b Key Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical Institute of Physics & Chemistry, Chinese Academy of Sciences, Xinjiang Key Laboratory of Electronic Information Materials and Devices, Urumqi 830011, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Since the advent in the last century, lasers have played an irreplaceable role in various frontier and industrial fields, due to their superior performances in brightness, directionality, monochromaticity and coherence [1-4]. Thereinto, all-solid-state lasers with characteristics of a high power density and a long working lifetime are important coherent light sources [5-7]. Thus, as a core component of all-solid-state lasers, nonlinear optical (NLO) crystals, especially short-wave ultraviolet (UV) NLO ones, with excellent properties are urgently needed [8-11]. As a good short-wave UV NLO crystal, it should possess a short absorption cutoff edge less than 280 nm, a stronger NLO response than that of KH2PO4 (KDP), and a moderate birefringence (0.050–0.100) to counteract the phase-mismatch resulting from the refractive index dispersion [12-15]. Sulfates with a wide UV transparency window always exhibit a strong NLO response, which become a promising short-wave UV NLO candidate [16-18]. However, they simultaneously exhibit an insufficient birefringence, which is hardly enough to satisfy the phase-matching condition, owing to the low polarizability anisotropy of the sulfate group [19-21]. Hence, it is necessary to develop strategies to improve sulfate's birefringence.
In recent years, a few related methods have been proposed. By introducing the strong electronegative group of [NH2]− to the [SO4]2− one, the SO2(NH2)2 crystal with a moderate birefringence (0.066@589.3 nm) was explored [22]. The (NH4)2SO3S crystal with [SO3S]2− heteroleptic groups could also exhibit an appropriate birefringence (0.077@546 nm) by replacing the O atom with the S atom in SO4 [23]. After combining the distorted [SbO4F2]7− octahedral group, the CsSbF2SO4 crystal with a large birefringence (0.112@1064 nm) was reported [24]. Because of the integration of the [BO3]3− triangular group [25,26], the (NH4)2B4SO10 crystal exhibits a moderate birefringence (0.053@1064 nm) [27]. In general, these methods could be summarized into two strategies, including the construction of heteroleptic tetrahedral groups to improve the configuration and the introduction of other groups with a large polarizability anisotropy.
Here, both strategies are adopted simultaneously to sharply improve the birefringence (Fig. 1). First, the dimeric heteroleptic tetrahedral group, [S3O6]2−, is designed. The dimerizing mode that uniformizes two heteroleptic groups could provide an innate uniformity, which is conducive to bringing about a large birefringence. Second, the organic triangular group, [C(NH2)3]+, is introduced. Its large polarizability anisotropy has been confirmed by a series of works, making for a large birefringence [28-31]. Thus, [C(NH2)3]2S3O6 (G2S3O6) crystal was designed and investigated.

|
Download:
|
| Fig. 1. Design idea: the construction of the [S3O6]2− group from the [SO4]2− one and the introduction of the [C(NH2)3]+ one (Eg, β and δ correspond to the highest occupied and the lowest unoccupied molecular orbitals gap, the hyperpolarization, and the polarizability anisotropy, respectively). | |
The G2S3O6 crystals were grown by an ion exchange method with raw materials of [C(NH2)3]2CO3 (aladdin, 99%) and Na2S3O6 (synthesized). The Na2S3O6 was obtained by a redox method with Na2S2O3 (Ourchem, 98.5%) and hydrogen peroxide solutions. Single crystal X-ray diffraction data were collected by a Rigaku Mercury single crystal diffractometer. Then, the crystal structure was established and refined by OLEX2 program [32], with detailed crystal data and structure refinement as listed in Table S1 (Supporting information). Powder X-ray diffraction patterns were collected by a Rigaku Mini-flex 600 diffractometer. UV diffuse reflectance spectra were obtained by a PerkinElmer Lamda-950 spectrometer with BaSO4 as the reference. The birefringence was studied on a ZEISS Axio Scope A1 polarizing microscope [33]. NLO responses were measured at different particle sizes by Kurtz-Perry method [34]. Based on the density functional theory, electronic structures and optical properties were calculated by CASTEP package [35]. The exchange-correlation potential was calculated by Perdew-Burke-Ernzerhof functional, based on the generalized gradient approximation [36]. By the norm-conserving pseudopotential, the interaction of the electrons with the ion cores was analyzed [37]. The NLO coefficients and second harmonic generation (SHG)-weighted electron density at zero frequency limit were calculated [38,39].
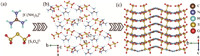
The G2S3O6 crystals crystallize in an asymmetric monoclinic space group of Pc (No. 7). Their purity was confirmed by the X-ray diffraction pattern (Fig. S1 in Supporting information). As shown in Figs. 2a and b, the structure of G2S3O6 crystals is characterized by a wrinkled layer that is constituted by organic triangular groups of [C(NH2)3]+ and arcuate heteroleptic tetrahedral ones of [S3O6]2− with multiple hydrogen bonds. Thereinto, three oxygen atoms bonding with the S2 atom in the [S3O6]2− group additionally link three [C(NH2)3]+ groups by O···H—N hydrogen bonds. Analogously, in the same [S3O6]2− group, two oxygen atoms associating with the S1 atom further connect three [C(NH2)3]+ groups in the adjacent layer. Hence, the powerful covalent bonds of S2-S3 (the S3 atom in [S3O6]2− group is the bridged one) become the interlayer force, which could entirely eliminate the layered habit (Fig. 2c).
|
Download:
|
| Fig. 2. Crystal structures: (a) The functional building blocks, (b) the wrinkled layer; (c) the three-dimensional structure. | |
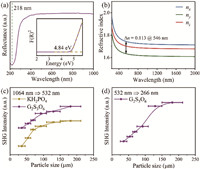
Theoretically, the multi-layered structure of the G2S3O6 crystal could result in an improved optical performance. Hence, the linear and nonlinear optical properties of G2S3O6 crystal were investigated. It exhibits a short absorption cutoff edge around 212 nm (corresponding to a band gap of 4.84 eV), as shown in the UV diffuse reflection spectrum (Fig. 3a), which satisfies the precondition for the 266 nm coherent output. Further, the birefringence was also examined, as shown in Fig. 3b and Fig. S2 (Supporting information). The sample with a thickness of 128.77 µm exhibits an optical path difference of 12.44 µm, indicating a corresponding birefringence of 0.097@546 nm, which is close to the calculated one of 0.113@546 nm. This large birefringence could manifest the strong phase-matching ability. Then, the NLO response was also investigated. As shown in Figs. 3c and d, the I-type phase-matching behaviors were verified under 1064 nm and 532 nm lasers, respectively. Simultaneously, a large NLO response of 1.4 × KDP under a 1064 nm laser was obtained, which was further confirmed by the theoretical results, as listed in Table S2 (Supporting information).
|
Download:
|
| Fig. 3. Optical properties: (a) The diffuse reflectance spectrum; (b) the refractive index dispersion curves; the powder NLO response at different particle sizes (c) under a 1064 nm laser with KDP crystals as a reference and (d) under a 532 nm laser. | |
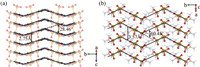
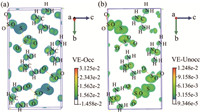
In terms of the excellent optical properties, the structure-property relationship was thoroughly explored. First, the electronic structure and density of states were investigated (Fig. S3 in Supporting information). The G2S3O6 crystal is a direct band gap compound, with the top of the valence band mainly dominated by N-2p, S-3p and O-2p orbitals, and the bottom of the conduction band mainly constituted by O-2p and S-3p orbitals. It indicates the strong interactions of S-S and S-O bonds in the [S3O6]2− groups, which are further confirmed by the electron localization function (Fig. S4 in Supporting information) with a high electron density in the vicinity of S-S and S-O bonds. Besides, the electron density of C—N bonds is also apparent, manifesting the great mutual effect. Thus, the cooperation of [C(NH2)3]+ and [S3O6]2− groups makes for the excellent optical properties. As mentioned before, [C(NH2)3]+ and [S3O6]2− groups with a large polarizability anisotropy are expected to bring about a sufficient birefringence. Here, their arrangements are specially discussed. For [C(NH2)3]+ groups, they mainly make up the wrinkled layers with an intralayer angle of 28.46° and a layer spacing of 2.75 Å (Fig. 4a), for [S3O6]2− groups, their arrangement could also be taken as layers perpendicular to the guanidine ones with an intralayer angle of 60.45° and a layer spacing of 3.57 Å (Fig. 4b). This perfect configuration is in favor of a great optical anisotropy, and then a decent birefringence. At the same time, the collaboration of [C(NH2)3]+ and [S3O6]2− groups also results in the strong NLO response. As shown in Fig. 5, the SHG-weighted electron density of the virtual electron (VE) process, the electrons concentrate on [C(NH2)3]+ and [S3O6]2− groups in both occupied and unoccupied states, indicating their contribution to the NLO response.
|
Download:
|
| Fig. 4. The arrangement of (a) [C(NH2)3]+ and (b) [S3O6]2− groups. | |
|
Download:
|
| Fig. 5. The SHG-weighted electron density of the VE process: (a) The occupied states and (b) the unoccupied states. | |
In order to improve the birefringence of short-wave UV NLO sulfates, a novel semi-organic sulfate, G2S3O6, was obtained by uniting the heteroleptic tetrahedral group and the triangular one. It exhibits a wrinkle-layered structure with excellent optical properties, including a short UV absorption cutoff edge, a strong NLO response, and a remarkable birefringence. These originate from the cooperation of [C(NH2)3]+ and [S3O6]2− groups. Consequently, this work provides an effective approach for enhancing the birefringence of UV NLO sulfates. It will favor the exploitation of new short-wave UV NLO crystals.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe sincerely thank professor Shilie Pan and Zhihua Yang for theoretical calculations. This work was supported by the National Natural Science Foundation of China (Nos. 52172009 and 12275274), the Science and Technology Project of Fujian Province (Nos. 2021J01519 and 2021H0043), and the Instrument Developing Project of Chinese Academy of Sciences (No. YJKYYQ20210033).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109730.
| [1] |
J. Hecht, Opt. Eng. 49 (2010) 091002. DOI:10.1117/1.3483597 |
| [2] |
G. Shi, Y. Wang, F. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 10645-10648. DOI:10.1021/jacs.7b05943 |
| [3] |
B. Zhang, G. Shi, Z. Yang, F. Zhang, S. Pan, Angew. Chem. Int. Ed. 56 (2017) 3916-3919. DOI:10.1002/anie.201700540 |
| [4] |
Z. Zhang, Y. Wang, B. Zhang, et al., Angew. Chem. Int. Ed. 57 (2018) 6577-6581. DOI:10.1002/anie.201803392 |
| [5] |
J.F. Holzrichter, Nature 316 (1985) 309-314. DOI:10.1038/316309a0 |
| [6] |
G. Huber, C. Kränkel, K. Petermann, J. Opt. Soc. Am. B 27 (2010) B93-B105. DOI:10.1364/JOSAB.27.000B93 |
| [7] |
B. Cheng, Z. Li, Y. Chu, et al., Natl. Sci. Rev. 9 (2022) nwac110. DOI:10.1093/nsr/nwac110 |
| [8] |
P.A. Franken, A.E. Hill, C.W. Peters, G. Weinreich, Phys. Rev. Lett. 7 (1961) 118-119. DOI:10.1103/PhysRevLett.7.118 |
| [9] |
V.G. Dmitriev, G.G. Gurzadyan, D.N. Nikogosyan, Handbook of Nonlinear Optical Crystals. Berlin, Heidelberg: Springer, 1999.
|
| [10] |
B.H. Lei, S. Pan, Z. Yang, et al., Phys. Rev. Lett. 125 (2020) 187402. DOI:10.1103/PhysRevLett.125.187402 |
| [11] |
W. Cai, A. Abudurusuli, C. Xie, et al., Adv. Funct. Mater. 32 (2022) 2200231. DOI:10.1002/adfm.202200231 |
| [12] |
P.S. Halasyamani, W. Zhang, Inorg. Chem. 56 (2017) 12077-12085. DOI:10.1021/acs.inorgchem.7b02184 |
| [13] |
L. Kang, F. Liang, Z. Lin, et al., Inorg. Chem. 57 (2018) 15001-15008. DOI:10.1021/acs.inorgchem.8b02795 |
| [14] |
H. Cheng, F. Li, Z. Yang, S. Pan, Angew. Chem. Int. Ed. 61 (2022) e202115669. DOI:10.1002/anie.202115669 |
| [15] |
J. Jiao, M. Zhang, S. Pan, Angew. Chem. Int. Ed. 62 (2023) e202217037. DOI:10.1002/anie.202217037 |
| [16] |
Y. Li, C. Yin, X. Yang, et al., CCS Chem. 3 (2021) 2298-2306. DOI:10.31635/ccschem.020.202000436 |
| [17] |
X. Dong, L. Huang, H. Zeng, et al., Angew. Chem. Int. Ed. 61 (2022) e202116790. DOI:10.1002/anie.202116790 |
| [18] |
Y. Sun, C. Lin, H. Tian, et al., Chem. Mater. 34 (2022) 3781-3788. DOI:10.1021/acs.chemmater.2c00225 |
| [19] |
H. Sha, J. Xu, L. Huang, et al., Scripta Mater. 217 (2022) 114764. DOI:10.1016/j.scriptamat.2022.114764 |
| [20] |
H. Sha, J. Xu, Z. Xiong, et al., Adv. Optical Mater. 10 (2022) 2200228. DOI:10.1002/adom.202200228 |
| [21] |
W. Zhang, Z. Zhang, W. Jin, Sci. China Chem. 64 (2021) 1498-1503. DOI:10.1007/s11426-021-1024-5 |
| [22] |
H. Tian, N. Ye, M. Luo, Angew. Chem. Int. Ed. 61 (2022) e202200395. DOI:10.1002/anie.202200395 |
| [23] |
S. Ke, H. Fan, C. Lin, N. Ye, M. Luo, Inorg. Chem. Front. 10 (2023) 2811-2817. DOI:10.1039/d3qi00172e |
| [24] |
X. Dong, L. Huang, C. Hu, et al., Angew. Chem. Int. Ed. 58 (2019) 6528-6534. DOI:10.1002/anie.201900637 |
| [25] |
A. Tudi, S. Han, Z. Yang, S. Pan, Coordin. Chem. Rev. 459 (2022) 214380. DOI:10.1016/j.ccr.2021.214380 |
| [26] |
F. Zhang, X. Chen, M. Zhang, et al., Light Sci. Appl. 11 (2022) 252. DOI:10.1038/s41377-022-00941-2 |
| [27] |
Z. Li, W. Jin, F. Zhang, et al., Angew. Chem. Int. Ed. 61 (2022) e202112844. DOI:10.1002/anie.202112844 |
| [28] |
C. Wu, X. Jiang, Z. Wang, et al., Angew. Chem. Int. Ed. 60 (2021) 14806-14810. DOI:10.1002/anie.202102992 |
| [29] |
Y. Song, M. Luo, D. Lin, et al., ACS Omega 6 (2021) 9263-9268. DOI:10.1021/acsomega.1c00736 |
| [30] |
M. Luo, C. Lin, D. Lin, N. Ye, Angew. Chem. Int. Ed. 59 (2020) 15978-15981. DOI:10.1002/anie.202006671 |
| [31] |
M. Mutailipu, J. Han, Z. Li, et al., Nat. Photonics 17 (2023) 694-701. DOI:10.1038/s41566-023-01228-7 |
| [32] |
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A. Howard, H. Puschmann, J. Appl. Cryst. 42 (2009) 339-341. DOI:10.1107/S0021889808042726 |
| [33] |
H. Sha, R. Su, Z. Xiong, et al., Adv. Optical Mater. 9 (2021) 2100080. DOI:10.1002/adom.202100080 |
| [34] |
S.K. Kurtz, T.T. Perry, J. Appl. Phys. 39 (1968) 3798-3813. DOI:10.1063/1.1656857 |
| [35] |
M.D. Segall, P.J.D. Lindan, M.J. Probert, et al., J. Phys. Condens. Matter. 14 (2002) 2717-2744. DOI:10.1088/0953-8984/14/11/301 |
| [36] |
M. Ernzerhof, G.E. Scuseria, J. Chem. Phys. 110 (1999) 5029-5036. DOI:10.1063/1.478401 |
| [37] |
A.M. Rappe, K.M. Rabe, E. Kaxiras, J.D. Joannopoulos, Phys. Rev. B 41 (1990) 1227-1230. DOI:10.1103/PhysRevB.41.1227 |
| [38] |
C. Aversa, J.E. Sipe, Phys. Rev. B 52 (1995) 14636-14645. DOI:10.1103/PhysRevB.52.14636 |
| [39] |
B. Zhang, M.H. Lee, Z. Yang, et al., Appl. Phys. Lett. 106 (2015) 031906. DOI:10.1063/1.4906427 |
 2025, Vol. 36
2025, Vol. 36 

