vigneswaran, J.D. Harris, et al., Cartilage 4 (2013) 193-203. DOI:10.1177/1947603513481603 |
| [12] |
D. Goyal, A. Goyal, S. Keyhani, et al., Arthroscopy 29 (2013) 1872-1878. |
| [13] |
S.S. Lin, C.M. Bono, R. Treuting, et al., Foot Ankle Int. 21 (2000) 742-748. DOI:10.1177/107110070002100905 |
| [14] |
W. Bartlett, J.A. Skinner, C.R. Gooding, et al., J. Bone Joint Surg. Br. 87 (2005) 640-645. |
| [15] |
H.S. Vasiliadis, J. Wasiak, G. Salanti, Knee Surg. Sports Traumatol. Arthrosc. 18 (2010) 1645-1655. DOI:10.1007/s00167-010-1050-3 |
| [16] |
L. Zhou, V.O. Gjvm, J. Malda, et al., Adv. Healthc. Mater. 9 (2020) e2001008. |
| [17] |
Y.S. Zhang, A. Khademhosseini, Science 356 (2017) eaaf3627. |
| [18] |
K. Yue, G. Trujillo-de Santiago, M.M. Alvarez, et al., Biomaterials 73 (2015) 254-271. |
| [19] |
S. Chen, P. Fu, R. Cong, et al., Gene. Dis. 2 (2015) 76-95. |
| [20] |
C.B. Carballo, Y. Nakagawa, I. Sekiya, et al., Clin. Sport. Med. 36 (2017) 413-425. |
| [21] |
A.R. Armiento, M.J. Stoddart, M. Alini, et al., Acta Biomater. 65 (2018) 1-20. |
| [22] |
H. Muir, Bioessays 17 (1995) 1039-1048. DOI:10.1002/bies.950171208 |
| [23] |
A. Preitschopf, D. Schörghofer, K. Kinslechner, et al., Stem Cell. Transl. Med. 5 (2016) 580-590. DOI:10.5966/sctm.2015-0262 |
| [24] |
M. Brittberg, P. Aglietti, R. Gambardella, et al., ICRS cartilage injury evaluation package, in: Proceedings of 3rd ICRS Meeting, Göteborg, Sweden, 2000, pp. 5–8.
|
| [25] |
A.J. Krych, D.B.F. Saris, M.J. Stuart, et al., J. Am. Acad. Orthop. Surg. 28 (2020) 914-922. DOI:10.5435/jaaos-d-20-00266 |
| [26] |
Y. Xiong, B.-B. Mi, Z. Lin, et al., Mil. Med. Res. 9 (2022) 65. |
| [27] |
E.A. Makris, A.H. Gomoll, K.N. Malizos, et al., Nat. Rev. Rheumatol. 11 (2015) 21-34. DOI:10.1038/nrrheum.2014.157 |
| [28] |
A.F. Steinert, S.C. Ghivizzani, A. Rethwilm, et al., Arthritis Res. Ther. 9 (2007) 213. DOI:10.1186/ar2195 |
| [29] |
M. Krampera, G. Pizzolo, G. Aprili, et al., Bone 39 (2006) 678-683. |
| [30] |
A. Andrzejewska, B. Lukomska, M. Janowski, Stem Cell. 37 (2019) 855-864. DOI:10.1002/stem.3016 |
| [31] |
L. Moroni, P.M. Fornasari, J. Cell Physiol. 228 (2013) 680-687. DOI:10.1002/jcp.24223 |
| [32] |
D. Paul, S.M. Samuel, N. Maulik, Antioxid. Redox Signal. 11 (2009) 1841-1855. DOI:10.1089/ars.2009.2455 |
| [33] |
Y. Yang, Y. Wu, D. Yang, et al., Bioact. Mater. 27 (2023) 98-112. |
| [34] |
J. Li, M. Pei, Tissue Eng. Part B: Rev. 18 (2012) 270-287. DOI:10.1089/ten.teb.2011.0583 |
| [35] |
S. Grogan, J. Kopcow, D. D'Lima, Stem Cell. Transl. Med. 11 (2022) 1186-1195. DOI:10.1093/stcltm/szac078 |
| [36] |
Q. Lian, E. Lye, K.Suan Yeo, et al., Stem Cell. 25 (2007) 425-436. DOI:10.1634/stemcells.2006-0420 |
| [37] |
X. Chen, X.-H. Song, Z. Yin, et al., Stem Cell. 27 (2009) 1276-1287. DOI:10.1002/stem.61 |
| [38] |
Y. Jung, G. Bauer, J.A. Nolta, Stem Cell. 30 (2012) 42-47. DOI:10.1002/stem.727 |
| [39] |
L.G. Villa-Diaz, S.E. Brown, Y. Liu, et al., Stem Cell. 30 (2012) 1174-1181. DOI:10.1002/stem.1084 |
| [40] |
H.M. Blau, G.Q. Daley, N. Engl. J. Med. 380 (2019) 1748-1760. DOI:10.1056/nejmra1716145 |
| [41] |
E. Koyama, Y. Shibukawa, M. Nagayama, et al., Dev. Biol. 316 (2008) 62-73. |
| [42] |
Y. Li, J. Li, Y. Chang, et al., Chin. Chem. Lett. 35 (2024) 109414. |
| [43] |
A.H. Gomoll, G. Filardo, L. de Girolamo, et al., Knee Surg. Sports Traumatol. Arthrosc. 20 (2012) 450-466. DOI:10.1007/s00167-011-1780-x |
| [44] |
L. da Silva Meirelles, T.T. Sand, R.J. Harman, et al., Tissue Eng. Part A 15 (2009) 221-229. DOI:10.1089/ten.tea.2008.0103 |
| [45] |
K.-Y. Saw, A. Anz, C. Siew-Yoke Jee, et al., Arthroscopy 29 (2013) 684-694. |
| [46] |
A. Banfi, A. Muraglia, B. Dozin, et al., Exp. Hematol. 28 (2000) 707-715. |
| [47] |
M.A. Baxter, R.F. Wynn, S.N. Jowitt, et al., Stem Cell. 22 (2004) 675-682. |
| [48] |
L. He, T. He, J. Xing, et al., Stem Cell Res. Ther. 11 (2020) 276. |
| [49] |
A. Vega, M. Angel Martin-Ferrero, F. Del Canto, et al., Transplantation 99 (2015) 1681-1690. |
| [50] |
L. Orozco, A. Munar, R. Soler, et al., Transplantation 95 (2013) 1535-1541. |
| [51] |
R. Bastos, M. Mathias, R. Andrade, et al., Knee Surg. Sport. Traumatol. Arthrosc. 26 (2018) 3342-3350. DOI:10.1007/s00167-018-4883-9 |
| [52] |
J. Chahal, A. Gómez-Aristizábal, K. Shestopaloff, et al., Stem Cell. Transl. Med. 8 (2019) 746-757. DOI:10.1002/sctm.18-0183 |
| [53] |
M.F. Pittenger, A.M. Mackay, S.C. Beck, et al., Science 284 (1999) 143-147. |
| [54] |
E.T. Hurley, Y. Yasui, A.L. Gianakos, et al., Knee Surg. Sport. Traumatol. Arthrosc. 26 (2018) 3499-3507. DOI:10.1007/s00167-018-4955-x |
| [55] |
M. Zaim, S. Karaman, G. Cetin, et al., Ann. Hematol. 91 (2012) 1175-1186. DOI:10.1007/s00277-012-1438-x |
| [56] |
Y. Li, N. Charif, D. Mainard, et al., Biomed. Mater. Eng. 24 (2014) 47-52. |
| [57] |
F. Bruna, D. Contador, P. Conget, et al., Stem Cell. Int. 2016 (2016) 1461648. |
| [58] |
C.H. Jo, Y.G. Lee, W.H. Shin, et al., Stem Cell. 32 (2014) 1254-1266. DOI:10.1002/stem.1634 |
| [59] |
T.S. de Windt, L.A. Vonk, I.C.M. Slaper-Cortenbach, et al., Stem Cell. 35 (2017) 256-264. DOI:10.1002/stem.2475 |
| [60] |
T. Chen, D. Xiao, Y. Li, et al., Chin. Chem. Lett. 33 (2022) 2517-2521. |
| [61] |
J. Pak, J.H. Lee, N. Pak, et al., Int. J. Mol. Sci. 19 (2018) 2146. DOI:10.3390/ijms19072146 |
| [62] |
P.B. Fodor, S.G. Paulseth, Aesthet. Surg. J. 36 (2016) 229-236. DOI:10.1093/asj/sjv135 |
| [63] |
S. Khaldoyanidi, Cell Stem Cell 2 (2008) 198-200. |
| [64] |
A. Sohni, C.M. Verfaillie, Stem Cell. Int. 2013 (2013) 130763. |
| [65] |
W. Zhou, J. Lin, K. Zhao, et al., Am. J. Sport. Med. 47 (2019) 1722-1733. DOI:10.1177/0363546519848678 |
| [66] |
Z.-J. Wei, Q.-Q. Wang, Z.-G. Cui, et al., Ann. Transl. Med. 9 (2021) 452. DOI:10.21037/atm-20-5116 |
| [67] |
M. Jeyaraman, S. Muthu, P.A. Ganie, Cartilage 13 (2021) 1532S-1547S. DOI:10.1177/1947603520951623 |
| [68] |
L. Lu, C. Dai, Z. Zhang, et al., Stem Cell Res. Ther. 10 (2019) 143. |
| [69] |
W.S. Lee, H.J. Kim, K.I. Kim, et al., Stem Cell. Transl. Med. 8 (2019) 504-511. DOI:10.1002/sctm.18-0122 |
| [70] |
B. Sadri, M. Hassanzadeh, A. Bagherifard, et al., Stem Cell Res. Ther. 14 (2023) 162. |
| [71] |
J.P. Wang, Y.T. Liao, S.H. Wu, et al., Stem Cell Rev. Rep. 17 (2021) 1796-1809. DOI:10.1007/s12015-021-10169-z |
| [72] |
L.L. Black, J. Gaynor, D. Gahring, et al., Vet. Ther. 8 (2007) 272-284. |
| [73] |
A.D. Martin, M.Z. Daniel, D.T. Drinkwater, et al., Int. J. Obes. Relat. Metab. Disord. 18 (1994) 79-83. |
| [74] |
K.A. Iwen, A.C. Priewe, M. Winnefeld, et al., Exp. Dermatol. 23 (2014) 395-400. DOI:10.1111/exd.12406 |
| [75] |
W.J.F.M. Jurgens, M.J. Oedayrajsingh-Varma, M.N. Helder, et al., Cell Tissue Res. 332 (2008) 415-426. DOI:10.1007/s00441-007-0555-7 |
| [76] |
D. Miller, A. Grant, S. Durgam, et al., Am. J. Phys. Med. Rehabil. 101 (2022) 879-887. DOI:10.1097/phm.0000000000001930 |
| [77] |
D. Baksh, R. Yao, R.S. Tuan, Stem Cell. 25 (2007) 1384-1392. DOI:10.1634/stemcells.2006-0709 |
| [78] |
S. Roura, J.M. Pujal, C. Gálvez-Montón, et al., Stem Cell Res. Ther. 6 (2015) 123. |
| [79] |
L. Avercenc-Léger, P. Guerci, J.M. Virion, et al., Stem Cell Res. Ther. 8 (2017) 161. |
| [80] |
S. Kern, H. Eichler, J. Stoeve, et al., Stem Cell. 24 (2006) 1294-1301. DOI:10.1634/stemcells.2005-0342 |
| [81] |
X. Zhang, M. Hirai, S. Cantero, et al., J. Cell Biochem. 112 (2011) 1206-1218. DOI:10.1002/jcb.23042 |
| [82] |
C.Y. Fong, A. Subramanian, K. Gauthaman, et al., Stem Cell Rev. Rep. 8 (2012) 195-209. DOI:10.1007/s12015-011-9289-8 |
| [83] |
C.Y. Fong, L.L. Chak, A. Biswas, et al., Stem Cell Rev. Rep. 7 (2011) 1-16. DOI:10.1007/s12015-010-9166-x |
| [84] |
J. Bartolucci, F.J. Verdugo, P.L. González, et al., Circ. Res. 121 (2017) 1192-1204. |
| [85] |
P.L. González, C. Carvajal, J. Cuenca, et al., Stem Cell. Transl. Med. 4 (2015) 1109-1121. DOI:10.5966/sctm.2015-0022 |
| [86] |
S.M. Richardson, G. Kalamegam, P.N. Pushparaj, et al., Methods 99 (2016) 69-80. |
| [87] |
J. Matas, M. Orrego, D. Amenabar, et al., Stem Cell. Transl. Med. 8 (2019) 215-224. DOI:10.1002/sctm.18-0053 |
| [88] |
Y.B. Park, C.W. Ha, C.H. Lee, et al., Stem Cell. Transl. Med. 6 (2017) 613-621. DOI:10.5966/sctm.2016-0157 |
| [89] |
M. Zavatti, F. Beretti, F. Casciaro, et al., Biofactors 46 (2020) 106-117. DOI:10.1002/biof.1576 |
| [90] |
H.E. Lu, M.S. Tsai, Y.C. Yang, et al., Exp. Cell Res. 317 (2011) 1895-1903. |
| [91] |
S. Arnhold, S. Glüer, K. Hartmann, et al., Stem Cell. Int. 2011 (2011) 715341. |
| [92] |
P.A.B. Klemmt, V. Vafaizadeh, B. Groner, Expert. Opin. Biol. Ther. 11 (2011) 1297-1314. DOI:10.1517/14712598.2011.587800 |
| [93] |
M. Cananzi, P. De Coppi, Organogenesis 8 (2012) 77-88. DOI:10.4161/org.22426 |
| [94] |
T. Maraldi, F. Beretti, M. Guida, et al., Stem Cell. Transl. Med. 4 (2015) 539-547. DOI:10.5966/sctm.2014-0266 |
| [95] |
M. Rosner, K. Schipany, M. Hengstschläger, Stem Cell. Transl. Med. 3 (2014) 553-559. DOI:10.5966/sctm.2013-0194 |
| [96] |
A. Bajek, J. Olkowska, M. Walentowicz-Sadłecka, et al., J. Cell Biochem. 118 (2017) 116-126. DOI:10.1002/jcb.25618 |
| [97] |
A. Inui, T. Iwakura, A.H. Reddi, Cells 1 (2012) 994-1009. DOI:10.3390/cells1040994 |
| [98] |
F. Yang, D. Zhang, Q. Zhou, et al., Chin. Chem. Lett. 33 (2022) 2901-2905. |
| [99] |
F.A. Petrigliano, N.Q. Liu, S. Lee, et al., NPJ. Regen. Med. 6 (2021) 77. |
| [100] |
Y. Wang, Z.B. Han, Y.P. Song, et al., Stem Cell. Int. 2012 (2012) 652034. |
| [101] |
S. Roberts, P. Genever, A. McCaskie, et al., Regen. Med. 6 (2011) 351-366. DOI:10.2217/rme.11.21 |
| [102] |
W.S. Toh, E.H. Lee, T. Cao, Stem Cell Rev. Rep. 7 (2011) 544-559. DOI:10.1007/s12015-010-9222-6 |
| [103] |
M. Yoshihara, Y. Hayashizaki, Y. Murakawa, Stem Cell Rev. Rep. 13 (2017) 7-16. DOI:10.1007/s12015-016-9680-6 |
| [104] |
S. Moradi, H. Mahdizadeh, T. Šarić, et al., Stem Cell. Res. Ther. 10 (2019) 341. |
| [105] |
G. Liu, B.T. David, M. Trawczynski, et al., Stem Cell. Rev. Rep. 16 (2020) 3-32. DOI:10.1007/s12015-019-09935-x |
| [106] |
X. Liu, W. Li, X. Fu, et al., Front. Immunol. 8 (2017) 645. |
| [107] |
A.E. Omole, A.O.J. Fakoya, PeerJ 6 (2018) e4370. DOI:10.7717/peerj.4370 |
| [108] |
M. Csobonyeiova, S. Polak, A. Nicodemou, et al., Biomedicines 9 (2021) 186. DOI:10.3390/biomedicines9020186 |
| [109] |
A. Kamaraj, H. Kyriacou, K.T.M. Seah, et al., Cytotherapy 23 (2021) 647-661. |
| [110] |
M. Lach, T. Trzeciak, M. Richter, et al., J. Tissue Eng. 5 (2014) 2041731414552701. |
| [111] |
Y. Nam, Y.A. Rim, J. Lee, et al., Stem Cell. Int. 2018 (2018) 8490489. |
| [112] |
Y. Liu, M. Zhu, M. Meng, et al., Chin. Chem. Lett. 34 (2023) 107583. |
| [113] |
S. Nedunchezian, P. Banerjee, C.Y. Lee, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 124 (2021) 112072. |
| [114] |
J. Lam, N.F. Truong, T. Segura, Acta Biomater. 10 (2014) 1571-1580. |
| [115] |
H. Zhu, N. Mitsuhashi, A. Klein, et al., Stem Cell. 24 (2006) 928-935. DOI:10.1634/stemcells.2005-0186 |
| [116] |
C. Chung, J.A. Burdick, Tissue Eng. Part A 15 (2009) 243-254. DOI:10.1089/ten.tea.2008.0067 |
| [117] |
J.M. Lamo-Espinosa, G. Mora, J.F. Blanco, et al., J. Transl. Med. 16 (2018) 213. |
| [118] |
K.-Y. Saw, A.W. Anz, R.C.-S. Ng, et al., Arthroscopy 37 (2021) 2502-2517. |
| [119] |
R.B. Jakobsen, A. Shahdadfar, F.P. Reinholt, et al., Knee Surg. Sports Traumatol. Arthrosc. 18 (2010) 1407-1416. DOI:10.1007/s00167-009-1017-4 |
| [120] |
M. Kim, I.E. Erickson, A.H. Huang, et al., Tissue Eng. Part A 24 (2018) 1693-1703. DOI:10.1089/ten.tea.2017.0520 |
| [121] |
Q. Feng, S. Lin, K. Zhang, et al., Acta Biomater. 53 (2017) 329-342. |
| [122] |
T.N. Snyder, K. Madhavan, M. Intrator, et al., J. Biol. Eng. 8 (2014) 10. |
| [123] |
S. Cheng, M. Pan, D. Hu, et al., Chin. Chem. Lett. 34 (2023) 108276. |
| [124] |
N. Iwasaki, Y. Kasahara, S. Yamane, et al., Polymers 3 (2011) 100-113. |
| [125] |
K.Y. Lee, D.J. Mooney, Chem. Rev. 101 (2001) 1869-1880. |
| [126] |
S. Yamane, N. Iwasaki, T. Majima, et al., Biomaterials 26 (2005) 611-619. |
| [127] |
S. Hsu, S.W. Whu, S.-C. Hsieh, et al., Artif. Organ. 28 (2004) 693-703. |
| [128] |
J. Yang, Y.S. Zhang, K. Yue, et al., Acta Biomater. 57 (2017) 1-25. |
| [129] |
E.J. Sheehy, T. Mesallati, T. Vinardell, et al., Acta Biomater. 13 (2015) 245-253. |
| [130] |
G.R. Ragetly, G.J. Slavik, B.T. Cunningham, et al., J. Biomed. Mater. Res. A 93 (2010) 46-55. DOI:10.1002/jbm.a.32514 |
| [131] |
B. Choi, S. Kim, B. Lin, et al., Acta Biomater. 12 (2015) 30-41. |
| [132] |
S. Gao, P. Zhao, C. Lin, et al., Tissue Eng. Part A 20 (2014) 1271-1284. DOI:10.1089/ten.tea.2012.0773 |
| [133] |
M. Giretova, L. Medvecky, E. Petrovova, et al., Appl. Biochem. Biotechnol. 189 (2019) 556-575. DOI:10.1007/s12010-019-03021-1 |
| [134] |
Z. Yang, Y. Wu, C. Li, et al., Tissue Eng. Part A 18 (2012) 242-251. DOI:10.1089/ten.tea.2011.0315 |
| [135] |
S. Huang, X. Song, T. Li, et al., Stem Cell Res. Ther. 8 (2017) 264. |
| [136] |
G.R. Ragetly, D.J. Griffon, H.B. Lee, et al., Acta Biomater. 6 (2010) 1430-1436. |
| [137] |
Y. Huang, D. Seitz, F. König, et al., Int. J. Mol. Sci. 20 (2019) 4487. DOI:10.3390/ijms20184487 |
| [138] |
G.C.B. Medrado, C.B. Machado, P. Valerio, et al., Biomed. Mater. 1 (2006) 155-161. DOI:10.1088/1748-6041/1/3/010 |
| [139] |
N. Gao, Y. Zhang, Z. Yang, et al., Chin. Chem. Lett. 35 (2024) 108820. |
| [140] |
B. Trica, C. Delattre, F. Gros, et al., Mar. Drugs 17 (2019) 405. DOI:10.3390/md17070405 |
| [141] |
R. Ahmad Raus, W.M.F. Wan Nawawi, R.R. Nasaruddin, Asian J. Pharm. Sci. 16 (2021) 280-306. |
| [142] |
N. Sahu, P. Agarwal, F. Grandi, et al., Adv. Healthc. Mater. 10 (2021) e2002118. |
| [143] |
M.H. Park, R. Subbiah, M.J. Kwon, et al., Carbohydr. Polym. 202 (2018) 488-496. |
| [144] |
D. El Khoury, H.D. Goff, S. Berengut, et al., Eur. J. Clin. Nutr. 68 (2014) 613-618. DOI:10.1038/ejcn.2014.53 |
| [145] |
T. Zehnder, B. Sarker, A.R. Boccaccini, et al., Biofabrication 7 (2015) 025001. DOI:10.1088/1758-5090/7/2/025001 |
| [146] |
C.H. Jang, Y. Koo, G. Kim, Carbohydr. Polym. 248 (2020) 116776. |
| [147] |
S. Khatab, M.J. Leijs, G. van Buul, et al., Cell Biol. Toxicol. 36 (2020) 553-570. DOI:10.1007/s10565-020-09532-6 |
| [148] |
K. Witte, M.C. de Andrés, J. Wells, et al., Biofabrication 12 (2020) 045034. DOI:10.1088/1758-5090/abb653 |
| [149] |
Z. Huang, P. Nooeaid, B. Kohl, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 50 (2015) 160-172. |
| [150] |
F. Ma, X. Pang, B. Tang, Carbohydr. Polym. 206 (2019) 229-237. |
| [151] |
S. Jahangir, D. Eglin, N. Pötter, et al., Stem Cell Res. Ther. 11 (2020) 436. |
| [152] |
K. Ma, A.L. Titan, M. Stafford, et al., Acta Biomater. 8 (2012) 3754-3764. |
| [153] |
Y.Y. Lin, C.Y. Kuan, C.T. Chang, et al., Int. J. Mol. Sci. 24 (2023) 7062. DOI:10.3390/ijms24087062 |
| [154] |
C. Krömmelbein, M. Mütze, R. Konieczny, et al., Carbohydr. Polym. 263 (2021) 117970. |
| [155] |
P. Zarrintaj, S. Manouchehri, Z. Ahmadi, et al., Carbohydr. Polym. 187 (2018) 66-84. |
| [156] |
G.R. López-Marcial, A.Y. Zeng, C. Osuna, et al., ACS Biomater. Sci. Eng. 4 (2018) 3610-3616. DOI:10.1021/acsbiomaterials.8b00903 |
| [157] |
M.A. Salati, J. Khazai, A.M. Tahmuri, et al., Polymers 12 (2020) 1150. DOI:10.3390/polym12051150 |
| [158] |
A.A. Amini, L.S. Nair, Biomed. Mater. 7 (2012) 024105. DOI:10.1088/1748-6041/7/2/024105 |
| [159] |
P. Zarrintaj, B. Bakhshandeh, I. Rezaeian, et al., Sci. Rep. 7 (2017) 17187. |
| [160] |
A.H. Huang, M. Yeger-McKeever, A. Stein, et al., Osteoarthrit. Cartil. 16 (2008) 1074-1082. |
| [161] |
A.H. Huang, A. Stein, R.L. Mauck, Tissue Eng. Part A 16 (2010) 2699-2708. DOI:10.1089/ten.tea.2010.0042 |
| [162] |
A. Forget, A. Blaeser, F. Miessmer, et al., Adv. Healthc. Mater. 6 (2017) 1700255. |
| [163] |
Y. Ge, Y. Li, Z. Wang, et al., Front. Bioeng. Biotechnol. 9 (2021) 697281. |
| [164] |
T. Vinardell, S.D. Thorpe, C.T. Buckley, et al., Ann. Biomed. Eng. 37 (2009) 2556-2565. DOI:10.1007/s10439-009-9791-1 |
| [165] |
K.L. Moffat, K. Goon, F.T. Moutos, et al., Macromol. Biosci. 18 (2018) e1800140. |
| [166] |
M. Oliver-Ferrándiz, L. Milián, M. Sancho-Tello, et al., Biomedicines 9 (2021) 834. DOI:10.3390/biomedicines9070834 |
| [167] |
A.H. Huang, A. Stein, R.S. Tuan, et al., Tissue Eng. Part A 15 (2009) 3461-3472. DOI:10.1089/ten.tea.2009.0198 |
| [168] |
Y.H. Yang, A.J. Lee, G.A. Barabino, Stem Cell. Transl. Med. 1 (2012) 843-854. DOI:10.5966/sctm.2012-0083 |
| [169] |
K.M. Pawelec, S.M. Best, R.E. Cameron, J. Mater. Chem. B 4 (2016) 6484-6496. |
| [170] |
W. Zhao, X. Jin, Y. Cong, et al., J. Chem. Technol. Biotechnol. 88 (2013) 327-339. DOI:10.1002/jctb.3970 |
| [171] |
U. Nöth, L. Rackwitz, A. Heymer, et al., J. Biomed. Mater. Res. A 83 (2007) 626-635. DOI:10.1002/jbm.a.31254 |
| [172] |
C.H. Lee, A. Singla, Y. Lee, Int. J. Pharm. 221 (2001) 1-22. |
| [173] |
Z. Piperigkou, D. Bainantzou, N. Makri, et al., Mol. Biol. Rep. 50 (2023) 5125-5135. DOI:10.1007/s11033-023-08461-x |
| [174] |
X. Chen, F. Zhang, X. He, et al., Injury 44 (2013) 540-549. |
| [175] |
L. Valot, M. Maumus, L. Brunel, et al., Gels 7 (2021) 73. DOI:10.3390/gels7020073 |
| [176] |
Q. Liu, W. Dai, Y. Gao, et al., Acta Biomater. 154 (2022) 194-211. |
| [177] |
L. Zhang, T. Yuan, L. Guo, et al., J. Biomed. Mater. Res. A 100 (2012) 2717-2725. DOI:10.1002/jbm.a.34194 |
| [178] |
J. Liu, C. Yu, Y. Chen, et al., J. Mater. Chem. B 5 (2017) 9130-9140. |
| [179] |
A.B. Bello, D. Kim, D. Kim, et al., Tissue Eng. Part B: Rev. 26 (2020) 164-180. DOI:10.1089/ten.teb.2019.0256 |
| [180] |
M.C. Echave, L. Saenz del Burgo, J.L. Pedraz, et al., Curr. Pharm. Des. 23 (2017) 3567-3584. |
| [181] |
F. Gao, Z. Xu, Q. Liang, et al., Adv. Sci. 6 (2019) 1900867. |
| [182] |
L. Han, J. Xu, X. Lu, et al., J. Mater. Chem. B 5 (2017) 731-741. |
| [183] |
K. Song, L. Li, W. Li, et al., Mater. Sci. Eng. C Mater. Biol. Appl. 55 (2015) 384-392. |
| [184] |
H. Lin, A.W.M. Cheng, P.G. Alexander, et al., Tissue Eng. Part A 20 (2014) 2402-2411. DOI:10.1089/ten.tea.2013.0642 |
| [185] |
X. Liang, C. Huang, H. Liu, et al., Chin. Chem. Lett. 35 (2024) 109442. |
| [186] |
M.C. Echave, R. Hernáez-Moya, L. Iturriaga, et al., Expert Opin. Biol. Ther. 19 (2019) 773-779. DOI:10.1080/14712598.2019.1610383 |
| [187] |
L.S. Wang, C. Du, W.S. Toh, et al., Biomaterials 35 (2014) 2207-2217. |
| [188] |
N. Tsuzuki, J. Seo, K. Yamada, et al., Can. Vet. J. 54 (2013) 573-580. |
| [189] |
B.J. Klotz, D. Gawlitta, A.J.W.P. Rosenberg, et al., Trend. Biotechnol. 34 (2016) 394-407. |
| [190] |
L. Pang, H. Jin, Z. Lu, et al., Adv. Healthc. Mater. 12 (2023) e2300315. |
| [191] |
P. Chen, L. Zheng, Y. Wang, et al., Theranostics 9 (2019) 2439-2459. DOI:10.7150/thno.31017 |
| [192] |
A.D. Dikina, H.V. Almeida, M. Cao, et al., ACS Biomater. Sci. Eng. 3 (2017) 1426-1436. DOI:10.1021/acsbiomaterials.6b00654 |
| [193] |
A.K. Kudva, A.D. Dikina, F.P. Luyten, et al., Acta Biomater. 90 (2019) 287-299. |
| [194] |
Y.H. Chang, K.C. Wu, C.C. Wang, et al., J. Biomed. Mater. Res. A 108 (2020) 2069-2079. DOI:10.1002/jbm.a.36966 |
| [195] |
B. Conrad, L.H. Han, F. Yang, Tissue Eng. Part A 24 (2018) 1631-1640. DOI:10.1089/ten.tea.2018.0011 |
| [196] |
J. Melke, S. Midha, S. Ghosh, et al., Acta Biomater. 31 (2016) 1-16. |
| [197] |
D.N. Rockwood, R.C. Preda, T. Yücel, et al., Nat. Protoc. 6 (2011) 1612-1631. DOI:10.1038/nprot.2011.379 |
| [198] |
Z. Zhou, J. Cui, S. Wu, et al., Theranostics 12 (2022) 5103-5124. DOI:10.7150/thno.74548 |
| [199] |
R.M. Amsar, A. Barlian, H. Judawisastra, et al., Future Sci. OA 7 (2021) FSO734. |
| [200] |
O. Hasturk, K.E. Jordan, J. Choi, et al., Biomaterials 232 (2020) 119720. |
| [201] |
Y. Kambe, K. Yamamoto, K. Kojima, et al., Biomaterials 31 (2010) 7503-7511. |
| [202] |
A. Barlian, H. Judawisastra, A. Ridwan, et al., Sci. Rep. 10 (2020) 19449. |
| [203] |
M. Zang, Q. Zhang, G. Davis, et al., Acta Biomater. 7 (2011) 3422-3431. |
| [204] |
N. Bhardwaj, S.C. Kundu, Biomaterials 33 (2012) 2848-2857. |
| [205] |
J. Jaipaew, P. Wangkulangkul, J. Meesane, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 64 (2016) 173-182. |
| [206] |
N. Sawatjui, T. Damrongrungruang, W. Leeanansaksiri, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 52 (2015) 90-96. |
| [207] |
N. Bhattarai, F.A. Matsen, M. Zhang, Macromol. Biosci. 5 (2005) 107-111. DOI:10.1002/mabi.200400140 |
| [208] |
B. Reid, M. Gibson, A. Singh, et al., J Tissue Eng. Regen. Med. 9 (2015) 315-318. DOI:10.1002/term.1688 |
| [209] |
A.X. Sun, H. Lin, M.R. Fritch, et al., Acta Biomater. 58 (2017) 302-311. |
| [210] |
S. Lee, E. Choi, M.J. Cha, et al., Oxid. Med. Cell Longev. 2015 (2015) 632902. |
| [211] |
A.K. Kudva, F.P. Luyten, J. Patterson, J. Biomed. Mater. Res. A 106 (2018) 33-42. DOI:10.1002/jbm.a.36208 |
| [212] |
H. Yao, J. Xue, Q. Wang, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 79 (2017) 661-670. |
| [213] |
M.L. Kang, S.Y. Jeong, G.I. Im, Tissue Eng. Part A 23 (2017) 630-639. DOI:10.1089/ten.tea.2016.0524 |
| [214] |
E. Filová, M. Rampichová, A. Litvinec, et al., Int. J. Pharm. 447 (2013) 139-149. |
| [215] |
D. Harpaz, T. Axelrod, A.L. Yitian, et al., Materials 12 (2019) 343. DOI:10.3390/ma12030343 |
| [216] |
Y. Lu, X. Liu, G. Luo, J. Appl. Polym. Sci. 134 (2017) 45111. |
| [217] |
J. Chen, P. An, H. Zhang, et al., Biomed. Mater. 17 (2021) 014106. |
| [218] |
D.B.F. Saris, J. Vanlauwe, J. Victor, et al., Am. J. Sport. Med. 36 (2008) 235-246. DOI:10.1177/0363546507311095 |
| [219] |
H. Dashtdar, M.R. Murali, A.A. Abbas, et al., Knee Surg. Sport. Traumatol. Arthrosc. 23 (2015) 1368-1377. DOI:10.1007/s00167-013-2723-5 |
| [220] |
L. Peng, Y. Zhou, W. Lu, et al., BMC Musculoskelet. Disord. 20 (2019) 257. |
| [221] |
X. Yu, G. Qian, S. Chen, et al., Carbohydr. Polym. 159 (2017) 20-28. |
| [222] |
X. Chen, S. Huang, Y. Niu, et al., Tissue Eng. Regen. Med. 21 (2024) 171-183. DOI:10.1007/s13770-023-00574-5 |
| [223] |
C. Edwall-Arvidsson, J. Wroblewski, Anat. Embryol. 193 (1996) 453-461. |
| [224] |
Y.T. Men, Y.L. Jiang, L. Chen, et al., Mater. Sci. Eng. C: Mater. Biol. Appl. 78 (2017) 79-87. |
| [225] |
A. Guermazi, D. Hayashi, F.W. Roemer, et al., Arthrit. Rheumatol. 69 (2017) 560-564. DOI:10.1002/art.39970 |
 2025, Vol. 36
2025, Vol. 36 

