b Department of Chemistry, Graduate School of Science, Kyoto University, Kyoto 6068501, Japan
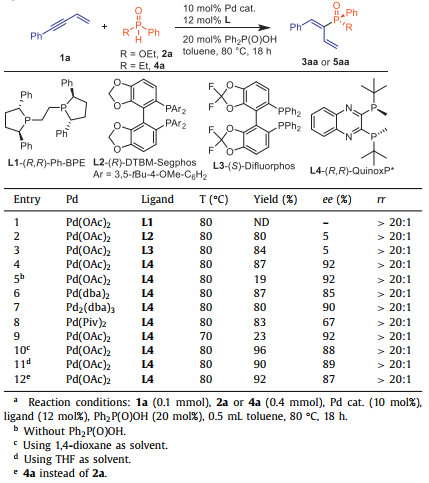
Phosphorous compounds have numerous applications in medicinal chemistry and photoluminescent materials, as well as in asymmetric transformations as ligands or organocatalysts [1-4]. Many chiral ligands with P-chirality have been extensively used in asymmetric catalysis [5-9]. Notable examples include (Rc,Sp)-Duanphos, (S,S,S,S)-BIBOP, (R,R)-QuinoxP* and (R,R)-BenzP* (Scheme 1A). Indeed, P-stereogenic organophosphorus compounds often outperform their chiral backbone substituted counterparts in the terms of coordination efficiency and enantio-control ability. As a result, numerous elegant methods have emerged for the construction of P-stereogenic compounds in recent years. These methods involve asymmetric addition of phosphorous nucleophiles to unsaturated bond [10-17], asymmetric C-P coupling [18-29], asymmetric C−H activation [30-52], asymmetric allylation [53-57], and other novel methods [58-74].
|
Download:
|
| Scheme 1. Asymmetric addition of P-nucleophiles to alkynes. | |
Alkenylphosphine derivatives serve as valuable building blocks in organic synthesis that can be easily transformed to important chiral 1,2-bisphosphine derivatives through processes such as asymmetric Hayashi-Miyaura reaction [75], asymmetric conjugate hydrophosphination [76] or double asymmetric hydrogenation [77,78]. Despite their importance, general and efficient methods for the chiral preparation of alkenylphosphine derivatives are rather limited. In recent years, the catalytic asymmetric addition of phosphorous nucleophiles to alkynes has emerged as a pivotal process for constructing alkenylphosphine derivatives with P-chirality in an efficient manner. However, due to the challenging regioselectivity of phosphorous nucleophiles’ addition to alkynes and the differing reaction properties of phosphorous nucleophiles, catalytic methodology leading to diverse P-stereogenic alkenylphosphine derivatives remains rare, thereby limiting their further application.
In 2002, Tanaka and Han developed a Pd-catalyzed addition of alkynes with (RP)-menthyl-phenylphosphinate to give enantiomerically pure P-chiral alkenylphosphinates while retaining the configuration at phosphorus atom [79]. In 2020, our group achieved asymmetric hydrophosphorylation of alkynes using a Pd/(R,R)-QuinoxP* catalyst for the synthesis of P-stereogenic phosphinates. However, secondary phosphine oxides exhibited poor yield and enantioselectivity under the same conditions [80]. Simultaneously, Zhang utilized their Pd/Xiao-Phos to give anti-Markovnikov addition products through enantioselective hydrophosphinylation of alkynes with phosphine oxides (Scheme 1B, a) [81]. Ni-catalysis has also proven to be a highly efficient catalyst for facilitating P-H addition. In 2021, Zhang successfully applied a Ni-catalyst in the asymmetric hydrophosphination of alkynes using a one-pot two-step process, resulting in the formation of P-stereogenic phosphines [82]. More recently, Zhang also realized anti-Markovnikov hydrophosphination of unactivated alkynes through NiⅡ catalysis with the addition of AgOTf (Scheme 1B, b) [83]. Besides, the same group reported the asymmetric addition of enynes with secondary phosphine oxides to give P-chiral alkenylphosphine oxides, where the alkene moiety served as a directing group by coordinating with the Ni catalyst (Scheme 1B, c) [84]. However, no examples using phosphinate as nucleophiles were provided. Recently, our group developed a Cu/CPA co-catalytic system for delivering axially chiral phosphorus-containing alkenes (Scheme 1B, d) [85]. Additionally, Duan has successfully developed a Ni-catalyzed asymmetric C(sp2)-P coupling of secondary phosphine oxides with alkenyl bromides for generating P-stereogenic alkenylphosphine oxides with high yields and enantioselectivities [86]. The aforementioned examples are more suitable for a specific type of phosphorous nucleophile in a catalytic system, and other phosphorous nucleophiles often yield unsatisfactory results. Therefore, it is particularly desirable to develop a more general approach to realize asymmetric addition of various phosphorous nucleophiles.
Conjugated enynes are unique compounds that possess easily functionalized alkyne and alkene groups, allowing for modular synthesis through a 1,4-functionalization strategy to obtain chiral allenes instead of chiral 1,3-dienes [87-89]. Although Pd-catalyzed enantioselective hydrofunctionalization of conjugated enynes with N-H [90,91] or C-H [92-94] nucleophile, mediated by the in situ formation of catalytically active palladium(Ⅱ)-hydride species, has been successful in delivering chiral allenes with good yields and enantioselectivities, the asymmetric construction of C-P bonds for the synthesis of chiral allenes or 1,3-dienes remains unexplored [95]. In recent years, our group has been dedicated to investigating the asymmetric addition of phosphorus nucleophiles to unsaturated bonds, including heterobicyclic alkenes [96], alkynes [80,85,97], allenes [98], cyclopropanes [99], alkenyl isoquinolines [100] and methylenecyclopropanes [101]. In this study, we present the catalytic enantioselective hydrophosphorylation and hydrophosphinylation of conjugated enynes using phosphinates or secondary phosphine oxides (SPO) in a Pd-catalyzed system (Scheme 1C). This methodology offers a highly efficient and versatile approach for the synthesis of P-chiral alkenylphosphinates and alkenylphosphine oxides with excellent yields and high enantioselectivities.
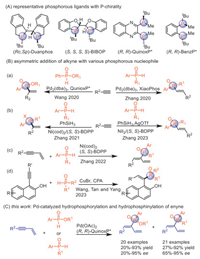
To begin the investigation, enyne 1a and ethyl phenylphosphinate 2a were chosen as the model substrates (Table 1). Based on our previous report [80], Ph2P(O)OH was included as an additive. Pd(OAc)2 was selected as the catalyst for screening chiral ligands (L1-L4, entries 1-4). To our delight, (R,R)-QuinoxP* L4 afforded chiral 1,3-dienes 3aa in 87% yield with 92% ee. Omitting Ph2P(O)OH resulted in a reduced yield of 19% without loss of enantioselectivity (entry 5). Other Pd precursors, such as Pd(dba)2, Pd2(dba)3 and Pd(Piv)2 were tested and Pd(OAc)2 was found to be the optimal choice (entry 4 vs. entries 6-8). Decreasing the temperature led to significantly diminished yields while enantioselectivity remained (entry 4 vs. entry 9). The reactions conducted in 1,4-dioxane, THF, and toluene provided product 3aa with similar high yields, but the reaction in toluene exhibited the highest ee (entry 4 vs. entries 10-11). Surprisingly, chiral 1,3-dienes 5aa were also obtained in 92% yield and 87% ee when using secondary phosphine oxide 4a instead of phosphinate 2a (entry 12), demonstrating the generality of this method.
|
|
Table 1 Optimization of the reaction conditions.a |
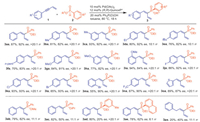
With the optimal conditions, the substrate scopes were then investigated. It was found that a large range of enynes were applicable in this reaction system (Scheme 2). Aryl enynes bearing electron-donating groups at the para-, meta- or ortho-positions (Me, Et, tBu, n-Pent and OMe) (3ba–3ia), as well as those substituted with electron-withdrawing groups (F, Cl, Br and CF3) (3ja–3na), were all well-tolerated and gave satisfactory results (55%-94% yields, 82%-93% ee). Substrate with thiophene moieties also afforded the desired products 3oa with good yield and good ee (89% yield, 87% ee). Encouraged by the success of enynes, we further expanded the scope of this catalytic system to include various H-phosphinates. Substrate with methyl ester were conducted smoothly to yield the desired product 3ab (79% yield, 82% ee). Isopropyl ester resulted in good yield but decreased enantioselectivity (3ac, 82% yield, 50% ee). Phenylphosphinate with a methyl substituent on the arene ring also worked and gave the product 3ad in 80% yield but only with 20% ee. This transformation was also compatible with ethyl naphthalen-1-ylphosphinate, providing the desired product 3ae in moderate yield and enantioselectivity (78% yield, 62% ee). Alky enyne, enzyl enyne 1p, showed relatively poor reactivity, affording 3pa in 20% yield with 40% ee.
|
Download:
|
| Scheme 2. Substrate scope of enyens and H-phosphinates. Reaction conditions: 1 (0.1 mmol), 2 (0.4 mmol), Pd(OAc)2 (10 mol%), (R,R)-Quinoxp* (12 mol%), Ph2P(O)OH (20 mol%), 0.5 mL toluene, 80 ℃, 18 h. | |
Encouraged by the success with H-phosphinates, the investigations were extended to include various secondary phosphine oxides (SPO) for the synthesis of alkenylphosphine oxides (Scheme 3). Employing the same condition, HP(O)EtPh 4a exhibited excellent reactivity with various aryl enynes substituted with electron-donating groups (Me, Et, tBu, n-Pent and OMe) (5ba–5ia) or electron-withdrawing groups (F, Cl and CF3) (5ja–5na) at the para-, meta- or ortho-positions, and gave the desired products with good yields with high ee values (27%-92% yields, 80%-91% ee). Notably, a thiophene-enyne also furnished the desired product in 44% yield and 71% ee (5oa). Second phosphine oxides with n-Bu or i-Pr were successfully transformed to alkenylphosphine oxides (5ab-5ac) without any significant steric hindrance effects. Furthermore, second phosphine oxides bearing various substituents on phenyl ring, such as Me, OMe, F, and Cl (5ad-5ai, 44%-83% yield, 79%-95% ee), were well tolerated with the reaction conditions. Interestingly, the introduction of bulky groups did not impact the reactivity. The use of secondary phosphine oxides with ortho-Me or 1-Nap substituent resulted in good yields and enantioselectivity (5ad and 5ai). Ethyl(thiophen-2-yl)phosphine oxide was also compatible, affording the desired product 5aj in 77% yield and 70% ee. The absolute configuration of 5af was confirmed as S-configuration by X-ray crystallography (CCDC: 2322969).

|
Download:
|
| Scheme 3. Substrate scope of enyens and SPO. Reaction conditions: 1 (0.1 mmol), 4 (0.4 mmol), Pd(OAc)2 (10 mol%), (R,R)-Quinoxp* (12 mol%), Ph2P(O)OH (20 mol%), 0.5 mL toluene, 80 ℃, 18 h. | |
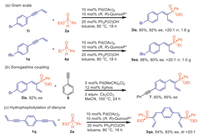
To estimate the synthetic potential of this catalytic system, gram–scale reactions were performed, giving 3la and 5ea in good yields without loss of enantioselectivity (Scheme 4a). Further transformations of the hydrophosphorylation products were also discovered. Product 3la underwent Sonogashira coupling to deliver compound 7 smoothly (Scheme 4b). In addition, the dihydrophosphorylation of dienynes 1q led to the formation of C-symmetric products 3qa in 54% yield and 83% ee (Scheme 4c).
|
Download:
|
| Scheme 4. Gram-scale version of the reaction and synthetic transformation. | |
To gain insights into the potential reaction mechanism, deuterium-labelling experiments were conducted using D2-1a or D-P(O)EtPh (D-4a) as starting materials. Since D-P(O)EtPh readily converted to H-P(O)EtPh at room temperature in the presence of Ph2P(O)OH, deuterium labelling experiment were conducted without the addition of Ph2P(O)OH. Firstly, enyne 1a and D-P(O)EtPh were subjected to the optimized reaction conditions without Ph2P(O)OH at 90 ℃, due to low reactivity of D-P(O)EtPh in standard condition. 80% deuterium incorporation was observed at the 4-position. Surprisingly, 30% deuterium incorporation was also observed at the terminal alkene positions, along with 20% incorporation of deuterium at the 2-position (Scheme 5A, a). These results suggested that the hydrophosphinylation reaction of enyne existed other reversible pathway. These results were different with previous report on hydrofunctionalization of conjugated enynes to give diene [84] or allenes [90-94], suggesting the reaction is not a direct hydrophosphinylation of the alkyne, but involves other reversible pathways. Then, when enyne D2-1a and D-P(O)EtPh were used as the starting material, deuterium incorporation was observed at the 4-positions to a 70% extent, deuterium incorporation at the terminal alkene positions to 70% (reduced from 87%), and 30% incorporation of deuterium at the 2-position (D-5aa-2). These results indicate that the hydropalladation of alkene in the enyne is a reversible process (Scheme 5A, b). Finally, when enyne D2-1a reacted with H-P(O)EtPh, the resulting product D-5aa-3 exhibited 10% incorporation of deuterium at the 2-position and 75% incorporation of deuterium at the terminal alkene positions. This observation confirms that the deuterium incorporation at the 2-position originates from the terminal alkene of D2-1a, further supporting the reversibility of the hydropalladation of the alkene in the enyne (Scheme 5A, c).
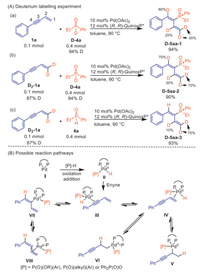
|
Download:
|
| Scheme 5. Deuterium labelling experiment and possible reaction pathways. | |
Based on previous reports about hydrofunctionalization of conjugated enynes [90-94], Pd-catalyzed intermolecular functionalization of conjugated enynes typically leads to the formation of allenes through the intermediate η3-butadienyl−Pd (Ⅷ), which is derived from η1-butadienyl−Pd (Ⅶ). However, the phosphinylallene product through η3-butadienyl−Pd (Ⅷ) with phosphinates or secondary phosphine oxides were not formed in current study, showing the active species in this transformation was intermediate Ⅶ rather than intermediate Ⅷ. Based on our previous work on palladium-catalyzed asymmetric hydrophosphorylation of alkynes [80] and deuterium labeling experiment, we hypothesized that a chiral palladium complex Ⅰ generates from Pd(OAc)2 and (R,R)-QuinoxP*. Then, the oxidative addition of P–H bond of phosphorous nucleophile or the O–H bond of Ph2P(O)OH to the palladium complex triggers the reaction, producing the hydropalladation intermediate Ⅱ. Intermediate Ⅱ coordinates with enyne to give intermediate Ⅲ and Ⅳ, which undergo tautomerism. Intermediate Ⅶ is formed by alkyne insertion into a palladium hydride species (Ⅲ), as confirmed by incorporation of deuterium of D-5aa-1 and D-5aa-2. Additionally, the intermediates Ⅴ and Ⅵ are produced via hydropalladation of alkene. These intermediate then undergo β-H-elimination to give intermediate Ⅳ that is coincided with the deuterium labelling experiment (Scheme 5B).
According to the deuterium labelling experiment and previous reports [80,90,102], we assumed chiral palladium complex Ⅰ undergoes oxidative addition of Ph2P(O)OH to generate the hydropalladation Ⅱ-1. After that, hydropalladation of enyne occurs, leading to the formation of the intermediate Ⅶ-1. Subsequent ligand exchange of intermediate Ⅶ-1 with phosphinates or secondary phosphine oxides gives the internal phosphorylpalladium intermediate Ⅶ-2 (Path A). Since the reaction also proceeds in the absence of Ph2P(O)OH, an alternative pathway is also possible, in which the intermediate Ⅱ-2 is generated directly through the oxidative addition of the P–H bond of phosphorous nucleophile to palladium. This is followed by hydropalladation of enyne, resulting in the same intermediate Ⅶ-2 (Path B). Finally, the reductive elimination of intermediate Ⅶ-2 gives the desired product and regenerates chiral palladium complex Ⅰ (Scheme 6).
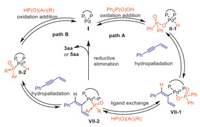
|
Download:
|
| 6. the proposed mechanism of hydrophosphorylation and hydrophosphinylation of conjugated enynes. | |
In summary, a versatile Pd/(R,R)-Quinoxp* system was developed for achieving highly regio- and enantioselective construction of alkenylphosphinates and alkenylphosphine oxides with P-chirality. The mechanistic study has revealed a different pathway for the Pd-catalyzed hydrofunctionalization reaction of enyne, which refreshes the understanding of hydrofunctionalization of conjugated enynes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementYanxin Jiang: Methodology. Kwai Wun Cheng: Methodology. Zhiping Yang: Writing – original draft, Project administration. Jun (Joelle) Wang: Writing – review & editing, Supervision.
AcknowledgmentsWe gratefully thank the National Natural Science Foundation of China (NSFC, No. 22271241), Yunnan Key Laboratory of Chiral Functional Substance Research and Application (No. 202402AN360010), Research Grants Council of Hong Kong (GRF, No. 12303422), and HKBU KRPS grant for financial support. Dr. Xiaoyong Chang from SUSTech is gratefully acknowledged for X-ray crystallographic analysis.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110231.
| [1] |
G.P. Horsman, D.L. Zechel, Chem. Rev. 117 (2017) 5704-5783. DOI:10.1021/acs.chemrev.6b00536 |
| [2] |
H. Ni, W.L. Chan, Y. Lu, Chem. Rev. 118 (2018) 9344-9411. DOI:10.1021/acs.chemrev.8b00261 |
| [3] |
C. Wang, M. Taki, Y. Sato, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 15817-15822. DOI:10.1073/pnas.1905924116 |
| [4] |
C. Fave, T.Y. Cho, M. Hissler, et al., J. Am. Chem. Soc. 125 (2003) 9254-9255. DOI:10.1021/ja035155w |
| [5] |
C.G. Newton, S.G. Wang, C.C. Oliveira, N. Cramer, Chem. Rev. 117 (2017) 8908-8976. DOI:10.1021/acs.chemrev.6b00692 |
| [6] |
J.J. Verendel, O. Pàmies, M. Diéguez, P.G. Andersson, Chem. Rev. 114 (2014) 2130-2169. DOI:10.1021/cr400037u |
| [7] |
G. Xu, C.H. Senanayake, W. Tang, Acc. Chem. Res. 52 (2019) 1101-1112. DOI:10.1021/acs.accounts.9b00029 |
| [8] |
D. Mandal, S. Roychowdhury, J.P. Biswas, S. Maiti, D. Maiti, Chem. Soc. Rev. 51 (2022) 7358-7426. DOI:10.1039/d1cs00923k |
| [9] |
M. Dutartre, J. Bayardon, S. Jugé, Chem. Soc. Rev. 45 (2016) 5771-5794. DOI:10.1039/C6CS00031B |
| [10] |
B. Join, D. Mimeau, O. Delacroix, A.C. Gaumont, Chem. Commun. (2006) 3249-3251. |
| [11] |
Y.B. Li, H. Tian, L. Yin, J. Am. Chem. Soc. 142 (2020) 20098-20106. DOI:10.1021/jacs.0c09654 |
| [12] |
R.H.X. Teo, H.J. Chen, Y. Li, S.A. Pullarkat, P.H. Leung, Adv. Synth. Catal. 362 (2020) 2373-2378. DOI:10.1002/adsc.202000131 |
| [13] |
C. Wang, K. Huang, J. Ye, W.L. Duan, J. Am. Chem. Soc. 143 (2021) 5685-5690. DOI:10.1021/jacs.1c02772 |
| [14] |
Z.H. Wu, A.Q. Cheng, M. Yuan, et al., Angew. Chem. Int. Ed. 60 (2021) 27241-27246. DOI:10.1002/anie.202111137 |
| [15] |
D. Ji, J. Jing, Y. Wang, et al., Chem 8 (2022) 3346-3362. DOI:10.1016/j.chempr.2022.08.019 |
| [16] |
Y. Wang, Z.Q. Wang, L. Yin, Synthesis 55 (2023) 2228-2240. DOI:10.3390/f14112228 |
| [17] |
Y.B. Li, Y. Li, L. Yin, Chin. Chem. Lett. 35 (2024) 109294-109300. DOI:10.1016/j.cclet.2023.109294 |
| [18] |
R. Beaud, R.J. Phipps, M.J. Gaunt, J. Am. Chem. Soc. 138 (2016) 13183-13186. DOI:10.1021/jacs.6b09334 |
| [19] |
Y. Zhang, H. He, Q. Wang, Q. Cai, Tetrahedron Lett. 57 (2016) 5308-5311. DOI:10.1016/j.tetlet.2016.10.048 |
| [20] |
Q. Dai, W. Li, Z. Li, J. Zhang, J. Am. Chem. Soc. 141 (2019) 20556-20564. DOI:10.1021/jacs.9b11938 |
| [21] |
W.Q. Cai, Q. Wei, Q.W. Zhang, Org. Lett. 24 (2022) 1258-1262. DOI:10.1021/acs.orglett.2c00209 |
| [22] |
Y. Li, X. Jin, P. Liu, et al., Angew. Chem. Int. Ed. 61 (2022) e202117093. DOI:10.1002/anie.202117093 |
| [23] |
R. Cui, Y. Wang, L. Yuwen, et al., Org. Lett. 25 (2023) 6139-6142. DOI:10.1021/acs.orglett.3c02216 |
| [24] |
J. Kang, K. Ding, S.M. Ren, B. Su, Angew. Chem. Int. Ed. 62 (2023) e202301628. DOI:10.1002/anie.202301628 |
| [25] |
J. Kang, S. Ren, B. Su, Synlett 35 (2024) 741-746. DOI:10.1055/a-2127-1305 |
| [26] |
B. Liu, P. Liu, X. Wang, et al., Org. Lett. 25 (2023) 2178-2183. DOI:10.1021/acs.orglett.3c00099 |
| [27] |
B. Zhang, W.Q. Zhou, X.T. Liu, Y. Sun, Q.W. Zhang, Chem. Sci. 14 (2023) 1286-1290. DOI:10.1039/d2sc05841c |
| [28] |
Q. Zhang, R.R. Cui, Q.W. Zhang, Synlett 34 (2023) 1819-1823. DOI:10.1055/s-0042-1751456 |
| [29] |
X. Zhang, S.Q. Wang, S.D. Yang, Sci. China Chem. 66 (2023) 2569-2575. DOI:10.1007/s11426-023-1678-1 |
| [30] |
Z.J. Du, J. Guan, G.J. Wu, et al., J. Am. Chem. Soc. 137 (2015) 632-635. DOI:10.1021/ja512029x |
| [31] |
Z.Q. Lin, W.Z. Wang, S.B. Yan, W.L. Duan, Angew. Chem. Int. Ed. 54 (2015) 6265-6269. DOI:10.1002/anie.201500201 |
| [32] |
L. Liu, A.A. Zhang, Y. Wang, et al., Org. Lett. 17 (2015) 2046-2049. DOI:10.1021/acs.orglett.5b00122 |
| [33] |
G. Xu, M. Li, S. Wang, W. Tang, Org. Chem. Front. 2 (2015) 1342-1345. DOI:10.1039/C5QO00142K |
| [34] |
Y.H. Chen, X.L. Qin, J. Guan, Z.J. Du, F.S. Han, Tetrahedron Asymmetry 28 (2017) 522-531. DOI:10.1016/j.tetasy.2017.03.008 |
| [35] |
Y.S. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 56 (2017) 15088-15092. DOI:10.1002/anie.201708440 |
| [36] |
Y. Lin, W.Y. Ma, Q.Y. Sun, Y.M. Cui, L.W. Xu, Synlett 28 (2017) 1432-1436. DOI:10.1055/s-0036-1588983 |
| [37] |
Y. Sun, L. Wozniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 56 (2017) 364-367. DOI:10.1002/anie.201606637 |
| [38] |
Y.S. Jang, Ł. Woźniak, J. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 57 (2018) 12901-12905. DOI:10.1002/anie.201807749 |
| [39] |
Y. Sun, N. Cramer, Chem. Sci. 9 (2018) 2981-2985. DOI:10.1039/c7sc05411d |
| [40] |
Z. Li, Z.Q. Lin, C.G. Yan, W.L. Duan, Organometallics 38 (2019) 3916-3920. DOI:10.1021/acs.organomet.9b00216 |
| [41] |
P. Hu, L. Kong, F. Wang, X. Zhu, X. Li, Angew. Chem. Int. Ed. 60 (2021) 20424-20429. DOI:10.1002/anie.202106871 |
| [42] |
S.Y. Song, Y. Li, Z. Ke, S. Xu, ACS Catal. 11 (2021) 13445-13451. DOI:10.1021/acscatal.1c03888 |
| [43] |
J.H. Chen, M.Y. Teng, F.R. Huang, et al., Angew. Chem. Int. Ed. 61 (2022) e202210106. DOI:10.1002/anie.202210106 |
| [44] |
Q.J. Yao, J.H. Chen, H. Song, F.R. Huang, B.F. Shi, Angew. Chem. Int. Ed. 61 (2022) e202202892. DOI:10.1002/anie.202202892 |
| [45] |
C.W. Zhang, X.Q. Hu, Y.H. Dai, et al., ACS Catal. 12 (2022) 193-199. DOI:10.1021/acscatal.1c05080 |
| [46] |
Y. Lin, T. von Münchow, L. Ackermann, ACS Catal. 13 (2023) 9713-9723. DOI:10.1021/acscatal.3c02072 |
| [47] |
T. von Münchow, S. Dana, Y. Xu, B. Yuan, L. Ackermann, Science 379 (2023) 1036-1042. DOI:10.1126/science.adg2866 |
| [48] |
G. Zhou, J.H. Chen, Q.J. Yao, et al., Angew. Chem. Int. Ed. 62 (2023) e202302964. DOI:10.1002/anie.202302964 |
| [49] |
T. Zhou, L.J. Fan, Z.J. Chen, et al., Org. Lett. 25 (2023) 5724-5729. DOI:10.1021/acs.orglett.3c01865 |
| [50] |
A. Das, R. Mandal, H.S. Ravi Sankar, et al., Angew. Chem. Int. Ed. 63 (2024) e202315005. DOI:10.1002/anie.202315005 |
| [51] |
T. Liu, W. Zhang, C. Xu, et al., Green Chem. 25 (2023) 3606-3614. DOI:10.1039/d3gc00455d |
| [52] |
Z.J. Chen, L.J. Fan, P.P. Xie, et al., Chem. Commun. 60 (2024) 1623-1626. DOI:10.1039/d3cc05052a |
| [53] |
X.T. Liu, Y.Q. Zhang, X.Y. Han, S.P. Sun, Q.W. Zhang, J. Am. Chem. Soc. 141 (2019) 16584-16589. DOI:10.1021/jacs.9b08734 |
| [54] |
Q. Zhang, X.T. Liu, Y. Wu, Q.W. Zhang, Org. Lett. 23 (2021) 8683-8687. DOI:10.1021/acs.orglett.1c02986 |
| [55] |
S. Zhang, J.Z. Xiao, Y.B. Li, C.Y. Shi, L. Yin, J. Am. Chem. Soc. 143 (2021) 9912-9921. DOI:10.1021/jacs.1c04112 |
| [56] |
X.L. Zhang, X. Qi, Y.X. Wu, P. Liu, Y. He, Cell Rep. Phys. Sci. 2 (2021) 100594-100610. DOI:10.1016/j.xcrp.2021.100594 |
| [57] |
Z. Huang, X.T. Liu, R. Cui, Q.W. Zhang, Org. Biomol. Chem. 21 (2023) 3096-3100. DOI:10.1039/d3ob00389b |
| [58] |
G. Nishida, K. Noguchi, M. Hirano, K. Tanaka, Angew. Chem. Int. Ed. 47 (2008) 3410-3413. DOI:10.1002/anie.200800144 |
| [59] |
J.S. Harvey, S.J. Malcolmson, K.S. Dunne, et al., Angew. Chem. Int. Ed. 48 (2009) 762-766. DOI:10.1002/anie.200805066 |
| [60] |
F. de Azambuja, R.C. Carmona, T.H.D. Chorro, G. Heerdt, C.R.D. Correia, Chem. Eur. J. 22 (2016) 11205-11209. DOI:10.1002/chem.201602572 |
| [61] |
K.M.H. Lim, T. Hayashi, J. Am. Chem. Soc. 139 (2017) 8122-8125. DOI:10.1021/jacs.7b04570 |
| [62] |
Z. Wang, T. Hayashi, Angew. Chem. Int. Ed. 57 (2018) 1702-1706. DOI:10.1002/anie.201712572 |
| [63] |
Y. Zheng, L. Guo, W. Zi, Org. Lett. 20 (2018) 7039-7043. DOI:10.1021/acs.orglett.8b02982 |
| [64] |
H. Fernández-Pérez, A. Vidal-Ferran, Org. Lett. 21 (2019) 7019-7023. DOI:10.1021/acs.orglett.9b02606 |
| [65] |
B.M. Trost, S.M. Spohr, A.B. Rolka, C.A. Kalnmals, J. Am. Chem. Soc. 141 (2019) 14098-14103. DOI:10.1021/jacs.9b07340 |
| [66] |
Y. Zhang, F. Zhang, L. Chen, et al., ACS Catal. 9 (2019) 4834-4840. DOI:10.1021/acscatal.9b00860 |
| [67] |
L.B. Balázs, Y. Huang, J.B. Khalikuzzaman, et al., J. Org. Chem. 85 (2020) 14763-14771. DOI:10.1021/acs.joc.0c00181 |
| [68] |
R.Y. Zhu, L. Chen, X.S. Hu, F. Zhou, J. Zhou, Chem. Sci. 11 (2020) 97-106. DOI:10.1039/c9sc04938j |
| [69] |
Q. Dai, L. Liu, J. Zhang, Angew. Chem. Int. Ed. 60 (2021) 27247-27252. DOI:10.1002/anie.202111957 |
| [70] |
Y.B. Li, H. Tian, S. Zhang, J.Z. Xiao, L. Yin, Angew. Chem. Int. Ed. 61 (2022) e202117760. DOI:10.1002/anie.202117760 |
| [71] |
L. Pang, Q. Sun, Z. Huang, et al., Angew. Chem. Int. Ed. 61 (2022) e202211710. DOI:10.1002/anie.202211710 |
| [72] |
L. Pang, Z. Huang, Q. Sun, et al., Nat. Commun. 14 (2023) 4437-4447. DOI:10.1038/s41467-023-40138-8 |
| [73] |
L. Pang, C. Wang, C. Ma, et al., Org. Lett. 25 (2023) 7705-7710. DOI:10.1021/acs.orglett.3c02998 |
| [74] |
S.B. Yan, R. Wang, Z.G. Li, et al., Nat. Commun. 14 (2023) 2264-2272. DOI:10.1038/s41467-023-37987-8 |
| [75] |
Y.Y. Sun, B. Zhang, L. Yu, et al., Chin. Chem. Lett. 33 (2022) 5084-5087. DOI:10.1016/j.cclet.2022.04.054 |
| [76] |
W.J. Yue, J.Z. Xiao, S. Zhang, L. Yin, Angew. Chem. Int. Ed. 59 (2020) 7057-7062. DOI:10.1002/anie.201916076 |
| [77] |
X. Yang, G. Liu, X. Xiang, et al., Org. Lett. 25 (2023) 738-743. DOI:10.1021/acs.orglett.2c04105 |
| [78] |
X. Liu, Z. Han, Z. Wang, K. Ding, Sci. China Chem. 57 (2014) 1073-1078. DOI:10.1007/s11426-014-5134-7 |
| [79] |
L.B. Han, C.Q. Zhao, S.Y. Onozawa, M. Goto, M. Tanaka, J. Am. Chem. Soc. 124 (2002) 3842-3843. DOI:10.1021/ja025816+ |
| [80] |
Z. Yang, X. Gu, L.B. Han, J. Wang, Chem. Sci. 11 (2020) 7451-7455. DOI:10.1039/d0sc01049a |
| [81] |
Q. Dai, L. Liu, Y. Qian, W. Li, J. Zhang, Angew. Chem. Int. Ed. 59 (2020) 20645-20650. DOI:10.1002/anie.202009358 |
| [82] |
X.T. Liu, X.Y. Han, Y. Wu, et al., J. Am. Chem. Soc. 143 (2021) 11309-11316. DOI:10.1021/jacs.1c05649 |
| [83] |
W.H. Wang, Y. Wu, P.J. Qi, Q.W. Zhang, ACS Catal. 13 (2023) 6994-7001. DOI:10.1021/acscatal.3c00539 |
| [84] |
Y.Q. Zhang, X.Y. Han, Y. Wu, et al., Chem. Sci. 13 (2022) 4095-4102. DOI:10.1039/D2SC00091A |
| [85] |
B. Cai, Y. Cui, J. Zhou, et al., Angew. Chem. Int. Ed. 62 (2023) e202215820. DOI:10.1002/anie.202215820 |
| [86] |
C. Wang, X. Hu, C. Xu, et al., Angew. Chem. Int. Ed. 62 (2023) e202300011. DOI:10.1002/anie.202300011 |
| [87] |
O. Pàmies, J. Margalef, S. Cañellas, et al., Chem. Rev. 121 (2021) 4373-4505. DOI:10.1021/acs.chemrev.0c00736 |
| [88] |
L. Fu, S. Greßies, P. Chen, G. Liu, Chin. J. Chem. 38 (2020) 91-100. DOI:10.1002/cjoc.201900277 |
| [89] |
G. Cera, G. Maestri, ChemCatChem 14 (2022) e202200295. DOI:10.1002/cctc.202200295 |
| [90] |
N.J. Adamson, H. Jeddi, S.J. Malcolmson, J. Am. Chem. Soc. 141 (2019) 8574-8583. DOI:10.1021/jacs.9b02637 |
| [91] |
Q. Li, X. Fang, R. Pan, H. Yao, A. Lin, J. Am. Chem. Soc. 144 (2022) 11364-11376. DOI:10.1021/jacs.2c03620 |
| [92] |
S.Q. Yang, Y.F. Wang, W.C. Zhao, G.Q. Lin, Z.T. He, J. Am. Chem. Soc. 143 (2021) 7285-7291. DOI:10.1021/jacs.1c03157 |
| [93] |
H. Tsukamoto, T. Konno, K. Ito, T. Doi, Org. Lett. 21 (2019) 6811-6814. DOI:10.1021/acs.orglett.9b02439 |
| [94] |
L. Li, S. Wang, P. Luo, et al., Nat. Commun. 12 (2021) 5667-5677. DOI:10.1038/s41467-021-25981-x |
| [95] |
S.N. Yang, S.H. Sun, C.H. Liu, et al., Chin. Chem. Lett. 34 (2023) 107914-107918. DOI:10.1016/j.cclet.2022.107914 |
| [96] |
Z. Lu, H. Zhang, Z. Yang, et al., ACS Catal. 9 (2019) 1457-1463. DOI:10.1021/acscatal.8b04787 |
| [97] |
X. Gu, K. Ngai, W. Wang, B. Li, J. Wang, Chem. Catal. 4 (2024) 100903-100916. DOI:10.1016/j.checat.2024.100903 |
| [98] |
Z. Yang, J. Wang, Angew. Chem. Int. Ed. 60 (2021) 27288-27292. DOI:10.1002/anie.202112285 |
| [99] |
Y. Zhang, Y. Jiang, M. Li, Z. Huang, J. Wang, Chem. Catal. 2 (2022) 3163-3173. DOI:10.1016/j.checat.2022.08.008 |
| [100] |
Q. Yang, J. Zhou, J. Wang, Chem. Sci. 14 (2023) 4413-4417. DOI:10.1039/d2sc06950d |
| [101] |
J. Zhou, L. Meng, S. Lin, B. Cai, J. Wang, Angew. Chem. Int. Ed. 62 (2023) e202303727. DOI:10.1002/anie.202303727 |
| [102] |
T. Chen, C.Q. Zhao, L.B. Han, J. Am. Chem. Soc. 140 (2018) 3139-3155. DOI:10.1021/jacs.8b00550 |
 2025, Vol. 36
2025, Vol. 36