b Department of Chemistry, University of Liverpool, Liverpool L69 7ZD, United Kingdom
Compounds containing chiral 1,2,3,4-tetrahydroquinoxalines have been discovered as core structure in a wide range of bioactive molecules and natural products [1–6]. As a result, the development of various approaches to synthesizing these chiral heterocycles has been ongoing for many years [7]. Homogeneous asymmetric hydrogenation (AH) of quinoxalines is a direct and atom economic method for the preparation of chiral tetrahydroquinoxaline [8–11]. Early in 1987, Murata reported the first rhodium-catalyzed AH of 2-methylquinoxalines, but with only 3% enantioselectivity observed [12]. Later, the ee value increased to 90% with an orthometalated dihydride iridium complex by Bianchini in 1998 [13]. Although some progress was made in the following several years [14–16], the reactions generally suffered from low conversions and/or low ee values. A breakthrough was achieved with an easily accessible Ir-diphosphinite catalyst by Chan in 2009, disclosing up to 98% enantioselectivity and unprecedented high catalytic activity (TOF up to 5620 h−1) [17]. Thereafter, the AH of quinoxaline derivatives with various transition metal catalysts, mainly based on Ir [18–23], Rh [24], Ru [25–28], and Mo [29] complexes, or using frustrated Lewis pairs (FLPs) [30], has been successfully demonstrated, providing good enantioselectivities and yields. However, given the high cost, limited supply and toxicity of late transition metals, and the often-complex synthetic processes and high loading of FLP catalysts, establishing a more sustainable and environmentally benign transition metal catalytic system is highly desirable.
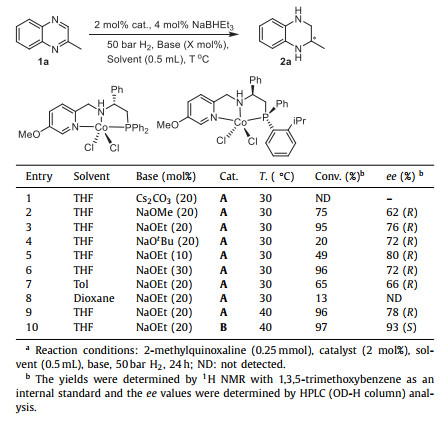
The replacement of precious metals with earth-abundant metals in asymmetric reactions has become an important topic [31–39]. Many catalysts comprised of one of the 3d transition-metals and a suitable chiral ligand have been reported, allowing for the AH of unsaturated substrates with C=C, C=O and C=N bonds [40–43]. However, for the AH of quinoxaline compounds, it remains a challenging task. In 2013, Beller reported an iron-catalyzed AH of quinoxalines, exploiting the concept of metal and chiral BrØnsted acid cooperative catalysis (Scheme 1A, left) [44–46]. Ten years later, Liu developed an efficient manganese-catalyzed AH of quinoxalines, using a chiral tridentate PNN ligand derived from ferrocene and an imidazole group (Scheme 1A, right) [47]. Cobalt, a congener of rhodium and iridium, is a promising transition metal for AH reactions because of its easier availability and reduced toxicity [43,48–65]. However, concerning the hydrogenation of quinoxalines, only one achiral Co catalyst has been reported until now (Scheme 1B, left) [58].
|
Download:
|
| Scheme 1. Catalysts based on 3d transition metals for the hydrogenation of quinoxalines. | |
Recently, we developed a cobalt catalyst bearing a chiral PNN pincer ligand based on an amino phosphine skeleton [54]. Whilst the cobalt catalyst showed low enantioselectivity in catalytic AH of aryl ketones, introducing an achiral monodentate phosphine ligand increased significantly the product enantioselectivity. This could be due to the coordination of the phosphine ligand to the cobalt, creating additional steric hindrance around the metal and thus enhancing the stereo-differentiating ability of the PNN ligand. We therefore thought that by increasing the steric bulkiness and the stereo-differentiating quality of the PNN ligand, we may be able to achieve higher enantioselectivities in AH reactions with a cobalt catalyst having such a PNN ligand. Herein, we present a new cobalt complex derived from a chiral pincer PNN ligand featuring both P and C-stereogenic centers (Scheme 1B, right). With this cobalt complex, the first examples of Co-catalyzed enantioselective hydrogenation of 2-substituent quinoxalines have been achieved, affording up to 99% ee values and full conversions under relatively mild reaction conditions.
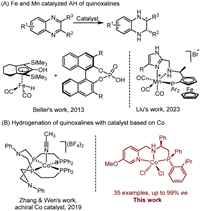
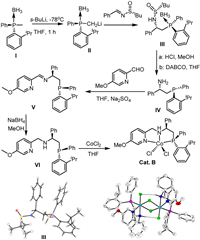
Following our pursuit of cobalt-catalyzed enantioselective hydrogenation reactions, we initially examined the AH of 2-methylquinoxaline (1a) with our previously reported cobalt catalyst Cat. A under conditions similar to those previously described for AH of ketones [54]. The Cat. A was firstly activated with a reducing reagent NaBHEt3, possibly giving a Co(Ⅰ)-H complex from Cat. A [66,67]. Disappointingly, no reaction was observed (Table 1, entry 1). Changing the weak base CsCO3 to a strong one, NaOMe, led to a significant change, with 2a being formed in 75% yield and 62% ee (Table 1, entry 2). Following this lead, a series of parameters were screened (Table 1, entries 1–9; see Supporting information for more details), and we eventually found that when using NaOEt as the base in THF at 50 bar, the AH afforded full conversion of 1a, although the enantioselectivity remained unsatisfactory, at 78% ee (Table 1, entry 9). In order to improve the enantioselectivity, we opted to alter the structure of the PNN ligand. With the thought to make the ligand sterically more demanding and differentiating, a stereogenic phosphorus atom was introduced to replace the original achiral phosphine moiety [68]. To this end, a new chiral PNN ligand Ⅵ bearing a P as well as a C-stereogenic center was synthesized and the corresponding cobalt complex, Cat. B, was prepared by reacting the ligand with CoCl2 in THF. The details of the ligand and complex synthesis are outlined in Scheme 2 (see Supporting information for experiment details) [69–72]. The absolute configurations of compound Ⅲ (CCDC: 2355942) and Cat. B (CCDC: 2355923) have been confirmed by X-ray crystallographic analysis. As with Cat. A (CCDC: 1997410) [54], the complex Cat. B exists as a dimer in the solid state with the two Co(Ⅱ) centers bridged by two chloride ions and each Co(Ⅱ) in a distorted octahedral geometry. The bridge is strengthened by each of the axial chloride hydrogen bonding with the neighboring NH proton. However, the dimer is expected to dissociate into a monomeric form in catalysis.
|
|
Table 1 Optimization of reaction conditions for cobalt-catalyzed hydrogenation of 2-methylquinoxaline.a |
|
Download:
|
| Scheme 2. Synthesis of a new chiral PNN ligand Ⅵ and its complex with Co(Ⅱ), Cat. B. The X-ray structures of compound Ⅲ and the dimeric form of Cat. B are shown. | |
The newly synthesized Cat. B was tested for the hydrogenation of 1a under the optimized reaction conditions for Cat. A. Delightfully, the enantioselectivity increased from 78% to 93% ee with 1a fully converted (Table 1, entries 9 vs. 10). Further optimization of the reaction condition did not lead to better enantioselectivities (see Supporting information for details).
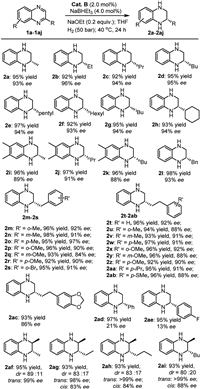
With the optimized reaction conditions in hand, we subsequently turned attention to examining the substrate scope of the AH reaction enabled by Cat. B (Scheme 3). 2-Alky substituted quinoxalines were firstly studied. All the tested 2-alky substituted quinoxalines could undergo smooth hydrogenation under the optimized reaction conditions, giving excellent yields and enantioselectivities (Scheme 3, 1a-1h). The enantioselectivity was relatively insensitive to the length (2a-2f) or bulkiness (2g-2h) of alkyl substituents. With increased steric bulkiness, the 6,7-dimethyl quinoxalines (1i-1k) could still be hydrogenated smoothly, affording excellent yields; however, the enantioselectivities decreased somewhat (2i-2k). For quinoxalines with 2-benzyl-substituted groups (1l-1s), the yields were excellent and the enantioselectivities were high, regardless of whether the phenyl ring contains an electron-donating group (1m-1r) or electron-withdrawing one (1s). The enantioselectivity of the product with a meta-substituted phenyl ring is slightly lower than para- or ortho-substituted ones (2q vs. 2p and 2r). The best enantioselectivity was observed for product 2o with a 2-para-methyl benzyl group (97% ee). Quinoxalines containing 2-substituted phenethyl groups were also investigated under the standard reaction conditions (1t-1ac). The substrates were hydrogenated smoothly, affording excellent yields (92%–97%), with the enantiomeric excesses varied between 86% and 92%. Interestingly, the substrate with a para-SMe group (1ab) underwent the AH smoothly with 2ab obtained in 88% ee, demonstrating the robustness of the Co(Ⅱ) catalyst. For 2-aryl substituted quinoxalines, the enantioselectivities were reduced considerably. Examples are seen in the AH of 1ad-1ae, which afforded 2ad-2ae in 21% and 13% ee, respectively, although the yield was excellent. Clearly, the chiral environment created by the PNN ligand Ⅵ around Co(Ⅱ) cannot effectively recognize the two arene faces when there is a 2-aryl group; the reason remains to be elucidated though. We also explored some 2,3-disubstiuted quinoxalines (1af-1ai) [27,73]. As can be seen from Scheme 3, diastereomeric products were formed, with trans isomers being the major products (2af-2ai). The ratios of trans/cis products ranged from 80:20 to 89:11. It is worth noting that the enantioselectivities of the trans-products are excellent, reaching up to > 99% ee, and are much higher than those of the cis isomers.
|
Download:
|
| Scheme 3. Scope of substrates. Reaction conditions: quinoxaline (0.25 mmol), Cat. B (0.005 mmol), NaBHEt3 (0.01 mmol), NaOEt (0.05 mmol), THF (0.5 mL), 40 ℃, H2 (50 bar), 24 h, isolated yields. The ee values were determined by HPLC analysis. | |
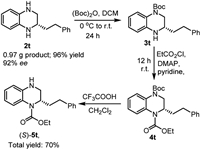
Finally, considering the importance of tetrahydroquinoxaline derivatives as biologically relevant molecules, the hydrogenation of 1t was carried out on a gram scale followed by the conversion to a potentially bioactive compound. Using the optimized reaction conditions, product (S)-2t was isolated in a high yield of 96% with 92% ee (Scheme 4). Following Ohshima's method [19], chemoselective N-Boc protection resulted in compound 3t, which was then treated with ethylchoroformate to give 4t. The deprotection of 4t with trifluoroacetic acid in dichloromethane led to the formation of 5t in 70% overall yield, which is an analogue of an inhibitor of cholesteryl ester transfer protein (CETP) [74].
|
Download:
|
| Scheme 4. A gram scale AH reaction and product derivatization. | |
In conclusion, we have developed the first cobalt-catalyzed AH of quinoxalines, with the asymmetry induced by a chiral PNN ligand featuring both a P and a C-stereogenic center. The introduction of chirality to the phosphine unit of the PNN ligand is critical for achieving high enantioselectivities in the AH of quinoxalines. The cobalt catalyst demonstrated a broad scope of quinoxalines and good to excellent enantioselectivities. The design strategy of combining P and C-stereogenic centers in a pincer ligand could shed a new light on the development of more effective chiral 3d metal catalysts for asymmetric catalysis in general.
Declaration of competing interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementMinghui Zhang: Formal analysis, Data curation. Na Zhang: Formal analysis, Data curation. Qian Zhao: Data curation. Chao Wang: Writing – review & editing. Alexander Steiner: Formal analysis. Jianliang Xiao: Writing – review & editing, Supervision. Weijun Tang: Writing – review & editing, Writing – original draft, Supervision.
AcknowledgmentsWe are grateful for the financial support of the National Natural Science Foundation of China (No. 21672133), the Opening Foundation of Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education (No. GK202205011), and the Fundamental Research Funds for the Central Universities (Nos. GK202307007 and GK202002003). We also thank Prof. Rui Cao for assistance in X-ray crystallographic experiment.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110081.
| [1] |
C.J. Abraham, D.H. Paull, M.T. Scerba, et al., J. Am. Chem. Soc. 128 (2006) 13370-13371. DOI:10.1021/ja065754d |
| [2] |
C.T. Eary, Z.S. Jones, R.D. Groneberg, et al., Bioorg. Med. Chem. Lett. 17 (2007) 2608-2613. DOI:10.1016/j.bmcl.2007.01.112 |
| [3] |
R.P. Law, S.J. Atkinson, P. Bamborough, et al., J. Med. Chem. 61 (2018) 4317-4334. DOI:10.1021/acs.jmedchem.7b01666 |
| [4] |
J.A. Sikorski, J. Med. Chem. 49 (2006) 1-22. DOI:10.1021/jm058224l |
| [5] |
K. Torisu, K. Kobayashi, M. Iwahashi, et al., Bioorg. Med. Chem. 12 (2004) 5361-5378. DOI:10.1016/j.bmc.2004.07.048 |
| [6] |
J.E. Wilson, R. Kurukulasuriya, M. Reibarkh, et al., ACS Med. Chem. Lett. 7 (2016) 261-265. DOI:10.1021/acsmedchemlett.5b00404 |
| [7] |
A.K. Sahoo, A. Bhattacharyya, Curr. Org. Chem. 28 (2024) 161-175. DOI:10.2174/0113852728285439240109071659 |
| [8] |
D.J. Ager, A.H.M. de Vries, J.G. de Vries, Chem. Soc. Rev. 41 (2012) 3340-3380. DOI:10.1039/c2cs15312b |
| [9] |
A.N. Kim, B.M. Stoltz, ACS Catal. 10 (2020) 13834-13851. DOI:10.1021/acscatal.0c03958 |
| [10] |
C.S.G. Seo, R.H. Morris, Organometallics 38 (2019) 47-65. DOI:10.1021/acs.organomet.8b00774 |
| [11] |
D.S. Wang, Q.A. Chen, S.M. Lu, et al., Chem. Rev. 112 (2012) 2557-2590. DOI:10.1021/cr200328h |
| [12] |
S. Murata, T. Sugimoto, S. Matsuura, Heterocycles 26 (1987) 763-766. DOI:10.3987/R-1987-03-0763 |
| [13] |
C. Bianchini, P. Barbaro, G. Scapacci, et al., Organometallics 17 (1998) 3308-3310. DOI:10.1021/om980219a |
| [14] |
H. Brunner, S. Rosenboem, Monatsh. Chem. 131 (2000) 1371-1382. DOI:10.1007/s007060070017 |
| [15] |
J.P. Henschke, M.J. Burk, C.G. Malan, et al., Adv. Synth. Catal. 345 (2003) 300-307. DOI:10.1002/adsc.200390025 |
| [16] |
L. Qiu, F.Y. Kwong, J. Wu, et al., J. Am. Chem. Soc. 128 (2006) 5955-5965. DOI:10.1021/ja0602694 |
| [17] |
W. Tang, L. Xu, Q.H. Fan, et al., Angew. Chem. Int. Ed. 48 (2009) 9135-9138. DOI:10.1002/anie.200904518 |
| [18] |
D. Cartigny, F. Berhal, T. Nagano, et al., J. Org. Chem. 77 (2012) 4544-4556. DOI:10.1021/jo300455y |
| [19] |
D. Cartigny, T. Nagano, T. Ayad, et al., Adv. Synth. Catal. 352 (2010) 1886-1891. DOI:10.1002/adsc.201000513 |
| [20] |
B. Li, G. Zhou, D. Zhang, et al., Org. Lett. 26 (2024) 2097-2102. DOI:10.1021/acs.orglett.4c00409 |
| [21] |
Y.B. Lu, C.F. Chen, Y.H. Hu, et al., ChemistrySelect 7 (2022) e202200912. DOI:10.1002/slct.202200912 |
| [22] |
N. Mrsic, T. Jerphagnon, A.J. Minnaard, et al., Adv. Synth. Catal. 351 (2009) 2549-2552. DOI:10.1002/adsc.200900522 |
| [23] |
S. Sun, P. Nagorny, Chem. Commun. 56 (2020) 8432-8435. DOI:10.1039/d0cc03088k |
| [24] |
A. Xu, C. Li, J. Huang, et al., Chem. Sci. 14 (2023) 9024-9032. DOI:10.1039/d3sc00803g |
| [25] |
N. Arai, Y. Saruwatari, K. Isobe, et al., Adv. Synth. Catal. 355 (2013) 2769-2774. DOI:10.1002/adsc.201300604 |
| [26] |
T. Okuma, N. Arai, K. Matsumura, Patent (2014) JP2014051458. |
| [27] |
J. Qin, F. Chen, Z. Ding, et al., Org. Lett. 13 (2011) 6568-6571. DOI:10.1021/ol2029096 |
| [28] |
S. Urban, N. Ortega, F. Glorius, Angew. Chem. Int. Ed. 50 (2011) 3803-3806. DOI:10.1002/anie.201100008 |
| [29] |
P. Viereck, G. Hierlmeier, P. Tosatti, et al., J. Am. Chem. Soc. 144 (2022) 11203-11214. DOI:10.1021/jacs.2c02007 |
| [30] |
Z. Zhang, H. Du, Angew. Chem. Int. Ed. 54 (2015) 623-626. DOI:10.1002/anie.201409471 |
| [31] |
K. Gopalaiah, Chem. Rev. 113 (2013) 3248-3296. DOI:10.1021/cr300236r |
| [32] |
H. Pellissier, H. Clavier, Chem. Rev. 114 (2014) 2775-2823. DOI:10.1021/cr4004055 |
| [33] |
Y.Y. Li, S.L. Yu, W.Y. Shen, et al., Acc. Chem. Res. 48 (2015) 2587-2598. DOI:10.1021/acs.accounts.5b00043 |
| [34] |
P.J. Chirik, Acc. Chem. Res. 48 (2015) 1687-1695. DOI:10.1021/acs.accounts.5b00134 |
| [35] |
W. Ai, R. Zhong, X. Liu, et al., Chem. Rev. 119 (2019) 2876-2953. DOI:10.1021/acs.chemrev.8b00404 |
| [36] |
W. Sun, Q. Sun, Acc. Chem. Res. 52 (2019) 2370-2381. DOI:10.1021/acs.accounts.9b00285 |
| [37] |
J. Guo, Z. Cheng, J. Chen, et al., Acc. Chem. Res. 54 (2021) 2701-2716. DOI:10.1021/acs.accounts.1c00212 |
| [38] |
L.J. Li, Y. He, Y. Yang, et al., CCS Chem. 6 (2023) 537-584. |
| [39] |
J. Han, Y.M. He, Y. Pan, et al., CCS Chem. 5 (2023) 2088-2100. DOI:10.31635/ccschem.023.202302781 |
| [40] |
J. Wen, F. Wang, X. Zhang, Chem. Soc. Rev. 50 (2021) 3211-3237. DOI:10.1039/d0cs00082e |
| [41] |
H. Wang, J. Wen, X. Zhang, Chem. Rev. 121 (2021) 7530-7567. DOI:10.1021/acs.chemrev.1c00075 |
| [42] |
P. Behera, D.S. Ramakrishna, M.M. Chandrasekhar, et al., Chirality 35 (2023) 477-497. DOI:10.1002/chir.23559 |
| [43] |
S. Chakrabortty, B. de Bruin, J.G. de Vries, Angew. Chem. Int. Ed. 63 (2024) e202315773. DOI:10.1002/anie.202315773 |
| [44] |
S. Fleischer, S. Zhou, S. Werkmeister, et al., Chem. Eur. J. 19 (2013) 4997-5003. DOI:10.1002/chem.201204236 |
| [45] |
C. Li, B. Villa-Marcos, J. Xiao, J. Am. Chem. Soc. 131 (2009) 6967-6969. DOI:10.1021/ja9021683 |
| [46] |
W. Tang, S. Johnston, J.A. Iggo, et al., Angew. Chem. Int. Ed. 52 (2013) 1668-1672. DOI:10.1002/anie.201208774 |
| [47] |
C. Liu, X. Liu, Q. Liu, Chem 9 (2023) 2585-2600. DOI:10.1016/j.chempr.2023.05.006 |
| [48] |
L. Zeng, M. Zhao, B. Lin, et al., Org. Lett. 25 (2023) 6228-6233. DOI:10.1021/acs.orglett.3c02530 |
| [49] |
H. Yang, Y. Hu, Y. Zou, et al., JACS Au 3 (2023) 2981-2986. DOI:10.1021/jacsau.3c00524 |
| [50] |
T. Chen, Y. Zou, Y. Hu, et al., Angew. Chem. Int. Ed. 62 (2023) e202303488. DOI:10.1002/anie.202303488 |
| [51] |
S. Chakrabortty, K. Konieczny, F.J. de Zwart, et al., Angew. Chem. Int. Ed. 62 (2023) e202301329. DOI:10.1002/anie.202301329 |
| [52] |
P. Lu, H. Wang, Y. Mao, et al., J. Am. Chem. Soc. 144 (2022) 17359-17364. DOI:10.1021/jacs.2c08525 |
| [53] |
D. Timelthaler, C. Topf, Synthesis 54 (2021) 629-642. |
| [54] |
T. Du, B. Wang, C. Wang, et al., Chin. Chem. Lett. 32 (2021) 1241-1244. DOI:10.1016/j.cclet.2020.09.011 |
| [55] |
H. Zhong, M. Shevlin, P.J. Chirik, J. Am. Chem. Soc. 142 (2020) 5272-5281. DOI:10.1021/jacs.9b13876 |
| [56] |
K. Murugesan, V.G. Chandrashekhar, C. Kreyenschulte, et al., Angew. Chem. Int. Ed. 59 (2020) 17408-17412. DOI:10.1002/anie.202004674 |
| [57] |
X. Du, Y. Xiao, J.M. Huang, et al., Nat. Commun. 11 (2020) 3239-3248. DOI:10.1038/s41467-020-17057-z |
| [58] |
Y.N. Duan, X. Du, Z. Cui, et al., J. Am. Chem. Soc. 141 (2019) 20424-20433. DOI:10.1021/jacs.9b11070 |
| [59] |
I. Sorribes, L. Liu, A. Domenech-Carbo, et al., ACS Catal. 8 (2018) 4545-4557. DOI:10.1021/acscatal.7b04260 |
| [60] |
M.R. Friedfeld, H. Zhong, R.T. Ruck, et al., Science 360 (2018) 888-893. DOI:10.1126/science.aar6117 |
| [61] |
M.R. Friedfeld, M. Shevlin, G.W. Margulieux, et al., J. Am. Chem. Soc. 138 (2016) 3314-3324. DOI:10.1021/jacs.5b10148 |
| [62] |
J. Chen, C. Chen, C. Ji, et al., Org. Lett. 18 (2016) 1594-1597. DOI:10.1021/acs.orglett.6b00453 |
| [63] |
F. Chen, A.E. Surkus, L. He, et al., J. Am. Chem. Soc. 137 (2015) 11718-11724. DOI:10.1021/jacs.5b06496 |
| [64] |
M.R. Friedfeld, M. Shevlin, J.M. Hoyt, et al., Science 342 (2013) 1076-1080. DOI:10.1126/science.1243550 |
| [65] |
S. Monfette, Z.R. Turner, S.P. Semproni, et al., J. Am. Chem. Soc. 134 (2012) 4561-4564. DOI:10.1021/ja300503k |
| [66] |
L. Zhang, Z. Huang, J. Am. Chem. Soc. 137 (2015) 15600-15603. DOI:10.1021/jacs.5b11366 |
| [67] |
J. Guo, B. Cheng, X. Shen, et al., J. Am. Chem. Soc. 139 (2017) 15316-15319. DOI:10.1021/jacs.7b09832 |
| [68] |
J. Wang, X. Shen, X. Chen, et al., J. Am. Chem. Soc. 145 (2023) 24958-24964. |
| [69] |
Q. Dai, W. Li, Z. Li, et al., J. Am. Chem. Soc. 141 (2019) 20556-20564. DOI:10.1021/jacs.9b11938 |
| [70] |
T. Imamoto, H. Tsuruta, Chem. Lett. 25 (2006) 707-708. |
| [71] |
Y. Wada, T. Imamoto, H. Tsuruta, et al., Adv. Synth. Catal. 346 (2004) 777-788. DOI:10.1002/adsc.200404043 |
| [72] |
T. Imamoto, T. Oshiki, T. Onozawa, et al., J. Am. Chem. Soc. 112 (1990) 5244-5252. DOI:10.1021/ja00169a036 |
| [73] |
S. Li, W. Meng, H. Du, Org. Lett. 19 (2017) 2604-2606. DOI:10.1021/acs.orglett.7b00935 |
| [74] |
G. Chang, M.T. Didiuk, J.I. Finneman, et al., Patent (2004) WO2004085401. |
 2025, Vol. 36
2025, Vol. 36