b State Key Laboratory of Flexible Electronics (KLOFE) & Institute of Advanced Materials (IAM), Nanjing Tech University (NanjingTech), Nanjing 211816, China
Hydrogen bonding, as one of the most ubiquitous molecular interactions, plays an important role in modulating the properties and functions of advanced materials for numerous applications [1-8]. Especially, in the field of luminescent materials, the modulation of hydrogen bonds (H-bond) is linked with performances in the aspects of photoswitching [9,10], emission wavelength [11,12], luminescence lifetime [13,14], and quantum yield [15,16]. Since the energy of ordinary H-bonds is normally less than 15 kcal/mol [17], environmental stimuli such as the temperature [18], humidity [19], vapor [20,21], force [22,23], and solvents [24,25] can interfere with the established H-bonds of the chromophores, making their emissions environment-sensitive.
Excited-state intramolecular proton transfer (ESIPT) is known as an H-bond dependent photophysical process that requires an intramolecular H-bond to trigger the enol to keto conversion upon photoexcitation, leading to a relatively large emission shift and high quantum yield [26-30]. The strategy of shutting intramolecular H-bond via isomerization has been utilized to design smart ESIPT materials for anti-counterfeiting [31], sensing [32-34], and force-response luminescent material [35]. In addition, Akutagawa et al. reported an example of an ESIPT chromophore containing two different H-bond acceptors, whose intramolecular H-bonding was switched in responding to acid/base stimulation, achieving a dual emission [36]. Furthermore, a recent study showed that the 2-(((1H-benzo[d]imidazol-2-yl)imino)methyl)-4-methoxyphenol system, which contains multiple hydrogen-bond donors and acceptors, demonstrated a unique ability to differentiate heavy water from normal water by regulating intermolecular hydrogen bonds [37]. Despite the successes in their specific applications, these ESIPT chromophores possess limited emission alterations due to insufficient diversity in the regulation of intra- and intermolecular H-bonds. It is worth noting that intermolecular H-bond governed molecular packing reconstruction is a major strategy to accomplish emission alterations in many fluorescent materials [38,39] other than ESIPT fluorophores, which suggests a potential but challenging route to extend the tunability in photochemical properties and application scenarios of ESIPT systems via regulating intra- and intermolecular H-bonds.
Surfaces are fundamental and prevalent in nature and industry, whose surface properties, especially hydrophobicity/hydrophilicity, are of great importance in many practical applications, such as biological adhesion [40,41], coating [42,43], anti-fogging [44], oil-water separation [45,46], and catalysis [47]. Thereby, characterizations of surface hydrophobicity/hydrophilicity are critical in understanding and controlling the surface behaviors. However, the conventional contact angle method falls short in offering a comprehensive measurement on such surfaces, while the heterogeneity might also lead to uncertainties in measurements [48]. Considering that the abundance of interfacial H-bond acceptors is closely relevant to the surficial hydrophobicity/hydrophilicity [49-51]. fluorescence-based method that is sensitive to H-bonding might offer a solution to complement the contact angle method in the scenario of large-scale heterogeneous surfaces.
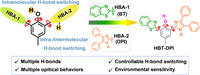
In this study, we explored the potential of multi-dimensional regulation of intra- and intermolecular H-bonds in single molecule ESIPT systems to expand the variety of emissions. We designed and synthesized a model ESIPT chromophore HBT-DPI by incorporating a diphenylimidazole (DPI) group into HBT (Scheme 1). Unlike conventional ESIPT chromophores, the presence of two H-bond accepting groups (HBAs), benzothiazole (BT) (HBA-1) and DPI (HBA-2), provides an extra choice for the intramolecular H-bond with the hydroxyl group in the phenol core. Moreover, the N-H in imidazole, as an H-bond donor, along with the bulky diphenyl group could offer flexibility in subtle modulation of intermolecular H-bond formation. In response to different solvent environments, four types of HBT-DPI crystal/cocrystals showed diversity in the contents of structural isomers, molecular packing modes, and photophysical properties, resulting from the varied behaviors in intra- and intermolecular H-bonds formation. Notably, we successfully employed HBT-DPI to visualize the surficial hydrophobicity/hydrophilicity distribution along with their quantification on heterogeneously modified poly(1,1-difluoroethylene) (PVDF) membranes, demonstrating a new approach for hydrophobicity/hydrophilicity monitoring and measurement on a large-scale surface with heterogeneous modification. Overall, this study provides a new strategy to construct ESIPT-inspired chromophores whose single molecular emissions are regulated by multiple-dimensional H-bonds, and demonstrated the unique application in hydrophobicity/hydrophilicity mapping on a large-scale heterogeneous surface.
|
Download:
|
| Scheme 1. Design of model molecule HBT-DPI with multi-dimensional H-bonds. | |
To enable the multi-dimensional regulation of H-bonds, DPI was attached to a central HBT core to obtain the model ESIPT chromophore HBT-DPI. Although both DPI and BT contain unsaturated N atoms, endowing them with excellent H-bond accepting capability, whereas the presence of sulfur atom in BT weakens its hydrogen accepting ability [52,53]. Moreover, the DPI group contains an extra H-bond donor (N-H) that can form either an intramolecular H-bond with the oxygen of the phenol core or an intermolecular H-bond with other molecules, which could extend the possible molecular packing behaviors in the solid state. By such a molecular design, the manipulation of multi-dimensional H-bonds (intra- and intermolecular) would render the environment sensitive material with a variety of photophysical properties. The ESIPT chromophore HBT-DPI was synthesized by three-step reactions according to Scheme S1 (Supporting information) and characterized using 1H nuclear magnetic resonance spectroscopy (NMR), 13C NMR and high-resolution mass spectrometry (Figs. S21–S23 in Supporting information).
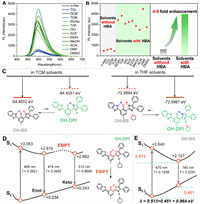
Then, we investigated the intramolecular H-bond switching in solution. Although HBT-DPI exhibits similar absorption bands (280–400 nm) in the tested solvents (Fig. S1 in Supporting information), the fluorescent emission spectra of HBT-DPI were found to be much stronger in the solvents containing O or N atoms that can act as H-bond acceptor (including ethyl acetate (EtOAc), tetrahydrofuran (THF), dioxane (DIO), acetone (ACE), alcohol (EtOH), methanol (MeOH), acetonitrile (ACN), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO)) than in those without H-bond acceptor (including n-hexane (n-Hex), toluene (TOL), dichloromethane (DCM), and chloroform (TCM)) (Figs. 1A and B), implying different molecular status in the two types of environments. Quantum chemical calculations reveal that, in TCM (without an H-bond acceptor), HBT-DPI tends to form the isomer OH-BS which is stabilized by two intramolecular H-bonds to achieve the lowest potential energy (Fig. 1C). In contrast, the O atom in THF competes for the free hydrogen of DPI, forming a strong N-H···O intermolecular H-bond (Fig. S2 in Supporting information); while the imidazole, a stronger H-bond acceptor than BT, preferentially forms the intramolecular H-bond with the hydroxyl in phenol core, representing the isomer OH-DPI (Fig. 1C). After demonstrating the two isomers with switched intramolecular H-bonds, further analysis shows that the keto form of OH-DPI has a significantly larger oscillator strength (f) of 0.8695 than the enol form of OH-DPI and the vertical keto*→keto photoluminescence peak is calculated to be 513 nm for OH-DPI (Fig. 1D), which is consistent with the experimental data, indicating the emissive ESIPT process of OH-DPI isomer. Whereas OH-BS possesses a weak emission mainly due to the fast non-radiative decay process caused by the large reorganization energy of 0.964 eV (Fig. 1E). Besides, we also found that the energy barrier of possible transforming routes between OH-DPI and OH-BS is relatively small in TCM (Fig. S3 in Supporting information). The small energy barrier would allow a partial transition from OH-BS to OH-DPI, contributing to the retention of fluorescence emission in TCM and possibly other solvents without H-bond acceptor. Additionally, when increasing the content of THF in the n-Hex solution, the fluorescence intensity of HBT-DPI significantly increased (Fig. S4 in Supporting information), indicating the occurrence of isomerization from OH-BS to OH-DPI. These results demonstrate the success of our molecular design on intramolecular H-bond switching, which can be controlled via the hydrogen accepting capability of solvent. Furthermore, scanning electron microscope (SEM) indicated the formation of nanoparticles with an size of ~3 µm in n-Hex and ~3 µm in THF (Fig. S5 in Supporting information), demonstrating comparable particle sizes in both solutions but exhibiting distinct fluorescent behaviors. Thus, the traditional explanation of aggregation-induced emission for these intensity variations could be ruled out.
|
Download:
|
| Fig. 1. Solvent-induced isomerization and theoretical calculation. (A) Fluorescence spectra (10 µmol/L) of HBT-DPI in different solvents, green lines: with strong emission, blue lines: with weak emission, n-Hex: n-hexane, TOL: toluene, DCM: dichloromethane, TCM: chloroform, EtOAc: ethyl acetate, THF: tetrahydrofuran, DIO: dioxane, ACE: acetone, EtOH: alcohol, MeOH: methanol, ACN: acetonitrile, DMF: N,N-dimethylformamide, DMSO: dimethyl sulfoxide, λex = 365 nm, slit: 5 nm/5 nm. (B) Statistics of fluorescence and wavelength changes in various solvents. (C) Chemical structure and the energy of OH-BS and OH-DPI in different solvents. (D) The schematic representation of the ESIPT state of OH-DPI in THF solvents. (E) Potential energy surfaces of OH-BS in TCM solvents. The relative energies (in eV) and oscillator strengths (f) were evaluated at the level of (TD)DFT/O3LYP/def2-SVP. | |
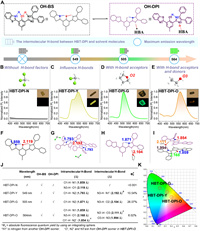
To further demonstrate the multi-dimensional regulation of intra- and intermolecular H-bonds in HBT-DPI and the resulting diverse photochemical properties, we prepared single crystals of HBT-DPI in various solvents. As expected, we successfully obtained four types of HBT-DPI single crystals (HBT-DPI-N, HBT-DPI-Y, HBT-DPI-G, and HBT-DPI-O) with distinct photophysical behaviors by the solvent-induced method. Through the single-crystal X-ray diffraction (SXRD) analysis, we found that the crystals exhibit varieties in the contents of HBT-DPI isomers and the molecular packing behaviors (Fig. 2, Figs. S6–S9 and Tables S9 and S10 in Supporting information), which are largely influenced by the strong H-bonds (distance between hydrogen atom and H-bond acceptor < 2.2 Å, bond energy around −4 kcal/mol to −15 kcal/mol) [17]. As shown in Fig. 2A, We first labeled different atoms in OH-BS and OH-DPI isomers for ease of description. The single crystal of HBT-DPI-N was obtained from a gas (n-pentane)-liquid (TCM) diffusion system. Due to the absence of competitive intermolecular H-bonds between imidazole moiety and solvent molecules, two types of strong intramolecular H-bonds including O1-H···N1 (1.859 Å) and N3-H···O1 (2.119 Å) were constructed in HBT-DPI-N corresponding to OH-BS isomer (Fig. 2C). The specific double intramolecular H-bonds system endows considerable reorganization energy, suppressing the emission of the OH-BS isomer (Figs. 2B and F). Moreover, the severe π-π stacking (r1 = 3.541 Å) of crossing packing mode further consumes the excited state energies (Fig. S6 in Supporting information), thus quenching the fluorescence emission in the crystalline state (ΦF < 0.001) (Figs. 2J and K). While an enhanced fluorescence quantum yield (ΦF = 10.54%) can be obtained in HBT-DPI-Y cocrystals prepared by evaporating n-Hex and DCM mixture (Figs. 2C, J and K). The HBT-DPI-Y cocrystals formed a new intramolecular H-bond (N2···H-O1, 1.793 Å), which corresponds to the OH-DPI isomer (Fig. 2G), facilitating the activation of ESIPT process. Unlike TCM, DCM molecules were trapped in the HBT-DPI-Y cocrystals, providing multiple interactions containing halogen bond (Cl···π 4.007 Å), H-bond (C-H···N2 2.637 Å, C-H···Cl 2.852 Å, C-H···Cl 2.915 Å) and C-H···π (2.807 Å) (Fig. S7A in Supporting information), along with the intermolecular H-bonds (N3-H···N1, 2.192 Å) among OH-DPI isomers, triggering the formation of an interlock crossing packing mode, which contributes a yellow fluorescence emission at 549 nm (Fig. 2C and Fig. S7 in Supporting information) and targets at (0.33, 0.53) in CIE 1931 (Fig. 2K).
|
Download:
|
| Fig. 2. Multimodal H-Bonds induced multiple optical behaviors in crystals. (A) Isomerization of HBT-DPI between OH-BS (black) and OH-DPI (purple). Fluorescence spectra of the crystal obtained in (B) TCM (HBT-DPI-N), (C) DCM (HBT-DPI-Y), (D) THF (HBT-DPI-G), (E) EtOH (HBT-DPI-O), λex = 365 nm, slit: 5 nm/5 nm, inset: crystal pictures (top right-hand corner) and fluorescence photographs (bottom right-hand corner). Crystal structures and intra-/intermolecular H-bonds of (F) HBT-DPI-N, (G) HBT-DPI-Y, (H) HBT-DPI-G, (I) HBT-DPI-O composed by OH-BS (black) and OH-DPI (purple). (J) Table of the wavelength, molecular states, H-bonds and absolute fluorescence quantum yields of crystals. (K) The fluorescence spectra of HBT-DPI-G, HBT-DPI-Y, and HBT-DPI-O tagged on a CIE 1931 chromaticity diagram. | |
In response to THF that offers H-bond acceptors, a type of green fluorescence cocrystals HBT-DPI-G (λem = 505 nm) was collected (Fig. 2D) and targeted at (0.21, 0.59) in CIE 1931 (Fig. 2K). The THF molecule formed an intermolecular H-bond with HBT-DPI between N3-H and the oxygen atom (O2) of THF (Fig. 2H), benefiting the formation of the OH-DPI isomer. The antiparallel packing mode found in the cocrystals HBT-DPI-G enlarges the centroid distance of adjacent molecules to avoid the π-π stacking (Figs. S8B-C in Supporting information), rendering the promoted ΦF of 26.07% (Figs. 2J and K). Intriguingly, a triad cocrystal species HBT-DPI-O was obtained from the protic EtOH. As shown in Fig. 2I, both the OH-BS and OH-DPI isomers were found in HBT-DPI-O at a 1:1 molar ratio. Possessing both the H-bond donor and acceptor, EtOH tethers a pair of OH-BS and OH-DPI via two intermolecular H-bonds (N3-H···O3 2.168 Å, O3-H···N2 1.994 Å) (Fig. 2I); and the paired units pack organized, leading to red-shifted fluorescence emission at 564 nm with a reduced ΦF (6.02%) (Figs. 2E, J and K) targeted at (0.40, 0.45) in CIE 1931 (Fig. 2K), which could be due to the existence of OH-BS isomers, π-π stacking and other concomitantly abundant intermolecular interactions (Fig. S9 in Supporting information). The discovery and characterizations of the four crystals demonstrated the success of tuning the photophysical behaviors of HBT-DPI via multi-dimensional regulation of intra- and intermolecular H-bonds.
Further attempts revealed that HBT-DPI-N can also grow in the solvent without H-bond acceptor, such as TOL (Figs. S10 and S12A, Table S11 in Supporting information); in contrast, the solvents with H-bond acceptor, including ACN, DIO, and ACE, favor the formation of HBT-DPI-G (Figs. S11 and S12B–D, Tables S11 and S12 in Supporting information). The orange emission similar to HBT-DPI-O was also observed in powders obtained from MeOH, which was confirmed to have a similar structure with HBT-DPI-O by X-ray diffraction (XRD) (Figs. S13 and S14 in Supporting information), suggesting the packing mode of HBT-DPI-O might be favorable in alcohols. Whereas the distinct packing mode of HBT-DPI-Y was only found in the crystal prepared in DCM. Overall, these results prove that the multi-dimensional regulation of intra-/intermolecular H-bonds offers extra diversity in constructing multi-emissive environment sensitive materials.
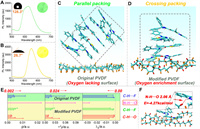
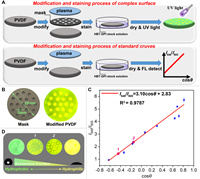
Inspired by the H-bond acceptor sensitive nature of HBT-DPI, we tried to sense the surficial hydrophilicity and hydrophobicity using HBT-DPI. HBT-DPI emitted green emission upon polycarbonate (PA) membrane (hydrophilic surface) and yellow emission upon hydrophilic membranes containing PVDF, polypropylene (PP) and polytetrafluoroethylene (PTFE), demonstrating the outstanding property of our fluorophore (Fig. 3A and Fig. S15 in Supporting information). On this basis, we tried our fluorophore to monitor the modified membranes, which showed extensive requirements and applications in material science [42,54,55]. Subsequently, we used PVDF membrane as the model surface and created hydrophilic areas using Sub-Atmospheric Microthermal Plasma (SAMP) to further investigate the optical behaviors of HBT-TCF upon hydrophilic and hydrophobic PVDFs. The original PVDF membrane surface was hydrophobic with a contact angle of around 128.3° (Fig. 3A), and the subsequent treatment of SAMP increased the surface hydrophilicity, rendering a contact angle of around 26.7° (Fig. 3B). The oxygen contents intensified by the SAMP treatment at the membrane surface (Tables S1 and S2 in Supporting information) increase the hydrophilicity while also acting as the H-bond acceptors. Consequently, by sensing the H-bond acceptors, the green coating of HBT-DPI on the original PVDF membrane surface (Fig. 4A) turned to yellow on the modified surface (Fig. 3B), demonstrating the potential of HBT-DPI on distinguishing between hydrophilic and hydrophobic areas.
|
Download:
|
| Fig. 3. Monitoring hydrophilic/hydrophobic surface. The fluorescence spectra of membrane (A) before and (B) after modification of PVDF membrane staining by HBT-DPI, inset: diagram of contact angle measurement (left) and colored membrane (right). (C) Stabilized packing mode of HBT-DPI adsorption on the original PVDF, blue background: solvents (DCM). (D) Stabilized packing mode of HBT-DPI adsorption on the modified PVDF, blue background: solvents (DCM). (E) Topological parameters of the interaction between PVDF (green background) or modified PVDF (yellow background) and HBT-DPI. (F) HBT-DPI adsorbed on the modified PVDF surface and the parameter of the N-H···O H-bond. Color code: white, H; sky blue, C; yellow, S; blue, N; red, O; orange, F. | |
|
Download:
|
| Fig. 4. Quantitative evaluation of surficial hydrophobicity/hydrophilicity. (A) Process of surficial modification, top: the process of obtaining complex surface, bottom: the process of obtaining standard curve. Concentration of HBT-DPI in DCM solution: 1 mmol/L. (B) Complex surface modified by coving a mask, left: the mask, right: complex surface after stained by HBT-DPI. (C) The standard curve between fluorescent ratio and contact angle upon the PVDF membrane. (D) The comparison of hydrophobicity between various large-scale surfaces. | |
Further theoretical calculations explored the molecular behaviors of HBT-DPI on the model surfaces to explain the changed fluorescent emissions (details of theoretical calculations are available in section 14 of the supplemental information). Since the original PVDF membrane lacks efficient HBAs, only weak H-bonds (C-H···F) formed between HBT-DPI molecules and the membrane (Fig. 3E), leading to parallelly piled HBT-DPI molecules which emit green fluorescence on the membrane surface (Fig. 3C, Figs. S16A–D and Video S1 in Supporting information). In contrast, the abundant oxygen (Tables S1 and S2) on the modified PVDF surface leads to the formation of abundant H-bonds (C-H···F, C-H···O and N-H···O) with HBT-DPI molecules, resulting in much stronger affinity between the modified surface and HBT-DPI (Fig. 3E, Figs. S17 and S18 in Supporting information). Especially, the bond energy and length of the N-H···O bond is calculated as −4.27 kcal/mol and 2.06 Å (Fig. 3F and Fig. S19, Table S3 in Supporting information), providing dominant interactions to trigger the cross-stacking mode of molecular assembly (Fig. 3D and Figs. S15E–H, Video S2 in Supporting information), which corresponds to a yellow fluorescence according to the aforementioned SXRD analysis (Fig. 2E).
Considering the relevance between the abundance of oxygens and hydrophilicity, the intensity ratio between the external H-bond-induced yellow emission (λem= 549 nm) and the green emission (λem= 505 nm) of HBT-DPI should be able to quantitatively evaluate the surficial hydrophobicity. As a proof of concept, several PVDF membranes with varying surficial hydrophobicity were prepared via plasma modification from 0 to 180 s. The contact angle (θ) for each modified PVDF membrane surface was measured within the range of 20°–130° (Fig. S20 and Table S4 in Supporting information). Afterward, the membranes were coated with HBT-DPI as mentioned above to collect the ratios of I549/I505 (Fig. 4A). By plotting I549/I505 versus cosθ, the calibration curve between the I549/I505 and its corresponding θ was defined by a linear equation, I549/I505=3.10cosθ + 2.83 (Fig. 4C, Fig. S20, Tables S4 and S6 in Supporting information). Owing to the influence of non-uniform surface textures, the measurement of the fluorescence intensity showed variations, resulting in an R2 of 0.9787 for the calibration curve. Despite the slightly low R2, we can still conclude the linear relationship between I549/I505 and cosθ, and the HBT-DPI fluorescence intensity ratio of I549/I505 increases along with the increasing cosθ, which represents enhanced hydrophilicity.
On this basis, we further prepared a PVDF membrane with a partially modified surface by covering a porous mask before plasma treatment (Figs. 4A and B). The purpose is to create unevenly distributed hydrophilic areas on the membrane surface. As expected, after merging the membrane in DCM solution containing HBT-DPI and drying, yellower patterns consistent with the pores on the mask were seen while the covered area, which is less irradiated, maintained green color (Figs. 4B and D). The observation convincingly demonstrates the unique merits of HBT-DPI in mapping the distribution of hydrophilic and hydrophobic areas on a large-scale surface, which is not feasible with the conventional contact angle method. We also estimated a mean hydrophobicity of the heterogeneous surface by corresponding the I549/I505 ratio to a calculated contact angle (Fig. 4C and Table S8 in Supporting information). For instance, the modified membrane 1 exhibited an I549/I505 ratio of 1.29 corresponding to a θ of 126.2°, while the modified membrane 2 with more hydrophilic areas gave an I549/I505 ratio of 2.28 corresponding to a θ of 104.6° (Figs. 4C and D, Table S8). Although the calculated contact angle via HBT-DPI mapping is not representing a specific hydrophobicity of any point on the surface, it can provide information on the overall hydrophobicity of a heterogenous and large-scale surface, which is not able to be estimated before. Taken together, our examples demonstrate the potential of HBT-DPI as a convenient and useful tool to evaluate the surficial hydrophobicity/hydrophilicity, and its advantages over the conventional method in the aspects of mapping the hydrophobic/hydrophilic areas as well as quantification on a large-scale surface, even the surficial modification is heterogeneous.
In summary, we proposed to extend the diversity in constructing single-molecule ESIPT systems by the multi-dimensional regulation of intra- and intermolecular H-bonds. The model molecule, HBT-DPI, reported in this work contains two functional groups, DPI and BT, which render the feature of switchable intramolecular H-bonds. Two isomers, OH-DPI and OH-BS, were obtained respectively in solvents with or without H-bond acceptors, possessing distinct intramolecular H-bonds that influence the ESIPT process to the emissive properties of the two isomers. In response to different solvent environments, four types of HBT-DPI crystal/cocrystals (HBT-DPI-N, HBT-DPI-Y, HBT-DPI-G, and HBT-DPI-O) were further obtained via crystal growth as a result of the regulation of both intra- and intermolecular H-bonds. The multi-dimensional H-bonds regulation largely influenced the molecular packing of HBT-DPI molecules, resulting in multimode crystal structures that are non-emissive or emit variable fluorescence ranging from green to orange. This work provides new insights into the H-bonds regulation induced structure-packing-performance relationship, which offers a molecular-level design strategy to construct single molecule light-emitting materials with multi-emissions. Furthermore, HBT-DPI was shown to map the hydrophobic/hydrophilic areas on large-scale heterogeneous surfaces and quantitatively estimate the surficial hydrophobicity/hydrophilicity, offering a new approach advantageous over the conventional contact angle measurement.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementHao Gu: Writing – original draft, Visualization, Validation, Investigation. Rui Li: Investigation, Formal analysis. Qiuying Li: Investigation. Sheng Lu: Writing – review & editing, Funding acquisition. Yahui Chen: Visualization. Xiaoning Yang: Software. Huili Ma: Writing – review & editing, Supervision, Funding acquisition. Zhijun Xu: Supervision, Software, Funding acquisition. Xiaoqiang Chen: Writing – review & editing, Supervision, Funding acquisition.
AcknowledgmentsThis work is supported by the National Key R&D Program of China (No. 2021YFC2103600), the National Natural Science Foundation of China (Nos. 21878156, 21978131, 22275085, and 22278224), the Natural Science Foundation of Jiangsu Province (Nos. BK20200089 and BK20200691) and the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the State Key Laboratory of Materials-Oriented Chemical Engineering (No. KL21-08). Thank Dr. Jiapeng Cao for his help in resolving single-crystals. Thank Nanjing Suman Plasma Technology Co., Ltd. for providing Sub-Atmospheric Microthermal Plasma (SAMP) equipment. We are grateful to the High Performance Computing Center in Nanjing Tech University for supporting the computational resources.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110116.
| [1] |
X. Song, Y. Wang, C. Wang, et al., J. Am. Chem. Soc. 144 (2022) 10663-10687. DOI:10.1021/jacs.2c02598 |
| [2] |
A.Y. Chan, I.B. Perry, N.B. Bissonnette, et al., Chem. Rev. 122 (2022) 1485-1542. DOI:10.1021/acs.chemrev.1c00383 |
| [3] |
Y.H. Wang, S. Zheng, W.M. Yang, et al., Nature 600 (2021) 81-85. DOI:10.1038/s41586-021-04068-z |
| [4] |
X. Zhang, Y. Cheng, J. You, et al., Nat. Commun. 13 (2022) 1117. DOI:10.1038/s41467-022-28759-x |
| [5] |
Z. Wang, Y. Zhang, C. Wang, et al., Adv. Mater. 32 (2020) 1907355. DOI:10.1002/adma.201907355 |
| [6] |
D. Li, Y. Yang, J. Yang, et al., Nat. Commun. 13 (2022) 347. DOI:10.1038/s41467-022-28011-6 |
| [7] |
B.C. Gibb, Nat. Chem. 12 (2020) 665-667. DOI:10.1038/s41557-020-0524-2 |
| [8] |
Z. Song, D. Mao, S.H.P. Sung, et al., Adv. Mater. 28 (2016) 7249-7256. DOI:10.1002/adma.201601214 |
| [9] |
H. Park, D. Lee, Chem. Sci. 12 (2021) 590-598. DOI:10.1039/d0sc05067a |
| [10] |
Z.Y. Liu, J.W. Hu, C.H. Huang, et al., J. Am. Chem. Soc. 141 (2019) 9885-9894. DOI:10.1021/jacs.9b02765 |
| [11] |
Y. Han, T. Zhang, X. Chen, et al., ACS Appl. Mater. Interfaces 13 (2021) 32270-32277. DOI:10.1021/acsami.1c08316 |
| [12] |
A. Shukla, V.T.N. Mai, V.V. Divya, et al., J. Am. Chem. Soc. 144 (2022) 13499-13510. DOI:10.1021/jacs.2c02163 |
| [13] |
K. Wu, T. Zhang, Z. Wang, et al., J. Am. Chem. Soc. 140 (2018) 8877-8886. DOI:10.1021/jacs.8b04795 |
| [14] |
L. Gu, H. Shi, C. Miao, et al., J. Mater. Chem. C 6 (2018) 226-233. DOI:10.1039/c7tc04452f |
| [15] |
J. Chen, T. Yu, E. Ubba, et al., Adv. Opt. Mater. 7 (2019) 1801593. DOI:10.1002/adom.201801593 |
| [16] |
X. Qiu, Y. Xu, C. Wang, et al., J. Mater. Chem. C 7 (2019) 5461-5467. DOI:10.1039/c9tc00357f |
| [17] |
G.R. Desiraju, T. Steiner, The Weak Hydrogen bond: in Structural Chemistry and Biology, Oxford University Press, NewYork, 2001.
|
| [18] |
M. Mieczkowski, C. Steinmetzger, I. Bessi, et al., Nat. Commun. 12 (2021) 3549-3559. DOI:10.1038/s41467-021-23932-0 |
| [19] |
A. Wang, R. Fan, Y. Dong, et al., ACS Appl. Mater. Interfaces 9 (2017) 15744-15757. DOI:10.1021/acsami.7b01254 |
| [20] |
Y. Zhang, H. Yang, H. Ma, et al., Angew. Chem. Int. Ed. 58 (2019) 8773-8778. DOI:10.1002/anie.201902890 |
| [21] |
K. Liu, G. Wang, N. Ding, et al., ACS Appl. Mater. Interfaces 13 (2021) 19342-19350. DOI:10.1021/acsami.1c03331 |
| [22] |
K. Nagura, S. Saito, H. Yusa, et al., J. Am. Chem. Soc. 135 (2013) 10322-10325. DOI:10.1021/ja4055228 |
| [23] |
G. Huang, Y. Jiang, J. Wang, et al., J. Mater. Chem. C 7 (2019) 12709-12716. DOI:10.1039/c9tc04501e |
| [24] |
J. Herbich, C.Y. Hung, R.P. Thummel, J. Waluk, J. Am. Chem. Soc. 118 (1996) 3508-3518. DOI:10.1021/ja952797d |
| [25] |
M. Das, S. Sahu, G. Krishnamoorthy, Phys. Chem. Chem. Phys. 21 (2019) 15669-15677. DOI:10.1039/c9cp02281c |
| [26] |
A.C. Sedgwick, L. Wu, H.H. Han, et al., Chem. Soc. Rev. 47 (2018) 8842-8880. DOI:10.1039/c8cs00185e |
| [27] |
Y. Chen, Y. Fang, H. Gu, et al., ACS Appl. Mater. Interfaces 12 (2020) 55094-55106. DOI:10.1021/acsami.0c16585 |
| [28] |
C.H. Wang, Z.Y. Liu, C.H. Huang, et al., J. Am. Chem. Soc. 143 (2021) 12715-12724. DOI:10.1021/jacs.1c05602 |
| [29] |
H. Gu, W. Wang, W. Wu, et al., Chem. Commun. 59 (2023) 2056-2071. DOI:10.1039/d2cc06556h |
| [30] |
K. Wang, L. Wan, J. Wang, et al., Chin. Chem. Lett. 35 (2024) 109554. DOI:10.1016/j.cclet.2024.109554 |
| [31] |
Y. Chen, Y.R. Lee, W. Wang, et al., Angew. Chem. Int. Ed. 62 (2023) e202301765. DOI:10.1002/anie.202301765 |
| [32] |
Q. Huang, Q. Guo, J. Lan, et al., Mater. Horiz. 8 (2021) 1499-1508. DOI:10.1039/d0mh02032j |
| [33] |
X.M. Cai, Y. Lin, Y. Li, et al., Nat. Commun. 12 (2021) 1773-1781. DOI:10.1038/s41467-021-22061-y |
| [34] |
Y. Sun, Y. Jiang, J. Jiang, T. Li, M. Liu, Chin. Chem. Lett. 35 (2024) 108409. DOI:10.1016/j.cclet.2023.108409 |
| [35] |
H. Hu, X. Cheng, Z. Ma, R.P. Sijbesma, Z. Ma, J. Am. Chem. Soc. 144 (2022) 9971-9979. DOI:10.1021/jacs.2c03056 |
| [36] |
K.I. Sakai, S. Tsuchiya, T. Kikuchi, T. Akutagawa, J. Mater. Chem. C 4 (2016) 2011-2016. DOI:10.1039/C5TC04290A |
| [37] |
F. Zhou, P. Gu, Z. Luo, et al., Nat. Commun. 12 (2021) 2339. DOI:10.1038/s41467-021-22685-0 |
| [38] |
Y. Chen, Y. Xie, Z. Li, J. Phys. Chem. Lett. 13 (2022) 1652-1659. DOI:10.1021/acs.jpclett.2c00118 |
| [39] |
B. Tang, B. Liu, H. Liu, H. Zhang, Adv. Funct. Mater. 30 (2020) 2004116. DOI:10.1002/adfm.202004116 |
| [40] |
L. Han, M. Wang, L.O. Prieto-Lopez, X. Deng, J. Cui, Adv. Funct. Mater. 30 (2020) 1907064. DOI:10.1002/adfm.201907064 |
| [41] |
K.W. Millsap, G. Reid, H.C. vanderMei, H.J. Busscher, Biomaterials 18 (1997) 87-91. DOI:10.1016/S0142-9612(96)00105-6 |
| [42] |
L. Zhang, B. Tang, J. Wu, R. Li, P. Wang, Adv. Mater. 27 (2015) 4889-4894. DOI:10.1002/adma.201502362 |
| [43] |
G. Huang, Q. Yang, Q. Xu, S.H. Yu, H.L. Jiang, Angew. Chem. Int. Ed. 55 (2016) 7379-7383. DOI:10.1002/anie.201600497 |
| [44] |
A.M. Abdul-Fattah, R. Oeschger, H. Roehl, et al., Eur. J. Pharm. Biopharm. 85 (2013) 314-326. DOI:10.1016/j.ejpb.2013.06.007 |
| [45] |
X. Zhang, C. Liu, J. Yang, X.J. Huang, Z.K. Xu, J. Membr. Sci. 624 (2021) 118976. DOI:10.1016/j.memsci.2020.118976 |
| [46] |
X. Cheng, Y. Ye, Z. Li, et al., ACS Nano 16 (2022) 4684-4692. DOI:10.1021/acsnano.1c11388 |
| [47] |
Y. Peng, L. Wang, Q. Luo, et al., Chem 4 (2018) 613-625. DOI:10.1016/j.chempr.2018.01.019 |
| [48] |
S. Zhang, F. Ye, X. Wang, et al., Science 380 (2023) 404-409. DOI:10.1126/science.adg3755 |
| [49] |
R. Alcalde, A. Gutierrez, M. Atilhan, S. Aparicio, J. Mol. Liq. 290 (2019) 110916. DOI:10.1016/j.molliq.2019.110916 |
| [50] |
F.O. Farias, J.F.B. Pereira, J.A.P. Coutinho, L. Igarashi-Mafra, M.R. Mafra, Fluid Phase Equilib. 503 (2020) 112319. DOI:10.1016/j.fluid.2019.112319 |
| [51] |
Y. Yin, C. Wu, G. Yu, et al., J. Mater. Chem. A 9 (2021) 7881-7887. DOI:10.1039/d1ta00289a |
| [52] |
Q. Xing, W. Pei, R. Xu, J. Pei, Basic Organic Chemistry (Ⅱ). Beijing: Peking University Press, 2005.
|
| [53] |
S. Kabir, A.M. Sapse, J. Comput. Chem. 12 (1991) 1142-1146. DOI:10.1002/jcc.540120913 |
| [54] |
D. Wang, Q. Sun, M.J. Hokkanen, et al., Nature 582 (2020) 55-59. DOI:10.1038/s41586-020-2331-8 |
| [55] |
J. Zou, J. Wu, Y. Wang, et al., Chem. Soc. Rev. 51 (2022) 2972-2990. DOI:10.1039/d0cs01487g |
 2025, Vol. 36
2025, Vol. 36 

