b State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Phenanthrene is a high-value industrial compound, primarily obtained from the crude anthracene fraction of coal tar, which is widely applied in many fields, such as dyes, pesticides, and optoelectronic materials [1-4]. The close boiling points (about 340 ℃) of PHE and its linear isomer ANT make it difficult to separate them. Common methods for separation and purification include solvent extraction, distillation, chemical methods, zone melting, emulsion membrane methods, and chromatography [5-7], most of which consume a lot of energy or cause environmental pollution [8-11]. It is still challenging to achieve the enrichment and separation of ANT and PHE components in an efficient, and environmentally friendly manner.
Artificial cages with large and well-defined internal cavities [12-22], utilizing the confinement effect and preorganization effect of supramolecular cavities, show great promise in diverse applications including encapsulation [23-32], recognition [33-41], supramolecular catalysis [42-54], and drug loading, etc. [55-61] Wherein, water-soluble coordination cages become potential candidates for encapsulating insoluble water guests due to their hydrophobic cavities [62-64], and achieve efficient separation of specific polycyclic aromatic hydrocarbons (PAHs) from mixtures [65-69]. Such supramolecular host-guest strategy has also been used to enhance the solubility difference of PHE and ANT in water and separate them. Li’s group exploited the macrocycle (M-A52+), which selectively recognized and separated 99% PHE from its isomer ANT mixture [70]. Huang and co-workers extracted 91.1% purity of PHE from the isomer mixture using a photo-responsive water-soluble azophenyl macrocycle [71]. Mukherjee group reported 98% and 93% purity of PHE using water-soluble molecular boats (MB1) and molecular bowls (TB), respectively [72,73].
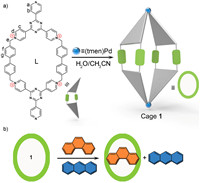
Inspired by enzymes in nature system, which can undergo conformation changes to fit target substrates, chemists are devoted to the design of adaptive functional cages to imitate adaptive guest binding and expand their functionality [74-81]. Design strategies that use macrocycle ligands with conformation dynamics as building blocks provide a new way to synthesize adaptive cages with cavities of lower symmetry [72,82,83]. Previously, we synthesized a series of water-soluble dynamic macrocycle-based ligands with rich conformational freedom, featuring two electron-deficient TPT panels bridged by either p-xylene linkers or m-xylene linkers [84,85]. These ligands were used to construct Pd2L2-type coordination supramolecular cages, which have adaptive cavities and exhibit guest-adaptive conformational changes and induced-fit cavity deformation. Here, we report controlled self-assembly of a new water-soluble Pd2L2 cage 1 from cis-Pd(Ⅱ) and extended macrocycle (L) with two bridging 4,4′-dimethylbiphenyl units. X-ray diffraction studies reveal the conformational changes of the ligand within the host compared to the free ligand. Host-guest studies demonstrate that cage 1 can selective recognize PHE instead of ANT. 1 is able to separate 99% purity of PHE from an equimolar mixture of PHE and isomers ANT by simple extraction. Furthermore, cage 1 can be recycled more than 5 times without loss of performance. This work demonstrates that 1 can serve as an excellent candidate for separation and purification of these isomer mixture with simple, energy-efficient, and cost-effective processes (Scheme 1).
|
Download:
|
| Scheme 1. (a) Self-assembly of Pd2L2 coordination cage 1 and (b) its separation of PHE from a mixture of ANT and PHE. | |
Macrocycle L (PF6− salt) was synthesized in 7.3% yield via a one-step reaction from 2,4,6-tris(4-pyridyl)-1,3,5-triazine (TPT, 1.0 equiv.) and 4,4′-bis(bromomethyl)biphenyl (1.0 equiv.), under N2 atmosphere in DMF at 115 ℃ for 24 h, followed by counter ion exchange with an excess amount of NH4·PF6 (see the Experimental Section in Supporting information for details). L·(NO3)4 or L·(BF4)4 were also obtained by counter ion exchange with [(n-C4H9)4N·NO3] or NaBF4, respectively. Its structure was fully characterized by NMR, ESI-MS and single-crystal X-ray diffraction studies (Figs. S1–S6 in Supporting information), where a trans-conformation of free ligand was revealed in the solid state.
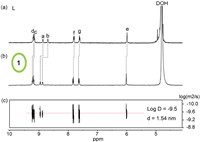
A mixture of L·(NO3)4 (1.62 µmol) and cis-(tmen)Pd(NO3)2 (tmen = tetramethylethylenediamine, 1.62 µmol) in 1 mL D2O was vigorously stirred at 85 ℃ until furnishing a clear light yellow solution. The 1H NMR spectrum showed the quantitative formation of a highly symmetric complex 1 and all the proton signals were fully assigned based on a 1H–1H COSY experiment (Fig. S8 in Supporting information). Compared with free ligand L, the downfield-shifting of the pyridinium doublets (9.13 and 9.12 ppm for Hd, 9.08 and 9.07 ppm for Hc) and the pyridyl doublets (8.86 and 8.85 ppm for Ha, 8.78 and 8.76 ppm for Hb) indicated the successful complexation with palladium. Diffusion-ordered spectroscopy (DOSY) NMR spectrum also confirmed the formation of a single species with a diffusion coefficient of 3.16 × 10−10 m2/s, corresponding to a diameter of 1.54 nm (Fig. 1 and Figs. S7–S9 in Supporting information).
|
Download:
|
| Fig. 1. 1H NMR (400 MHz, D2O, 298 K) spectra of (a) ligand L and (b) Pd2L2 coordination cage 1, (c) 1H DOSY spectrum of 1. | |
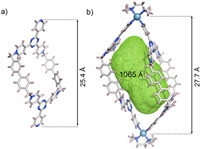
Fortunately, unambiguous evidence for complex 1 is provided by X-ray crystallographic analysis. Single crystals of 1 suitable for X-ray study were grown by slowly tetrahydrofuran vapor diffusion into the aqueous solution of 1 over several weeks. In the crystal structure (Fig. 2b), two cis-form ligands were glued together with two (tmen)Pd capping units, forming a three-dimensional (3D) macrotricycle. The distance between two Pd(Ⅱ) centers is measured to be 27.7 Å. The N(py)-Pd-N(py) bite angle is found to be 86.6°. Compared with the free ligand L (Fig. 2a), cage 1 exhibits conformational change from trans-conformation to the cis-conformation. Pd2L2-type cage 1 possesses the interior hydrophobic cavity of volume ca. 1065 Å3 calculated by MoloVol program (Fig. S10 in Supporting information) [86].
|
Download:
|
| Fig. 2. X-ray structure of (a) ligand L, (b) cage 1. For clarity, solvent molecules and counterions are omitted (Pd centers, cyan sphere; C, gray; N, blue; H, light pink). | |
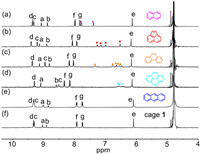
The large inner cavity of 1 is proposed to pull aromatic guests into the aqueous phase driven by the hydrophobic effect, which makes it possible for the separation of various aromatic hydrocarbons. Aiming to address the separation of PHE and ANT, we first explored the host-guest interactions between 1 and PHE or ANT by NMR binding studies. Excess amount of the acetonitrile solution of PHE and ANT were added into cage 1 in D2O and stirred for 1 h. During stirring, the suspension of PHE gradually turned dark yellow solution. 1H NMR spectra evidenced the inclusion of PHE inside the cage (Figs. S17–S19 in Supporting information). Due to poor water-solubility of PHE and ANT, they could hardly be detected by 1H NMR spectroscopy when suspended in D2O. In the presence of cage 1, upfield-shifted guests signals along with noticeable changes in the ligand’s Ha,b signal were clearly observed for PHE (Fig. 3c). DOSY NMR showed all the peaks of host and guest correspond to the same diffusion coefficient, indicating the formation of the host-guest complex (Fig. S19). At the same time, 1H NMR titrations showed gradual shifts of the peaks of 1 in PHE⊂1 upon the addition of increasing equivalents of PHE (Fig. S35 in Supporting information), therefore confirming a fast-exchange dynamic binding mode. The apparent association constant was calculated by Hill function fitting to be 905 L/mol (Fig. S36 in Supporting information). The Job’s plot based on 1H NMR titrations confirmed that the host-guest stoichiometry of 1 and PHE was determined to be 1:2 (Figs. S37 and S38 in Supporting information). However, ANT cannot be trapped in the cavity of Pd2L2 (Fig. S23 in Supporting information), as the 1H NMR spectrum showed no guest signals and no shifts of host. UV–vis spectra of host-guest complexes showed the appearance of a new shoulder band at around 293 nm for PHE⊂1 (Fig. S25 in Supporting information). In contrast, no coloration or new UV–vis absorbance band was observed upon the addition of ANT into cage 1.
|
Download:
|
| Fig. 3. 1H NMR (400 MHz, D2O/CD3CN = 4/1, 298 K) spectra of (a) naphthalene⊂1, (b) acenaphthylene⊂1, (c) phenanthrene⊂1 and (d) pyrene⊂1, (e) cage 1 with addition of 3 equiv. of anthracene and (f) empty cage 1. | |
In addition, cage 1 can encapsulate other three aromatic guests such as naphthalene, acenaphthylene, and pyrene, which were confirmed by 1H NMR, UV–vis, and 2D NMR studies (Figs. 3a, b, and d, and see the Experimental Section in Supporting information for details). In the cases of naphthalene, acenaphthylene, and pyrene, the host−guest stoichiometry was determined by Job’s plot analysis (Table S1 in Supporting information). Apparent binding constants for naphthalene⊂1, acenaphthylene⊂1, and pyrene⊂1 were determined by Hill function fitting to be 752, 794 and 912 L/mol, respectively (Figs. S28, S32 and S40 in Supporting information). In the UV–vis spectra, the appearance of new charge-transfer bands in the case of encapsulated PAHs confirms the CT interaction between the π-electron-rich PAHs and the π-electron-deficient pyridinium-based ligands of the cage (Fig. S24 in Supporting information).
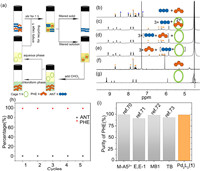
From the host-guest chemistry mentioned above, it is evident that cage 1 selectively encapsulate PHE over its isomer ANT. Multiple attempts to crystallize the host-guest complexes have failed. Molecular mechanical modeling (Fig. S26 in Supporting information) suggests that PHE⊂1 is more geometrically favorable than ANT⊂1 due to the presence of additional CH···π interactions between PHE guests and the macrocycle host compared to the hypothetical ANT⊂1 complex. According to the literature [87], the solubility of PHE (8.1 × 10−3 mmol/L) is about 21 times higher than that of ANT (3.9 × 10−4 mmol/L) in water. It is possible that such a difference in solubility may also contributed to the selective binding of PHE over ANT. These unexpected discoveries position cage 1 as a more favorable water-soluble supramolecular host for separating PHE from isomeric mixtures of PHE and ANT, as illustrate in Fig. 4a. Firstly, we conducted a competitive encapsulation experiment between PHE and ANT within cage 1. Initially, clear PHE⊂1 solution was treated with 3 equiv. of ANT and stirred for 1 h at room temperature. After the removal of the solid, the resulting clear solution exhibited nearly unchanged 1H NMR signals of PHE⊂1 (Figs. 4b and c and Fig. S44 in Supporting information). In reverse, the equivalent amount of PHE was added into the resulting mixture of cage 1 and 3 equiv. of ANT after stirring for 1 h. 1H NMR signals also clearly indicated that the host-guest signals were consistent with PHE⊂1 (Fig. 4e and Fig. S43 in Supporting information). Furthermore, when 3 equiv. of ANT and 3 equiv. of PHE were simultaneously added into the aqueous solution of cage 1, the supernatant obtained after centrifugation exhibited the identical signals to that of PHE⊂1 with no detectable ANT observed in the 1H NMR (Fig. 3d and Fig. S45 in Supporting information). These results clearly indicated that 1 selectively captured PHE from the mixture of PHE and ANT. The trapped PHE can be released by extracting the clear aqueous solution with chloroform, resulting in a high purity of 99% PHE, as detected by the 1H NMR spectrum using trimethoxy benzene as an internal standard. Additionally, the successfully recovered empty 1 in aqueous state was also evidenced by the 1H NMR spectrum. Moreover, the recovered 1 can be reused for subsequent separations of PHE from the mixture. We found that cage 1 can be recycled up to 5 times without loss of its performance in selectivity (99%) (Fig. 4h and Fig. S46 in Supporting information), suggesting that 1 was a stable and robust material for the selective separation of PHE. Compared with previous works on the separation of the isomer mixture [72,73], our cage exhibits equal or higher purity of PHE (Fig. 4i). Furthermore, our cage 1 selectively removed a small amount of PHE (10%) from ANT (90%), resulting in an impressive purity exceeding 99.9% (Figs. S47 and S48 in Supporting information). These findings demonstrate that cage 1 holds promise as an excellent candidate for the separation of the isomer mixture.
|
Download:
|
| Fig. 4. (a) Schematic presentation of separation of PHE from a mixture of ANT and PHE by cage 1. 1H NMR (400 MHz, 298 K) spectra of (b) the equimolar mixture of PHE and ANT in CDCl3, (c) PHE⊂1 followed by the addition of ANT, (d) cage 1 treated with equimolar amount of PHE and ANT, (e) the mixture of ANT and cage 1 followed by the addition of PHE, (f) CDCl3 extract of the aqueous solution formed by treating an equimolar mixture of PHE and ANT with 1. The signals of PHE and ANT are represented by red circle and blue square. The peak for trimethoxybenzene (internal standard) is shown by*, (g) cage 1 recovered after 5 cycles of PHE separation. (h) Percentage of PHE and ANT isolated by cage 1 after five recycles. (i) Purity of PHE from its ANT mixture using different supramolecular hosts. | |
In summary, we have introduced a novel water-soluble coordination cage 1 with a large cavity, built from macrocycle ligand L with biphenyl linkers and cis-Pd corner connections. Host-guest studies exhibited that Pd2L2-type cage 1 could selectively encapsulate PHE while not recognizing ANT. Based on host-guest recognition followed by CHCl3 extraction, cage 1 emerges an excellent candidate for separation, resulting in a remarkable 99% purity of PHE separation from its isomer ANT mixture in aqueous solution. Notably, recovered cage 1 can be reused more than 5 times without losing its performance in selectivity. Even when the initial mixture ratio of PHE and ANT was 1:9, cage 1 is also able to extract PHE with high purity (99%), further suggesting its exceptional selectivity toward PHE. This supramolecular host-guest recognition strategy offers a promising and sustainable separation platform for isolating PHE and ANT isomers.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementYing-Mei Zhong: Writing – original draft, Formal analysis, Data curation. Zi-Jun Xia: Data curation. Yu-Hang Hu: Formal analysis. Li-Peng Zhou: Formal analysis. Li-Xuan Cai: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Formal analysis. Qing-Fu Sun: Writing – review & editing, Supervision, Resources, Investigation.
AcknowledgmentsThis work was supported by the National Key Research and Development Program of China (Nos. 2022YFA1503300 and 2021YFA1500400), the National Natural Science Foundation of China (Nos. 22171262, 21825107 and 22171264), and the Science Foundation of Fujian Province (Nos. 2021J01516 and 2021J02016).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110164.
| [1] |
M. Li, C. An, T. Marszalek, et al., Chem. Mater. 27 (2015) 2218-2223. DOI:10.1021/acs.chemmater.5b00341 |
| [2] |
D.R. Vinayakumara, H. Ulla, S. Kumar, et al., Mater. Chem. Front. 2 (2018) 2297-2306. DOI:10.1039/c8qm00377g |
| [3] |
S. Kataoka, H. Fukumoto, T. Kawasaki-Takasuka, et al., J. Fluorine Chem. 218 (2019) 84-89. |
| [4] |
G. Asaithambi, V. Periasamy, Res. Chem. Intermed. 45 (2018) 1295-1308. |
| [5] |
A. Burel, S.J.T. Brugman, M. Mignot, et al., Chem. Eng. Technol. 39 (2016) 1317-1325. DOI:10.1002/ceat.201600033 |
| [6] |
N. Couvrat, A. Burel, S. Tisse, et al., J. Therm. Anal. Calorim. 112 (2012) 293-300. |
| [7] |
O.N. Pavlovich, Coke Chem. 55 (2012) 247-248. |
| [8] |
Y. Yang, M. Liu, L. Wang, et al., Mar. Pollut. Bull. 62 (2011) 1025-1031. |
| [9] |
M. Patejko, J. Jacyna, M.J. Markuszewski, J. Chromatogr. B 1043 (2017) 150-157. DOI:10.1016/j.jchromb.2016.09.029 |
| [10] |
C.E. Nazario, B.H. Fumes, M.R. da Silva, et al., J. Chromatogr. B 1043 (2017) 81-95. DOI:10.1016/j.jchromb.2016.10.041 |
| [11] |
O. Filippou, D. Bitas, V. Samanidou, J. Chromatogr. B 1043 (2017) 44-62. |
| [12] |
M. Yamashina, Y. Sei, M. Akita, et al., Nat. Commun. 5 (2014) 4662. |
| [13] |
D. Luo, B. Pan, J. Zhang, et al., Chin. Chem. Lett. 32 (2021) 1397-1399. |
| [14] |
Y.L. Lai, H.J. Zhang, J. Su, et al., Chin. Chem. Lett. 34 (2023) 107686. |
| [15] |
M. Morimoto, S.M. Bierschenk, K.T. Xia, et al., Nat. Catal. 3 (2020) 969-984. DOI:10.1038/s41929-020-00528-3 |
| [16] |
L. Jia, X. Tang, Y. Cui, et al., Sci. Chin. Chem. 66 (2023) 2169-2180. DOI:10.1007/s11426-023-1607-4 |
| [17] |
K. Wang, X. Tang, B.A. Anjali, et al., J. Am. Chem. Soc. 146 (2024) 6638-6651. DOI:10.1021/jacs.3c12555 |
| [18] |
M. Pan, K. Wu, J.H. Zhang, et al., Coord. Chem. Rev. 378 (2019) 333-349. |
| [19] |
Q. Chen, Z. Li, Y. Lei, et al., Nat. Commun. 14 (2023) 4627. DOI:10.1038/s41467-023-40255-4 |
| [20] |
Z. Zhang, Y. Yao, L. He, et al., Chin. Chem. Lett. 34 (2023) 107521. DOI:10.1016/j.cclet.2022.05.035 |
| [21] |
C. Li, Y. Wang, Y. Lu, et al., Chin. Chem. Lett. 31 (2020) 1183-1187. |
| [22] |
S.J. Hu, X.Q. Guo, L.P. Zhou, et al., Chin. J. Chem 41 (2023) 797-804. DOI:10.1002/cjoc.202200649 |
| [23] |
D. Samanta, D. Galaktionova, J. Gemen, et al., Nat. Commun. 9 (2018) 641-649. |
| [24] |
L.J. Wang, X. Li, S. Bai, et al., J. Am. Chem. Soc. 142 (2020) 2524-2531. DOI:10.1021/jacs.9b12309 |
| [25] |
A. Sorensen, A.M. Castilla, T.K. Ronson, et al., Angew. Chem. Int. Ed. 52 (2013) 11273-11277. DOI:10.1002/anie.201305245 |
| [26] |
O. Yanshyna, M.J. Bialek, O.V. Chashchikhin, et al., Commun. Chem. 5 (2022) 44. |
| [27] |
J. Wang, C. He, P. Wu, et al., J. Am. Chem. Soc. 133 (2011) 12402-12405. DOI:10.1021/ja2048489 |
| [28] |
D. Chakraborty, S. Ali, P. Choudhury, et al., J. Am. Chem. Soc. 145 (2023) 26973-26982. DOI:10.1021/jacs.3c10191 |
| [29] |
K. Hema, A.B. Grommet, M.J. Bialek, et al., J. Am. Chem. Soc. 145 (2023) 24755-24764. |
| [30] |
L.P. Zhou, X.S. Feng, S.J. Hu, et al., J. Am. Chem. Soc. 145 (2023) 17845-17855. DOI:10.1021/jacs.3c04921 |
| [31] |
Y. Yang, T.K. Ronson, J. Zheng, et al., Chem 9 (2023) 1972-1982. DOI:10.1016/j.chempr.2023.03.027 |
| [32] |
X. Huang, L. Chen, J. Jin, et al., Inorg. Chem. 61 (2022) 20237-20242. DOI:10.1021/acs.inorgchem.2c03701 |
| [33] |
B. Shi, K. Jie, Y. Zhou, et al., J. Am. Chem. Soc. 138 (2016) 80-83. DOI:10.1021/jacs.5b11676 |
| [34] |
S.M. Biros, J. Rebek Jr., Chem. Soc. Rev. 36 (2007) 93-104. DOI:10.1039/B508530F |
| [35] |
Z. Laughrey, B.C. Gibb, Chem. Soc. Rev. 40 (2011) 363-386. DOI:10.1039/C0CS00030B |
| [36] |
M. Whitehead, S. Turega, A. Stephenson, et al., Chem. Sci. 4 (2013) 2744-2751. DOI:10.1039/c3sc50546d |
| [37] |
C. Jose, M. Sarkar, P. Rajasekar, et al., Inorg. Chem. 62 (2023) 19375-19381. DOI:10.1021/acs.inorgchem.3c03105 |
| [38] |
P.J. Gilissen, Q. Duez, G.L. Tripodi, et al., Chem. Commun. 59 (2023) 13974-13977. DOI:10.1039/d3cc04934e |
| [39] |
Y.H. Huang, Y.L. Lu, X.D. Zhang, et al., Angew. Chem. Int. Ed. 63 (2024) e202315053. DOI:10.1002/anie.202315053 |
| [40] |
L. Yang, N. Song, D. Zhang, et al., Inorg. Chem. 62 (2023) 17705-17712. DOI:10.1021/acs.inorgchem.3c02331 |
| [41] |
D. Kim, J. Park, G. Kim, et al., Inorg. Chem. 62 (2023) 10605-10612. DOI:10.1021/acs.inorgchem.3c00823 |
| [42] |
C. Zhao, F.D. Toste, K.N. Raymond, et al., J. Am. Chem. Soc. 136 (2014) 14409-14412. DOI:10.1021/ja508799p |
| [43] |
W. Cullen, H. Takezawa, M. Fujita, Angew. Chem. Int. Ed. 58 (2019) 9171-9173. DOI:10.1002/anie.201904752 |
| [44] |
J. Guo, Y.W. Xu, K. Li, et al., Angew. Chem. Int. Ed. 56 (2017) 3852-3856. DOI:10.1002/anie.201611875 |
| [45] |
Z.J. Wang, C.J. Brown, R.G. Bergman, et al., J. Am. Chem. Soc. 133 (2011) 7358-7360. DOI:10.1021/ja202055v |
| [46] |
C. Tan, J. Jiao, Z. Li, et al., Angew. Chem. Int. Ed. 57 (2018) 2085-2090. DOI:10.1002/anie.201711310 |
| [47] |
K. Iizuka, H. Takezawa, M. Fujita, J. Am. Chem. Soc. 145 (2023) 25971-25975. DOI:10.1021/jacs.3c10720 |
| [48] |
Y. Yang, H. Li, Y. Shi, et al., Angew. Chem. Int. Ed. 63 (2024) e202319605. DOI:10.1002/anie.202319605 |
| [49] |
S.M. Bierschenk, J.Y. Pan, N.S. Settineri, et al., J. Am. Chem. Soc. 144 (2022) 11425-11433. DOI:10.1021/jacs.2c04182 |
| [50] |
Q.N.N. Nguyen, K.T. Xia, Y. Zhang, et al., J. Am. Chem. Soc. 144 (2022) 11413-11424. DOI:10.1021/jacs.2c04179 |
| [51] |
D. Liu, H. Ma, C. Zhu, et al., J. Am. Chem. Soc. 146 (2024) 2275-2285. DOI:10.1021/jacs.3c14254 |
| [52] |
Y.L. Lu, Y.H. Qin, S.-P. Zheng, et al., ACS Catal. 14 (2023) 94-103. |
| [53] |
D.N. Yan, L.X. Cai, P.M. Cheng, et al., J. Am. Chem. Soc. 143 (2021) 16087-16094. DOI:10.1021/jacs.1c06390 |
| [54] |
T.P. Sheng, C. He, Z. Wang, et al., CCS Chem. 4 (2022) 1098-1107. DOI:10.31635/ccschem.021.202100987 |
| [55] |
S.K. Samanta, D. Moncelet, V. Briken, et al., J. Am. Chem. Soc. 138 (2016) 14488-14496. DOI:10.1021/jacs.6b09504 |
| [56] |
D. Zhang, T.K. Ronson, J.R. Nitschke, Acc. Chem. Res. 51 (2018) 2423-2436. DOI:10.1021/acs.accounts.8b00303 |
| [57] |
F. Schmitt, J. Freudenreich, N.P. Barry, et al., J. Am. Chem. Soc. 134 (2012) 754-757. DOI:10.1021/ja207784t |
| [58] |
J.E.M. Lewis, E.L. Gavey, S.A. Cameron, et al., Chem. Sci. 3 (2012) 778-784. DOI:10.1039/C2SC00899H |
| [59] |
X. Li, W. Lin, V. Sharma, et al., Nat. Commun. 14 (2023) 3112. DOI:10.1109/tnet.2023.3270565 |
| [60] |
Y. Wang, R. Tang, D. Wang, et al., Inorg. Chem. 62 (2023) 1786-1790. DOI:10.1021/acs.inorgchem.2c01206 |
| [61] |
S. Fang, M. Wang, Y. Wu, et al., Chem. Sci. 13 (2022) 6254-6261. DOI:10.1039/d2sc01782b |
| [62] |
N. Kishi, Z. Li, K. Yoza, et al., J. Am. Chem. Soc. 133 (2011) 11438-11441. DOI:10.1021/ja2037029 |
| [63] |
P. Mal, D. Schultz, K. Beyeh, et al., Angew. Chem. Int. Ed. 47 (2008) 8297-8301. DOI:10.1002/anie.200803066 |
| [64] |
A. Walther, I. Regeni, J.J. Holstein, et al., J. Am. Chem. Soc. 145 (2023) 25365-25371. DOI:10.1021/jacs.3c09295 |
| [65] |
J.R. Wu, Y.W. Yang, Angew. Chem. Int. Ed. 60 (2021) 1690-1701. DOI:10.1002/anie.202006999 |
| [66] |
D. Zhang, T.K. Ronson, R. Lavendomme, et al., J. Am. Chem. Soc. 141 (2019) 18949-18953. DOI:10.1021/jacs.9b10741 |
| [67] |
L.J. Wang, S. Bai, Y.F. Han, J. Am. Chem. Soc. 144 (2022) 16191-16198. DOI:10.1021/jacs.2c07586 |
| [68] |
Y.L. Lai, J. Su, L.X. Wu, et al., Chin. Chem. Lett. 35 (2024) 108326. DOI:10.1016/j.cclet.2023.108326 |
| [69] |
L. Tong, D. Zhu, B. Chen, et al., Org. Lett. 23 (2021) 9343-9347. DOI:10.1021/acs.orglett.1c03154 |
| [70] |
G. Wu, C.Y. Wang, T. Jiao, et al., J. Am. Chem. Soc. 140 (2018) 5955-5961. DOI:10.1021/jacs.8b01651 |
| [71] |
Y. Liu, H. Wang, L. Shangguan, et al., J. Am. Chem. Soc. 143 (2021) 3081-3085. DOI:10.1021/jacs.1c01204 |
| [72] |
A.B. Sainaba, M. Venkateswarulu, P. Bhandari, et al., J. Am. Chem. Soc. 144 (2022) 7504-7513. DOI:10.1021/jacs.2c02540 |
| [73] |
D. Prajapati, P. Bhandari, N. Hickey, et al., Inorg. Chem. 62 (2023) 9230-9239. DOI:10.1021/acs.inorgchem.3c01156 |
| [74] |
D.D. Boehr, R. Nussinov, P.E. Wright, Nat. Chem. Biol. 5 (2009) 789-796. DOI:10.1038/nchembio.232 |
| [75] |
C.J. Brown, F.D. Toste, R.G. Bergman, et al., Chem. Rev. 115 (2015) 3012-3035. DOI:10.1021/cr4001226 |
| [76] |
S. De, K. Mahata, M. Schmittel, Chem. Soc. Rev. 39 (2010) 1555-1575. DOI:10.1039/b922293f |
| [77] |
J. Gemen, M.J. Bialek, M. Kazes, et al., Chem 8 (2022) 2362-2379. DOI:10.1016/j.chempr.2022.05.008 |
| [78] |
S.L. Huang, Y.J. Lin, T.S. Hor, et al., J. Am. Chem. Soc. 135 (2013) 8125-8128. DOI:10.1021/ja402630g |
| [79] |
H. Li, Z.J. Yao, D. Liu, et al., Coord. Chem. Rev. 293–294 (2015) 139-157. |
| [80] |
T.K. Ronson, J.P. Carpenter, J.R. Nitschke, Chem 8 (2022) 557-568. DOI:10.1016/j.chempr.2021.12.017 |
| [81] |
Y. Tamura, H. Takezawa, M. Fujita, J. Am. Chem. Soc. 142 (2020) 5504-5508. DOI:10.1021/jacs.0c00459 |
| [82] |
J. Mosquera, T.K. Ronson, J.R. Nitschke, J. Am. Chem. Soc. 138 (2016) 1812-1815. DOI:10.1021/jacs.5b12955 |
| [83] |
T. Sawada, H. Hisada, M. Fujita, J. Am. Chem. Soc. 136 (2014) 4449-4451. DOI:10.1021/ja500376x |
| [84] |
Z.J. Xia, Y.M. Zhong, S.J. Hu, et al., Inorg. Chem. 62 (2023) 8293-8299. DOI:10.1021/acs.inorgchem.3c00762 |
| [85] |
D.N. Yan, L.X. Cai, S.J. Hu, et al., Angew. Chem. Int. Ed. 61 (2022) e202209879. DOI:10.1002/anie.202209879 |
| [86] |
J.B. Maglic, R. Lavendomme, J. Appl. Crystallogr. 55 (2022) 1033-1044. DOI:10.1107/s1600576722004988 |
| [87] |
X. Wang, M.L. Brusseau, Environ. Sci. Technol. 29 (1995) 2346-2351. DOI:10.1021/es00009a029 |
 2025, Vol. 36
2025, Vol. 36 

