Photon upconversion [1] based on triplet-triplet annihilation (TTA-UC) is attracting continuous attention due to its widespread applications ranging from photovoltaics [2-5], photocatalysis [6-9] to bioimaging [10,11] and chemosensing through stimuli response [12,13], due to the advantages of TTA-UC over other upconversion methods [14-19]. UC efficiency is a critical parameter for TTA-UC and could often be improved by optimizing the photophysical properties of the sensitizers [20-26], such as enhancing visible light absorption [27-29] and extending the triplet-state lifetimes which are beneficial for the Dexter triplet-triplet energy transfer (TTET) process [30]. Supramolecular pre-assembling the sensitizers and annihilators to position them in close proximity exhibited an alternative strategy which made the enhancement of UC emission no longer strictly dependent on the optimization of excited state properties of the photosensitizer [31-38]. It also provides opportunities to overcome challenges related to intermolecular distances and the relative orientation of sensitisers and annihilators [39,40]. More importantly, the reversible response of supramolecular interactions toward the external stimulus provided possibilities to construct intelligent TTA-UC systems [41,42]. However, the equilibrium characteristics of the supramolecular interactions often led to high background UC signals as the unbounded sensitizers and annihilators could also contribute to the UC emissions [31,43], e.g., we have firstly demonstrated that TTA-UC efficiency could be improved by host−guest complexation between the sensitizer and annihilator, but the intensity was enhanced by no more than 5-fold at diluted solutions [44]. Considering the moderate affinity of most non-covalent interaction, achieving a switch-on effect of the UC emission in the supramolecular UC systems is highly challenging, but is very promising for the practical application of supramolecular TTA-UC system in such as molecular sensing [28,45,46].
We have demonstrated that the TTA-UC efficiency could be significantly improved either by chemically optimizing the excited state properties of the sensitizers [47] or by supramolecular self-assembly [48], and developed several stimulus-responsive TTA-UC systems for molecular sensing and lithographic process [49,50]. We also demonstrated that the well-organized chromophores by supramolecular interactions showed significantly different photochemical and photophysical properties [51,52]. Herein, in order to inhibit the UC emissions from the unbounded UC components in the supramolecular UC system, “mask” attaching photosensitizers were devised in which the triplet states were inactivated when they were free, but when the sensitizer was bounded by host−guest complexation, the mask could be uncovered and therefore the UC emission was switched on.
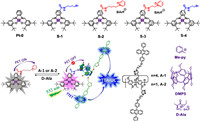
Pt(Ⅱ)−salophen complexes were employed as the sensitizer and pyridine cation was selected as a “mask” here. Subsequently, S-2 and S-3 (Scheme 1) were synthesized, photo-induced electron transfer (PET) was assumed to occur in both S-2 and S-3, and different alkyl chain lengths were devised to investigate the effect of PET efficiencies on the TTA-UC switch-on properties by host−guest complexation. Moreover, the pyridine cation also functioned served as a supramolecular-binding site for the 9,10-diphenylanthracene (DPA) tethered pillararene derivatives (A-1 and A-2, Scheme 1) [53]. S-1 and S-4 was the relevant control compounds for S-2 and S-3.
|
Download:
|
| Scheme 1. The chemical structures of sensitizers, emitters, DMP5, Me-py, and D-Ala, and a proposed mechanism of switching on TTA-UC by host-guest complexation. | |
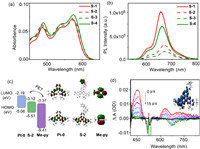
S-1~S-4 showed intense absorption in the visible region at 450~650 nm with peak molar extinction coefficient (ε) of 23,000–30,000 L mol−1 cm−1, assigning to the typical 1MLCT/1IL transitions (Fig. 1a). As anticipated, the phosphorescent intensity of both S-2 and S-3 significantly decreased when compared to that of S-1 and S-4, the phosphorescence quantum yields (ΦP) were determined to be only 13.0% and 9.3% for S-2 and S-3, vs. 28.8% and 24.4% for S-1 and S-4, respectively (Table S2 in Supporting information). DFT calculations showed that the charge distribution of the HOMO orbital for S-2 spread on Pt(Ⅱ)−salophen complex, while for LUMO orbital, it spread on pyridine cation (Fig. 1c, and Table S3 in Supporting information). The Gibbs free energy (ΔG) of the electron transfer from the singlet state of Pt(Ⅱ)-salophen complex Pt-0 to methylpyridine was calculated to be −0.23 V, while from the triplet state, it was −0.05 V (Table S1 and Fig. S49 in Supporting information), thus, we assumed that the PET occurred mainly between the singlet state of Pt(Ⅱ)-salophen complex to the pyridine cation in S-2 and S-3 and the decreased phosphorescence was mainly due to the spontaneous PET process [54]. The ΦP of S-3 was even lower than that of S-2, demonstrating that the PET should be more efficient in S-3 which was reasonable as the distance between the electron donor (Pt(Ⅱ)-salophen complex) and electron acceptor (pyridine cation) was shorter. Interestingly, adding pillar[5]arenes, which possessed an electron-rich cavity, to the solution of S-2 or S-3, the phosphorescence increased (Fig. S44 in Supporting information), indicating that the PET process could be inhibited by the supramolecular binding of pyridine cation into the cavity of pillar[5]arenes.
|
Download:
|
| Fig. 1. UV–vis absorption (a) and phosphorescence (b) spectra of sensitizers (S-1~S-4) in CHCl3, [sensitizer] = 10 µmol/L, 25 ℃. (c) The calculated energy level diagrams and MO diagrams of Pt-0, S-2, and Me-py. (d) Nanosecond time-resolved transient difference absorption spectra of S-2 (λex = 532 nm); inset: spin-density surface of S-2 at the triplet excited state. Calculation at the B3LYP/6–31 G/LANL2DZ level with Gaussian 09 W. | |
Nanosecond time-resolved transient absorption spectroscopy showed a characteristic absorption of Pt(Ⅱ)-salophen-complex localized triplet states (Fig. 1d, Figs. S45 and S46 in Supporting information), spin-density surface also showed that spin density distribution of S-2 and S-3 localized on the salophen ligand and Pt atom (Fig. 1d inset, and Figs. S45 and S46), not on pyridine cation, demonstrating that, despite of the decreased phosphorescence intensity, the inherent attribution of the triplet states was not changed by the introduction of pyridine cation on the salophen ligand.
A widely used annihilator DPA, was tethered on pillar[5]arenes to form supramolecular annihilator A-1 [55]. In order to prove the university of the proposed strategy to switch on the UC emission by host-guest interactions, A-2 with shorter alkyl chain length was also synthesized, the different distance between the two DPA units was expected to influence the encounter possibilities of the two-triplet excitons of DPA. The ε of A-1 and A-2 was about twice of DPA, proportional to the number of DPA units tethering on the pillar[5]arenes, demonstrating that there was no significant interaction between the DPA units at the ground state (Figs. S47 and S48 in Supporting information). More importantly, the fluorescence quantum yields of A-1 and A-2 were 89.49% and 94.18%, respectively (Table S2 in Supporting information), which were comparable with that of DPA [56]. The unreduced fluorescence quantum yield by derivatization will be highly beneficial for applications of these annihilators for TTA-UC.
The binding behavior of the supramolecular annihilators toward the sensitizers was investigated. Job’s plots showed a 1:1 complex stoichiometry for either S-2/A-1 or S-3/A-1 (Figs. S50 and S51 in Supporting information). By gradually adding A-1 to the solutions of S-2 or S-3, the absorption of the sensitizer at 582 nm continuously increased (Figs. S52 and S53 in Supporting information), and the binding constants were determined to be 3.5 × 104 L/mol for S-2/A-1 and 2.8 × 104 L/mol for S-3/A-1, respectively. A relatively moderate binding constant between the sensitizer and annihilator determined a relatively low complexation ratio at a normal concentration condition for TTA-UC, e.g., for [S-2] = 1 × 10−5 mol/L and [A-1] = 2 × 10−5 mol/L, a total amount of only 37.4% of S-2 was present in the form of 1:1 complex, while 62.6% of S-2 and 82.2% of A-1 were in free (Fig. S75 in Supporting information). Despite that, the host-guest complex showed much more efficient TTA-UC due to the accelerated TTET process, UC emission between abundant free sensitizers and annihilators could not be ignored. Thus, usually, the UC emission could only be enhanced to a certain extent, and switching on UC emission was highly difficult in the supramolecular UC system [31,44]. Herein, considering the inactivated excited state of the Pt(Ⅱ)−salophen complex by the pyridine cation, we tried to activate the triplet state of the sensitizer and thus switch on the UC emission by host-guest complexation.
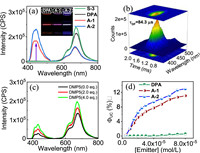
S-2 or S-3 alone showed weak phosphorescence emission at 600–750 nm when irradiated with a 532 nm diode pumped solid state laser (Fig. S54 in Supporting information). With expectation, when DPA was added to the chloroform solution of S-2 or S-3, negligible or no UC emission at 400–500 nm could be observed (Fig. 2a). However, if the sensitizer is changed to S-1 or S-4, much stronger UC emission could be observed (Fig. S55 in Supporting information), demonstrating that the PET effect of S-2 and S-3 significantly decreased the UC emission. Interestingly, when either A-1 or A-2 was used as the acceptor for S-2 and S-3, the emission at 400–500 nm boosted (Fig. 2a and Fig. S56 in Supporting information), irradiation of S-2 or S-3 alone at the same conditions won’t lead to such emission (Fig. S57 in Supporting information), and the lifetime of this emission was determined to be 84.3 µs (Fig. 2b), which was overwhelmingly longer than the prompt emission of A-1 (4.55 ns). These results undoubtedly demonstrated that the boosted blue emission at 400–500 nm originated from the delayed fluorescence of the annihilator sensitized by S-2 or S-3. The UC emission of A-2 was significantly higher than that of A-1 when either S-2 or S-3 was used as the sensitizer (Fig. 2a and Fig. S56a), demonstrating that the boosted UC emission intensities could be further optimized by fine tuning the structures of supramolecular annihilators. The boosted UC emission of A-1 and A-2 was visible to the naked eye (Fig. 2a inset). For the UC components of S-3/DPA, red phosphorescence of the sensitizer was observed, while for S-3/A-1, purple emission was observed due to the mixed emission of UC emission of A-1 and residual phosphorescence of S-3, and for S-3/A-2 system, bright blue emissions appeared due to the much higher UC emission than residual phosphorescence.
|
Download:
|
| Fig. 2. (a) TTA-UC emission of DPA, A-1, and A-2 sensitized by S-3 (10 µmol/L) in deaerated CHCl3, λex = 532 nm (662.4 mW/cm2). Inset: photographs of the emissions of the solution containing S-3 and acceptors DPA (40 µmol/L), A-1 (20 µmol/L) or A-2 (20 µmol/L). (b) Time-resolved emission spectra (TRES) of the upconverted emission of A-1 using S-3 as the sensitizer. (c) UC emission of S-3/DPA (200 µmol/L) system upon the addition of DMP5, λex = 532 nm, [sensitizer]= 10 µmol/L. (d) TTA-UC quantum yields as a function of acceptor concentrations. | |
The phosphorescence quenching of S-2/S-3 by A-1/A-2 was much more efficient than by DPA (Fig. 2a and Figs. S59-S64 in Supporting information), the observed kq values derived from the Stern−Volmer quenching of S-2 by A-1 was 5.8 × 1010 L mol−1 s−1 vs. 0.9 × 109 L mol−1 s−1 (Table S5 in Supporting information) for DPA, demonstrating that the TTET process of the S-2/A-1 system was significantly facilitated by the host-guest complexation. Interestingly, both the UC emission and phosphorescence of the S-2/DPA and S-3/DPA systems were significantly enhanced by adding pillar[5]arene (Fig. 2c and Fig. S58 in Supporting information), demonstrating that, besides the facilitated TTET process by host-guest complexation, the triplet states of sensitizer were also activated, which contributed greatly to the UC enhancement.
The TTA-UC quantum yields (ΦUC) of the supramolecular UC components was almost linearly increasing with acceptors at diluted concentrations (Fig. 2d and Fig. S56b), e.g., for S-3/A-2 system, ΦUC rapidly growing to 6.49% when increasing A-2 from 0 to 20 µmol/L to the solution of S-3 (10 µmol/L), while for S-3/DPA system, the UC emission could be hardly observable until 40 µmol/L DPA was added, still, ΦUC was determined to be only 0.05% at 40 µmol/L DPA (Fig. 2d). The ΦUC of S-3/A-2 system was almost 130-fold higher than that of the S-3/DPA system, showing a significant UC switch-on effect by supramolecular host-guest complexation. The optimized ΦUC of S-3/A-2 system reached to 12.9%, but for S-3/DPA system at the same conditions, ΦUC was only 0.6%. The increasing trend of ΦUC almost completely coincided with the increasing population of the 1:1 complex, contrasting significantly with our previous findings that exhibited a sigmoid-like change in ΦUC [44]. This observation demonstrated that in this UC system, the 1:1 complex of the sensitizer and annihilator mainly contributed to the TTA-UC emission, while the UC emission from the free UC components was negligible. Interestingly, when S-2 was used as the sensitizer, for which the PET efficiency should be lower, the UC emission of DPA as the annihilator was much higher (Fig. S56), therefore the ΦUC of S-2/A-2 system was only increased by 24-fold by host-guest complexation, demonstrating that PET inactivated the excited state of sensitizer played crucial roles in achieving switch-on effect of UC emission.
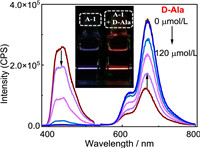
The switch-on effect of UC emission by supramolecular interactions was further validated by the addition of amino acid derivatives as the competitive guests. As exemplified by D-alanine (D-Ala), gradually adding D-Ala to the S-2/A-1 solution, the UC emission significantly decreased with the residual phosphorescence of the sensitizer increased (Fig. 3), while adding 120 µmol/L D-Ala, the UC emission was completely quenched, demonstrating that S-2 was squeezed out of the pillar[5]arene cavities, and the free S-2 and A-1 did not lead to UC emission. The intensities of both the UC and phosphorescence revealed good linear relationships with the D-Ala concentrations, endowing these UC systems as good candidates for ratiometric detecting of amino acid derivatives. The ratio intensity of UC and phosphorescence (IUC/IP) of the S-2/A-1 system as a function of D-Ala concentrations also showed a good linear relationship with a correlation coefficient (R2) of 0.993 in concentrations of 0-65 µmol/L (Fig. S76 in Supporting information). Moreover, the ON—OFF changing of the UC emission by supramolecular binding made the detection of competitive guests visualizable, e.g., under 532 nm irradiation, the bright blue emission obviously turned to deep red upon adding 120 µmol/L D-Ala, showing the unique advantages of these supramolecular UC systems for molecular sensing.
|
Download:
|
| Fig. 3. UC emissions of S-2/A-1 upon adding different concentrations of D-Ala (0.0–120.0 µmol/L) in deaerated CHCl3, [S-2] = 10 µmol/L, [A-1] = 30 µmol/L, λex = 532 nm (662.4 mW/cm2), 25 ℃. Inset: photographs of the emissions of the solution containing S-2 and acceptor A-1 in the absence and presence of D-Ala. | |
In conclusion, pyridine-appending Pt(Ⅱ)−salophen complexes (S-2, S-3) were devised with the purpose of suppressing the UC emission from the unbounded sensitizers and annihilators in the supramolecular UC systems. Pyridine cation served as a ‘mask’ of the excited states of sensitizer, and it quenched the excited state of the Pt(Ⅱ) complex by PET effect and therefore can’t sensitize the UC emission of DPA. However, the ‘mask’ could be uncovered by supramolecular binding of pyridine cation with an electron-rich cavity, and therefore DPA-tethered pillararene derivatives A-1 and A-2 showed boosted UC emission. The UC quantum yield of A-2 was up to 130 times higher than that of DPA when S-3 was the sensitizer, and the ON—OFF switching of UC emission could be achieved by adding competitive guests, showing good application potentials of these UC systems for molecular sensing or construction of intelligent stimulus-response system.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementCheng He: Writing – original draft. Renlan Huang: Investigation. Lingling Wei: Investigation. Qiuhui He: Investigation. Jinbo Liu: Methodology. Jiao Chen: Methodology. Ge Gao: Investigation. Cheng Yang: Writing – review & editing. Wanhua Wu: Writing – review & editing.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 22171194, 21971169, 92056116 and 21871194), the Fundamental Research Funds for the Central Universities (No. 20826041D4117) and the Science & Technology Department of Sichuan Province (Nos. 2022YFH0095 and 2021ZYD0052). Compound characterization was achieved with the support of the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, and Prof. Peng Wu of the Analytical & Testing Center, Sichuan University, which are greatly appreciated.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110103.
| [1] |
J. Zhou, Q. Liu, W. Feng, Y. Sun, F. Li, Chem. Rev. 115 (2015) 395-465. DOI:10.1021/cr400478f |
| [2] |
V. Gray, D. Dzebo, M. Abrahamsson, B. Albinsson, K. Moth-Poulsen, Phys. Chem. Chem. Phys. 16 (2014) 10345-10352. |
| [3] |
B.S. Richards, D. Hudry, D. Busko, A. Turshatov, I.A. Howard, Chem. Rev. 121 (2021) 9165-9195. DOI:10.1021/acs.chemrev.1c00034 |
| [4] |
A.J. Carrod, V. Gray, K. Börjesson, Energy. Environ. Sci. 15 (2022) 4982-5016. DOI:10.1039/d2ee01600a |
| [5] |
C. Gao, W.W.H. Wong, Z. Qin, et al., Adv. Mat. 33 (2021) 2100704. |
| [6] |
R.R. Islangulov, F.N. Castellano, Angew. Chem. Int. Ed. 45 (2006) 5957-5959. DOI:10.1002/anie.200601615 |
| [7] |
B.D. Ravetz, A.B. Pun, E.M. Churchill, et al., Nature 565 (2019) 343-346. DOI:10.1038/s41586-018-0835-2 |
| [8] |
O.S. Wenger, Chem. Eur. J. 17 (2011) 11692-11702. DOI:10.1002/chem.201102011 |
| [9] |
M. Cybularczyk-Cecotka, J. Szczepanik, M. Giedyk, Nat. Catal. 3 (2020) 872-886. DOI:10.1038/s41929-020-00515-8 |
| [10] |
J.H. Kim, J.H. Kim, J. Am. Chem. Soc. 134 (2012) 17478-17481. DOI:10.1021/ja308789u |
| [11] |
J. Park, M. Xu, F. Li, H.C. Zhou, J. Am. Chem. Soc. 140 (2018) 5493-5499. DOI:10.1021/jacs.8b01613 |
| [12] |
S.M. Borisov, C. Larndorfer, I. Klimant, Adv. Funct. Mater. 22 (2012) 4360-4368. DOI:10.1002/adfm.201200794 |
| [13] |
Z. Qiaoyu, L. Guiwen, C. Jinping, et al., Chin. Chem. Lett. 35 (2023) 109009. |
| [14] |
P. Bharmoria, H. Bildirir, K. Moth-Poulsen, Chem. Soc. Rev. 49 (2020) 6529-6554. DOI:10.1039/d0cs00257g |
| [15] |
X. Xiao, W. Tian, M. Imran, H. Cao, J. Zhao, Chem. Soc. Rev. 50 (2021) 9686-9714. DOI:10.1039/d1cs00162k |
| [16] |
L. Huang, T. Le, K. Huang, G. Han, Nat. Commun. 12 (2021) 1898. |
| [17] |
W.P. To, K.T. Chan, G.S.M. Tong, et al., Angew. Chem. Int. Ed. 52 (2013) 6648-6652. DOI:10.1002/anie.201301149 |
| [18] |
J.H. Kang, E. Reichmanis, Angew. Chem. Int. Ed. 51 (2012) 11841-11844. DOI:10.1002/anie.201205540 |
| [19] |
L. Liu, J. Chen, T. Yu, et al., Chin. Chem. Lett. 34 (2023) 107649-107653. |
| [20] |
X. Guo, Y. Liu, Q. Chen, D. Zhao, Y. Ma, Adv. Opt. Mater. 6 (2018) 1700981. DOI:10.1002/adom.201700981 |
| [21] |
J.K. Li, M.Y. Zhang, L. Zeng, L. Huang, X.Y. Wang, Angew. Chem. Int. Ed. 62 (2023) e202303093. |
| [22] |
H. Liang, X. Liu, L. Tang, et al., Chin. Chem. Lett. 34 (2023) 107515. |
| [23] |
X. Wang, F. Ding, T. Jia, et al., Nat. Commun. 15 (2024) 2157. DOI:10.1364/ol.518729 |
| [24] |
X. Zhang, Z. Wang, Y. Hou, et al., J. Mater. Chem. C 9 (2021) 11944-11973. DOI:10.1039/d1tc02535j |
| [25] |
J. Zhao, S. Ji, H. Guo, RSC Adv. 1 (2011) 937-950. |
| [26] |
S. Guo, L.H. Kong, P. Wang, et al., Angew. Chem. Int. Ed. 61 (2022) e202206193. DOI:10.1002/anie.202206193 |
| [27] |
P. Wang, S. Guo, H.J. Wang, et al., Nat. Commun. 10 (2019) 3155. |
| [28] |
H. Zhou, J. Lin, S. Wan, W. Lu, Phys. Chem. Chem. Phys. 24 (2022) 29151-29158. DOI:10.1039/d2cp04532j |
| [29] |
K.K. Chen, C.C. Qin, M.J. Ding, et al., Proc. Natl. Acad. Sci. U. S. A. 119 (2022) e2213479119. |
| [30] |
A. Monguzzi, R. Tubino, F. Meinardi, Phys. Rev. B 77 (2008) 155122. DOI:10.1103/PhysRevB.77.155122 |
| [31] |
H. Chen, I. Roy, M.S. Myong, et al., J. Am. Chem. Soc. 145 (2023) 10061-10070. DOI:10.1021/jacs.2c13846 |
| [32] |
H. Lai, T. Zhao, Y. Deng, et al., Chin. Chem. Lett. 30 (2019) 1979-1983. DOI:10.1016/j.cclet.2019.09.009 |
| [33] |
W. Xu, W. Liang, W. Wu, et al., Chem. Eur. J. 24 (2018) 16677-16685. DOI:10.1002/chem.201804001 |
| [34] |
D. Yang, J. Han, Y. Sang, et al., J. Am. Chem. Soc. 143 (2021) 13259-13265. DOI:10.1021/jacs.1c05927 |
| [35] |
X. Qin, J. Han, D. Yang, et al., Chin. Chem. Lett. 30 (2019) 1923-1926. |
| [36] |
B. Joarder, N. Yanai, N. Kimizuka, J. Phys. Chem. Lett. 9 (2018) 4613-4624. DOI:10.1021/acs.jpclett.8b02172 |
| [37] |
I. Roy, S. Bobbala, R.M. Young, et al., J. Am. Chem. Soc. 141 (2019) 12296-12304. DOI:10.1021/jacs.9b03990 |
| [38] |
T. Ogawa, N. Yanai, A. Monguzzi, N. Kimizuka, Sci. Rep. 5 (2015) 10882. |
| [39] |
V. Gray, K. Moth-Poulsen, B. Albinsson, M. Abrahamsson, Coord. Chem. Rev. 362 (2018) 54-71. DOI:10.1016/j.ccr.2018.02.011 |
| [40] |
Y.C. Simon, C. Weder, J. Mater. Chem. 22 (2012) 20817-20830. DOI:10.1039/c2jm33654e |
| [41] |
X. Cui, J. Zhao, Y. Zhou, J. Ma, Y. Zhao, J. Am. Chem. Soc. 136 (2014) 9256-9259. DOI:10.1021/ja504211y |
| [42] |
D. Yildiz, C. Baumann, A. Mikosch, et al., Angew. Chem. Int. Ed. 58 (2019) 12919-12923. DOI:10.1002/anie.201907436 |
| [43] |
I. Roy, A. Garci, Y. Beldjoudi, et al., J. Am. Chem. Soc. 142 (2020) 16600-16609. DOI:10.1021/jacs.0c05445 |
| [44] |
C. Fan, W. Wu, J.J. Chruma, J. Zhao, C. Yang, J. Am. Chem. Soc. 138 (2016) 15405-15412. DOI:10.1021/jacs.6b07946 |
| [45] |
S. Chen, F. Chen, P. Han, et al., RSC Adv. 9 (2019) 36410-36415. DOI:10.1039/c9ra06524e |
| [46] |
M.P. Jewell, M.D. Greer, A.L. Dailey, K.J. Cash, ACS Sens. 5 (2020) 474-480. DOI:10.1021/acssensors.9b02252 |
| [47] |
C. Fan, L. Wei, T. Niu, et al., J. Am. Chem. Soc. 141 (2019) 15070-15077. DOI:10.1021/jacs.9b05824 |
| [48] |
L. Wei, C. Fan, M. Rao, et al., Mater. Horiz. 9 (2022) 3048-3056. DOI:10.1039/d2mh00887d |
| [49] |
Y. Sun, L. Wei, S. Zhu, et al., Sens. Actuators B: Chem. 387 (2023) 133764. DOI:10.1016/j.snb.2023.133764 |
| [50] |
S. Zhu, L. Wei, Y. Sun, C. Yang, W. Wu, Adv. Opt. Mater. 11 (2023) 2300484. DOI:10.1002/adom.202300484 |
| [51] |
J. Ji, X. Wei, W. Wu, et al., J. Am. Chem. Soc. 144 (2022) 1455-1463. DOI:10.1021/jacs.1c13210 |
| [52] |
F. Gao, X. Yu, L. Liu, et al., Chin. Chem. Lett. 34 (2023) 107558. DOI:10.1016/j.cclet.2022.05.072 |
| [53] |
L. Shao, Y. Pan, B. Hua, et al., Angew. Chem. Int. Ed. 59 (2020) 11779-11783. DOI:10.1002/anie.202000338 |
| [54] |
X. Chen, X. Zhang, X. Xiao, Z. Wang, J. Zhao, Angew. Chem. Int. Ed. 62 (2023) e202216010. DOI:10.1002/anie.202216010 |
| [55] |
G. Li, C. Fan, G. Cheng, W. Wu, C. Yang, Beilstein J. Org. Chem. 15 (2019) 1601-1611. DOI:10.3762/bjoc.15.164 |
| [56] |
J.V. Morris, M.A. Mahaney, J.R. Huber, J. Phys. Chem. 80 (1976) 969-974. DOI:10.1021/j100550a010 |
 2025, Vol. 36
2025, Vol. 36 

