b College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, China
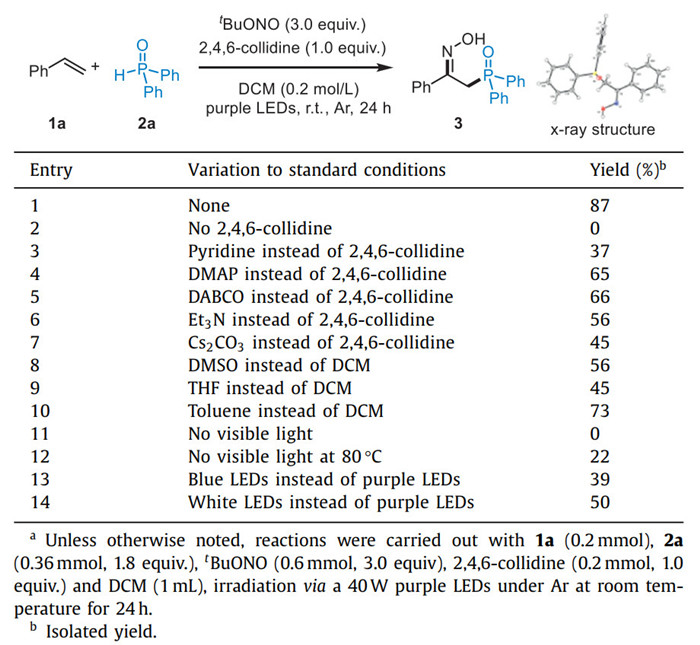
Over the past decades, radical-mediated difunctionalization of alkenes has emerged as a robust platform for constructing complex molecules from simple materials [1-11]. In this field, alkene functional-oximation represents a highly valued synthetic transformation, due to the importance of oxime scaffold in synthetic chemistry and pharmaceutical chemistry [12-20]. Remarkably, the early common strategy for this reaction was the radical coupling of alkenes with nitric oxide (NO) gas, which underwent the radical addition to C═C bond and the coupling with NO to provide the corresponding nitrosoalkane, followed by tautomerization to lead the final oxime product (Scheme 1a) [21-26]. However, the high toxicity, instability, and low solubility in common solvents of NO gas severely limit its application in organic synthesis [27,28]. A series of organic [NO] reagents, including N-nitrosamines/amides [29-33], nitroso alkanes [34-37], and nitrite esters [38-41], have been used as NO sources instead of NO gas for the synthesis of oximes via in situ generation of the persistent nitric oxide radical (Scheme 1a). In this context, the inexpensive and easily accessible tert-butyl nitrite (TBN) as a bifunctional reagent [42,43] triggered radical alkene difunctionalization has been exploited as a straightforward and efficient strategy for the rapid assembly of a wide range of structurally diverse oxime-containing compounds [44-54]. The reactive tert-butoxyl radical and NO radical, which showed the dual roles as the hydrogen atom transfer (HAT) reagent and the source of oximes, respectively, were generated by homolytic O—NO bond cleavage of TBN under high-energy ultraviolet light (UV) irradiation or elevated temperatures (Scheme 1b) [47-54]. Recently, Studer and co-workers reported a mild visible-light-driven radical silyloximation of electron-deficient alkenes with TBN and silanes, in which a light-promoted generated tert-butoxyl radical abstracts a hydrogen atom from silane to form silyl radical (Scheme 1c) [55]. Very recently, the Li group disclosed a visible-light-mediated radical sulfamoyloximation of alkenes using TBN as a bifunctional reagent [56]. Therefore, the development of novel visible-light-induced radical functional-oximation of alkenes with TBN as a bifunctional reagent, to efficiently access functionalized oxime compounds, remains highly desirable.
|
Download:
|
| Scheme 1. Radical functional-oximation of alkenes. | |
On the other hand, organophosphorus compounds have found widespread applications in various fields, including catalysis, organic synthesis, medicinal chemistry, and materials science [57-63]. Consequently, the synthesis of organophosphorus molecules has attracted significant attention and notable progress has been made [64-70]. In this context, P-centered radical-triggered difunctionalization of alkenes via transition metal-catalysis, photocatalysis and electrochemistry, emerges as an ideal strategy [71-91]. Given the significance of oxime compounds and phosphorus functionalities, we hypothesized that radical phosphinoyloximation of alkenes with secondaryl phosphine oxides and TBN would be an efficient route to α-phosphinoyl oximes [92-95]. It should be mentioned that Zou and co-workers elegantly realized this transformation with a noble silver catalyst [96]. Herein, we report a visible-light-induced radical phosphinoyloximation of alkenes with secondaryl phosphine oxides and TBN under photocatalyst- and metal-free conditions (Scheme 1d). This reaction shows broad substrate scope and good functional group compatibility, yielding various α-phosphinoyl oximes in moderate to good yields with high stereoselectivities.
We commenced the investigations using styrene (1a) and diphenylphosphine oxide (2a), in the presence of tert-butyl nitrite (TBN), as model substrates (Table 1). Encouragingly, after extensive screening of the reaction conditions, we succeeded in the desired phosphinoyloximation reaction using 2,4,6-collidine (1.0 equiv.) as base in DCM under irradiation of 40 W purple LEDs (Figs. S1 and S2 in Supporting information) at room temperature, affording the desired phosphinoyl oxime product 3 in 87% isolated yield with exclusive stereoselectivity (entry 1). The precise structure of 3 was unambiguously confirmed by X-ray crystallographic analysis (CCDC: 2354465), and the Z-stereoselectivity of oxime may be due to the intermolecular hydrogen-bonds between oxime and phosphinoyl functionalities. Notably, base was essential for this reaction as no desired product was detected in the absence of 2,4,6-collidine (entry 2). We proposed that the base may have a facilitative effect for the tautomerization of nitrosoalkane to oxime product. Various organic and inorganic bases were screened, but no improvement of the results was observed (entries 3–7). Then, the use of other solvents instead of DCM, such as DMSO, THF and toluene, resulted in diminished yields (entries 8–10). Control experiments confirmed that no desired product was obtained in the absence of visible light, and only 22% yield was afforded even upon increasing the temperature to 80 ℃ (entries 11 and 12). Other light sources, such as blue LEDs and white LEDs, gave lower reaction efficiencies relative to purple LEDs (entries 13 and 14).
|
|
Table 1 Optimization of the reaction conditions.a |
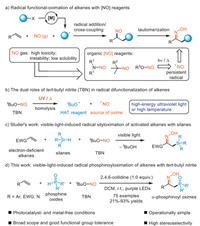
With the optimized conditions in hand, the alkene scope of this visible-light-induced phosphinoyloximation reaction was investigated (Scheme 2). A wide variety of styrene derivatives bearing both an electron-donating and electron-withdrawing group or two substituents at the different positions of benzene ring smoothly participated in this reaction to give the desired products 3–26 in moderate to good yields with high stereoselectivities. 1- and 2-vinylnaphthalene were also suitable for this reaction to produce the corresponding products 27 and 28 in 31% and 63% yields, respectively. Note that the introduction of a substituent at the ortho position of phenyl moiety had an obvious harmful influence on the reaction efficiencies (23, 24, 27), probably as a result of high steric hindrance. The alkenes bearing heterocycles such as pyridine, thiophene, benzofuran, and benzothiophene were found to be compatible with the present reaction conditions to provide the products 29–33 in 40%–93% yields. In addition to terminal alkenes, acyclic and cyclic internal alkenes were amenable to this protocol, albeit with low reaction efficiencies (34, 35), which might be due to the relatively slower addition rate of the phosphinoyl radical to double bond. A twofold phosphinoyloximation reaction of p-divinylbenzene was achieved to afford the product 36 in 55% yield. Notably, N-vinyl-2-pyrrolidone, -phthalimide and -carbazole proved to be suitable substrates in this reaction to generate the aminooxime products 37–39 in moderate yields. Moreover, a variety of electron-deficient alkenes, including acrylates, acrylamide, vinyl ketone, acrylonitrile, and vinyl sulfone, underwent this transformation smoothly to afford the corresponding products 40–45 in good yields, while a low stereoselectivity of 1.3:1 was observed for the reaction of acrylonitrile. Interestingly, the visible-light-induced phosphinoyloximation reaction of the allyl acrylate substrate, which contains two different types of carbon−carbon double bonds, preferentially occurred at the activated electron-deficient alkene rather than the unactivated one to give the product 41. When the unactivated aliphatic alkenes, such as 1-heptane and 4-phenyl-1-butane, were utilized in this reaction, no desired products were observed at the current stage. To demonstrate the potential applicability of this protocol, we examined more complex substrates for the phosphinoyloximation reaction. A variety of natural-products and drug derived alkenes reacted well, giving the desired products 46–54 in good yields.

|
Download:
|
| Scheme 2. Substrate scope of alkenes. The reactions were performed on a 0.2 mmol scale. Isolated yields. a The reaction was carried out with 1,4-divinylbenzene 1ah (0.2 mmol), 2a (0.72 mmol, 3.6 equiv.), tBuONO (1.2 mmol, 6.0 equiv.), 2,4,6-collidine (0.4 mmol, 2.0 equiv.) and DCM (2 mL). | |
As shown in Scheme 3, we next explored the scope of secondary phosphine oxides. A variety of monosubstituted and disubstituted diarylphosphine oxides took part in the reaction to afford the corresponding phosphinoyl oxime products 55−67 in good yields. Furthermore, the phosphine oxides substituted with naphthyl groups or heterocycles such as thiophene and benzothiophene were found to be compatible with this phosphinoyloximation approach. The asymmetric phosphine oxides such as substituted phenyl- or alkylphenylphosphine oxides were also suitable substrates to deliver the corresponding products 72–76 in 76%–84% yields. Note that the reaction of 6H-dibenz[c,e][1,2]oxaphosphorin 6-oxide (DOPO) proceeded smoothly to provide the desired product 77 in moderate yield, while dicyclohexylphosphine oxide, ethyl phenylphosphinate, and diethyl phosphite failed to participate in the present phosphinoyloximation reaction.
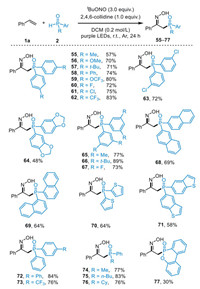
|
Download:
|
| Scheme 3. Substrate scope of secondary phosphine oxides. The reactions were performed on a 0.2 mmol scale. Isolated yields. | |
To further demonstrate the practical applicability of this protocol, we conducted a gram-scale reaction and explored the further transformations of α-phosphinoyl oxime product (Scheme 4). The model reaction of 1a and 2a was carried out on a 5.0 mmol scale under the optimal conditions, and 1.16 g desired product 3 was obtained in 69% yield. Then, the O-acetylations of oxime functionality of 3 with acetyl and benzoyl chlorides gave the oxime esters 78 and 79 in 80% and 73% yields, respectively. The phosphine oxide group in 3 could not only undergo the oxygen-sulfur exchange process to generate phosphine sulfide 80 in 56% yield in the presence of Lawesson reagent, but also be readily reduced by Si—H reagent to afford trivalent phosphine compound 81 in 63% yield. Furthermore, the rhodium-catalyzed aromatic C—H bond functionalization of 3 with oxime as directing group afforded the isoquinoline derivative 82 in 54% yield [32], followed by the reduction and protection with borane to obtain phosphine compound 83 as a new bidentate P, N-ligand precursor in good yield.
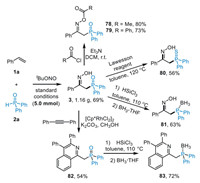
|
Download:
|
| Scheme 4. Utility of the reaction methodology. | |
To gain some insights into this reaction, mechanistic studies were conducted. First, UV–vis absorbance spectra revealed that TBN had a noticeable absorption under our visible LEDs (Scheme 5a). Second, when the radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxy (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) was added to the model reaction, the formation of product 3 was suppressed completely, and the adducts 84 and 85 of phosphinoyl radical by TEMPO and BHT were isolated in 35% and 26% yields, respectively, which indicated the phosphinoyl radical was involved in this reaction (Scheme 5b). Finally, a radical clock experiment using vinyl cyclopropane 86 provided the ring-opening product 87 in 24% yield, suggesting a benzyl radical might be generated after P-centered radical addition to the alkene (Scheme 5c).
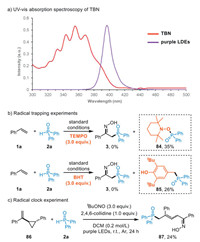
|
Download:
|
| Scheme 5. Mechanistic investigations. | |
Based on the above experiment results and previous studies [44-56,71-91], a possible reaction mechanism was proposed in Scheme 6. First, visible-light-induced homolytic cleavage of the O—NO bond of TBN produces a reactive tert-butoxyl radical (tBuO•) and the persistent NO radical. The subsequent HAT from diphenylphosphine oxide 2a to tBuO• [97], generates the phosphinoyl radical Ⅰ with the release of byproduct tBuOH, which was detected by GC in an amount comparable to the product 3 (Figs. S6–S8 in Supporting information). Then, the addition of radical Ⅰ to styrene 1a affords the benzyl radical intermediate Ⅱ, which is trapped by NO radical through a radical/radical cross-coupling process governed by the persistent radical effect [98]. Finally, the rapid tautomerization of nitrosoalkane Ⅲ delivers the final phosphinoyl oxime product 3.
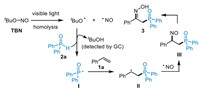
|
Download:
|
| Scheme 6. Proposed reaction mechanism. | |
In conclusion, we have developed an efficient visible-light-induced radical phosphinoyloximation of alkenes with secondary phosphine oxides and TBN. Under photocatalyst- and metal-free conditions, various α-phosphinoyl oximes were afforded in moderate to good yields and high stereoselectivities with broad substrate scope and good functional group compatibility. This method can be readily scalable and the phosphinoyl oxime products could serve as valuable building blocks for further transformations. Critical to this success is TBN's dual roles as HAT reagent and the source of oximes. We anticipate that this strategy for photochemical generation of phosphinoyl radicals with TBN provides a new perspective to rapidly construct organophosphorus compounds.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementHuaixiang Yang: Methodology, Formal analysis, Data curation. Miao-Miao Li: Writing – original draft, Project administration. Aijun Zhang: Methodology, Formal analysis, Data curation. Jiefei Guo: Methodology, Formal analysis, Data curation. Yongqi Yu: Writing – review & editing, Project administration. Wei Ding: Writing – review & editing, Project administration, Conceptualization.
AcknowledgmentsWe gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 22201265, 22201264) and the China Postdoctoral Science Foundation (Nos. 2022M710133, 2022TQ0287).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110425.
| [1] |
M.Y. Cao, X. Ren, Z. Lu, Tetrahedron Lett. 56 (2015) 3732-3742. DOI:10.1016/j.tetlet.2015.04.091 |
| [2] |
X.W. Lan, N.X. Wang, Y. Xing, Eur. J. Org. Chem. 2017 (2017) 5821-5851. DOI:10.1002/ejoc.201700678 |
| [3] |
T. Koike, M. Akita, Chem 4 (2018) 409-437. DOI:10.1016/j.chempr.2017.11.004 |
| [4] |
J. Lin, R.J. Song, M. Hu, J.H. Li, Chem. Rec. 19 (2018) 440-451. DOI:10.1002/tcr.201800053 |
| [5] |
X. Bao, J. Li, W. Jiang, C. Huo, Synthesis 51 (2019) 4507-4530. DOI:10.1055/s-0039-1690987 |
| [6] |
H. Yao, W. Hu, W. Zhang, Molecules 26 (2020) 105. DOI:10.3390/molecules26010105 |
| [7] |
S.O. Badir, G.A. Molander, Chem 6 (2020) 1327-1339. DOI:10.1016/j.chempr.2020.05.013 |
| [8] |
H. Jiang, A. Studer, Chem. Soc. Rev. 49 (2020) 1790-1811. DOI:10.1039/c9cs00692c |
| [9] |
Z.L. Li, G.C. Fang, Q.S. Gu, X.Y. Liu, Chem. Soc. Rev. 49 (2020) 32-48. DOI:10.1039/c9cs00681h |
| [10] |
S. Zhu, X. Zhao, H. Li, L. Chu, Chem. Soc. Rev. 50 (2021) 10836-10856. DOI:10.1039/d1cs00399b |
| [11] |
P. Gao, Y.J. Niu, F. Yang, L.N. Guo, X.H. Duan, Chem. Commun. 58 (2022) 730-746. DOI:10.1039/d1cc05730h |
| [12] |
K. Katakawa, M. Kitajima, N. Aimi, et al., J. Org. Chem. 70 (2004) 658-663. |
| [13] |
J.T. Bagdanoff, M.S. Donoviel, A. Nouraldeen, et al., J. Med. Chem. 53 (2010) 8650-8662. DOI:10.1021/jm101183p |
| [14] |
C. Kornhaaß, J. Li, L. Ackermann, J. Org. Chem. 77 (2012) 9190-9198. DOI:10.1021/jo301768b |
| [15] |
Y. Wei, N. Yoshikai, J. Am. Chem. Soc. 135 (2013) 3756-3759. DOI:10.1021/ja312346s |
| [16] |
Y. Xu, W. Hu, X. Tang, et al., Chem. Commun. 51 (2015) 6843-6846. DOI:10.1039/C5CC01661D |
| [17] |
N. Liu, L. Song, M. Liu, et al., Chem. Sci. 7 (2016) 482-488. DOI:10.1039/C5SC03021H |
| [18] |
D.Q. Dong, J.C. Song, S.H. Yang, et al., Chin. Chem. Lett. 33 (2022) 1199-1206. DOI:10.1016/j.cclet.2021.08.067 |
| [19] |
S. Yang, Y. Wang, W. Xu, et al., Org. Lett. 25 (2023) 8834-8838. DOI:10.1021/acs.orglett.3c03526 |
| [20] |
T. Zou, Y.S. He, R. Liu, et al., Chin. Chem. Lett. 34 (2023) 107822-107826. DOI:10.1016/j.cclet.2022.107822 |
| [21] |
K. Kato, T. Mukaiyama, Chem. Lett. 19 (1990) 1395-1398. DOI:10.1246/cl.1990.1395 |
| [22] |
M. Kijima, H. Yamashita, T. Sato, J. Organomet. Chem. 426 (1992) 399-404. DOI:10.1016/0022-328X(92)83072-P |
| [23] |
T. Okamoto, K. Kobayashi, S. Oka, S. Tanimoto, J. Org. Chem. 52 (2002) 5089-5092. |
| [24] |
C. de Salas, O. Blank, M.R. Heinrich, Chem. Eur. J. 17 (2011) 9306-9310. DOI:10.1002/chem.201101565 |
| [25] |
C. de Salas, M.R. Heinrich, Green Chem. 16 (2014) 2982-2987. DOI:10.1039/C3GC42432D |
| [26] |
J. Hartung, Chem. Rev. 109 (2009) 4500-4517. DOI:10.1021/cr900085j |
| [27] |
P. Scharlin, R. Battino, E. Silla, I. Tuñón, J.L. Pascual-Ahuir, Pure Appl. Chem. 70 (1998) 1895-1904. DOI:10.1351/pac199870101895 |
| [28] |
B. Weinberger, Toxicol. Sci. 59 (2001) 5-16. DOI:10.1093/toxsci/59.1.5 |
| [29] |
P.F. Yuan, T. Huang, J. He, et al., Org. Chem. Front. 8 (2021) 5785-5792. DOI:10.1039/d1qo01101d |
| [30] |
Z. Wang, N. Wierich, J. Zhang, C.G. Daniliuc, A. Studer, J. Am. Chem. Soc. 145 (2023) 8770-8775. DOI:10.1021/jacs.3c01129 |
| [31] |
H. Lan, X. Huo, Y. Jia, D. Wang, Org. Lett. 26 (2024) 1011-1016. DOI:10.1021/acs.orglett.3c04085 |
| [32] |
P.F. Yuan, X.T. Huang, L.H. Long, et al., Angew. Chem. Int. Ed. 63 (2024) e202317968. DOI:10.1002/anie.202317968 |
| [33] |
J.W. Sang, H. Chen, Y. Zhang, J. Wang, W.D. Zhang, Green Chem. 26 (2024) 7849-7856. DOI:10.1039/d4gc01976h |
| [34] |
H. Chakrapani, M.D. Bartberger, E.J. Toone, J. Org. Chem. 74 (2009) 1450-1453. DOI:10.1021/jo802517t |
| [35] |
D. Zheng, S. Plöger, C.G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 60 (2021) 8547-8551. DOI:10.1002/anie.202016955 |
| [36] |
R. Gao, F. Wang, X. Geng, C.Y. Li, L. Wang, Org. Lett. 24 (2022) 7118-7122. DOI:10.1021/acs.orglett.2c02703 |
| [37] |
S. Plöger, C. Mück-Lichtenfeld, C.G. Daniliuc, A. Studer, Chem. Sci. 13 (2022) 9749-9754. DOI:10.1039/d2sc03860a |
| [38] |
D.H.R. Barton, J.M. Beaton, J. Am. Chem. Soc. 82 (1960) 2641. DOI:10.1021/ja01495a062 |
| [39] |
D.H.R. Barton, J.M. Beaton, L.E. Geller, M.M. Pechet, J. Am. Chem. Soc. 82 (1960) 2640-2641. DOI:10.1021/ja01495a061 |
| [40] |
D.H.R. Barton, J.M. Beaton, L.E. Geller, M.M. Pechet, J. Am. Chem. Soc. 83 (1961) 4076-4083. DOI:10.1021/ja01480a030 |
| [41] |
G. Majetich, K. Wheless, Tetrahedron 51 (1995) 7095-7129. DOI:10.1016/0040-4020(95)00406-X |
| [42] |
E. Piers, Pure Appl. Chem. 60 (1988) 107-114. DOI:10.1351/pac198860010107 |
| [43] |
H.M. Huang, P. Bellotti, J. Ma, T. Dalton, F. Glorius, Nat. Rev. Chem. 5 (2021) 301-321. DOI:10.1038/s41570-021-00266-5 |
| [44] |
A. Dahiya, A.K. Sahoo, T. Alam, B.K. Patel, Chem. Asian J. 14 (2019) 4454-4492. DOI:10.1002/asia.201901072 |
| [45] |
N.G. Khaligh, Mini-Rev. Org. Chem. 17 (2020) 3-25. DOI:10.2174/1570193x15666181029141019 |
| [46] |
Z.L. Chen, Q.Q. Li, A. Studer, J. Xuan, Sci. China Chem. 68 (2025) 118-133. DOI:10.1007/s11426-024-2096-6 |
| [47] |
J. Yang, Y.Y. Liu, R.J. Song, Z.H. Peng, J.H. Li, Adv. Synth. Catal. 358 (2016) 2286-2292. DOI:10.1002/adsc.201600109 |
| [48] |
B. Wang, L. Tang, L. Liu, et al., Green Chem. 19 (2017) 5794-5799. DOI:10.1039/C7GC03051G |
| [49] |
J. Wysocki, J.H. Teles, R. Dehn, et al., ChemPhotoChem 2 (2018) 22-26. DOI:10.1002/cptc.201700151 |
| [50] |
L. Chen, Z. Wang, H. Liu, X. Li, B. Wang, Chem. Commun. 58 (2022) 9152-9155. DOI:10.1039/d2cc02823a |
| [51] |
Y. Liu, J. Cao, M. Zhang, et al., J. Org. Chem. 88 (2023) 15311-15317. DOI:10.1021/acs.joc.3c01815 |
| [52] |
Y. Tang, Y. Yang, Q. Zhou, et al., Org. Biomol. Chem. 21 (2023) 5254-5264. DOI:10.1039/d3ob00630a |
| [53] |
Y. Zhao, X. Li, S.L. Homölle, B. Wang, L. Ackermann, Chem. Sci. 15 (2024) 1117-1122. DOI:10.1039/d3sc05946d |
| [54] |
X.m. Chen, J. Huang, J. Pan, et al., Org. Lett. 26 (2024) 3883-3888. DOI:10.1021/acs.orglett.4c01038 |
| [55] |
S. Plöger, A. Studer, Org. Lett. 24 (2022) 8568-8572. DOI:10.1021/acs.orglett.2c03644 |
| [56] |
W. Li, Z. Huang, D. Zhong, H. Li, Org. Lett. 26 (2024) 2062-2067. DOI:10.1021/acs.orglett.4c00314 |
| [57] |
P.C.J. Kamer, P.W.N.M. van Leeuwen, J.N.H. Reek, Acc. Chem. Res. 34 (2001) 895-904. DOI:10.1021/ar000060+ |
| [58] |
P.W.N.M. van Leeuwen, P.C.J. Kamer, C. Claver, O. Pàmies, M. Diéguez, Chem. Rev. 111 (2011) 2077-2118. DOI:10.1021/cr1002497 |
| [59] |
C. Queffélec, M. Petit, P. Janvier, D.A. Knight, B. Bujoli, Chem. Rev. 112 (2012) 3777-3807. DOI:10.1021/cr2004212 |
| [60] |
G.P. Horsman, D.L. Zechel, Chem. Rev. 117 (2017) 5704-5783. DOI:10.1021/acs.chemrev.6b00536 |
| [61] |
H. Ni, W.L. Chan, Y. Lu, Chem. Rev. 118 (2018) 9344-9411. DOI:10.1021/acs.chemrev.8b00261 |
| [62] |
H. Guo, Y.C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 118 (2018) 10049-10293. DOI:10.1021/acs.chemrev.8b00081 |
| [63] |
P. Finkbeiner, J.P. Hehn, C. Gnamm, J. Med. Chem. 63 (2020) 7081-7107. DOI:10.1021/acs.jmedchem.0c00407 |
| [64] |
K. Moonen, I. Laureyn, C.V. Stevens, Chem. Rev. 104 (2004) 6177-6216. DOI:10.1021/cr030451c |
| [65] |
C.S. Demmer, N. Krogsgaard-Larsen, L. Bunch, Chem. Rev. 111 (2011) 7981-8006. DOI:10.1021/cr2002646 |
| [66] |
J.L. Montchamp, Acc. Chem. Res. 47 (2014) 77-87. DOI:10.1021/ar400071v |
| [67] |
H. Sun, Y. Li, W. Liu, Y. Zheng, Z. He, Chin. Chem. Lett. 29 (2018) 1625-1628. DOI:10.1016/j.cclet.2018.01.026 |
| [68] |
L. Chen, X.Y. Liu, Y.X. Zou, Adv. Synth. Catal. 362 (2020) 1724-1818. DOI:10.1002/adsc.201901540 |
| [69] |
J.D. Gbubele, T.K. Olszewski, Org. Biomol. Chem. 19 (2021) 2823-2846. DOI:10.1039/d1ob00124h |
| [70] |
B. Wang, L. Sun, Q. Cao, et al., Chin. Chem. Lett. 35 (2024) 109617. DOI:10.1016/j.cclet.2024.109617 |
| [71] |
D. Leca, L. Fensterbank, E. Lacôte, M. Malacria, Chem. Soc. Rev. 34 (2005) 858-865. DOI:10.1039/b500511f |
| [72] |
Y. Gao, G. Tang, Y. Zhao, Phosphorus, Sulfur, Silicon Relat. Elem. 192 (2017) 589-596. DOI:10.1080/10426507.2017.1295965 |
| [73] |
B.G. Cai, J. Xuan, W.J. Xiao, Sci. Bull. 64 (2019) 337-350. DOI:10.1016/j.scib.2019.02.002 |
| [74] |
C. Li, J. Wang, S.D. Yang, Chem. Commun. 57 (2021) 7997-8002. DOI:10.1039/d1cc02604f |
| [75] |
J. Liu, H.Z. Xiao, Q. Fu, D.G. Yu, Chem. Synth. 1 (2021) 9. DOI:10.20517/cs.2021.14 |
| [76] |
Y. Niu, S.-D. Yang, Chem. Synth. 1 (2021) 12. |
| [77] |
C. Zhang, Z. Li, L. Zhu, et al., J. Am. Chem. Soc. 135 (2013) 14082-14085. DOI:10.1021/ja408031s |
| [78] |
W.J. Yoo, S. Kobayashi, Green Chem. 15 (2013) 1844-1848. DOI:10.1039/c3gc40482j |
| [79] |
G. Zhang, L. Fu, P. Chen, J. Zou, G. Liu, Org. Lett. 21 (2019) 5015-5020. DOI:10.1021/acs.orglett.9b01607 |
| [80] |
Y.H. Li, C.H. Wang, S.Q. Gao, F.M. Qi, S.D. Yang, Chem. Commun. 55 (2019) 11888-11891. DOI:10.1039/c9cc06075h |
| [81] |
Q. Fu, Z.Y. Bo, J.H. Ye, et al., Nat. Commun. 10 (2019) 3592. DOI:10.1038/s41467-019-11528-8 |
| [82] |
W.Q. Liu, T. Lei, S. Zhou, et al., J. Am. Chem. Soc. 141 (2019) 13941-13947. DOI:10.1021/jacs.9b06920 |
| [83] |
N. Fu, L. Song, J. Liu, et al., J. Am. Chem. Soc. 141 (2019) 14480-14485. DOI:10.1021/jacs.9b03296 |
| [84] |
W. Yang, B. Li, M. Zhang, et al., Chin. Chem. Lett. 31 (2020) 1313-1316. DOI:10.1016/j.cclet.2019.10.022 |
| [85] |
P.Z. Wang, W.J. Xiao, J.R. Chen, Nat. Rev. Chem. 7 (2022) 35-50. DOI:10.1038/s41570-022-00441-2 |
| [86] |
Q. Cao, M.M. Li, X. Mao, Q.Q. Zhou, W. Ding, Org. Lett. 26 (2024) 4678-4683. DOI:10.1021/acs.orglett.4c01422 |
| [87] |
H. Zhang, X. Sun, C. Ma, et al., ACS Catal. 14 (2024) 3115-3127. DOI:10.1021/acscatal.3c05154 |
| [88] |
G.Q. Li, Z.Q. Li, M. Jiang, et al., Angew. Chem. Int. Ed. 63 (2024) e202405560. DOI:10.1002/anie.202405560 |
| [89] |
L. Liu, Y. Wu, C. Xiang, J.T. Yu, C. Pan, Chem. Commun. 60 (2024) 4687-4690. DOI:10.1039/d4cc00608a |
| [90] |
X.M. Chen, L. Song, J. Pan, et al., Chin. Chem. Lett. 35 (2024) 110112. DOI:10.1016/j.cclet.2024.110112 |
| [91] |
D. Liang, P. Gao, Z. Zhang, W. Xiao, J. Chen, Green Synth. Catal. 5 (2024). DOI:10.1016/j.gresc.2024.01.002 |
| [92] |
A.V. Il'yasov, Biophysics 58 (2013) 167-171. DOI:10.1134/S0006350913020103 |
| [93] |
J. Francos, J. Borge, S. Conejero, V. Cadierno, Eur. J. Inorg. Chem. 2018 (2018) 3176-3186. DOI:10.1002/ejic.201800398 |
| [94] |
Y.A. Naumovich, S.L. Ioffe, A.Y. Sukhorukov, J. Org. Chem. 84 (2019) 7244-7254. DOI:10.1021/acs.joc.9b00924 |
| [95] |
H. Lan, Y. Su, Y. Chen, X. He, D. Wang, Org. Chem. Front. 11 (2024) 4207-4213. DOI:10.1039/d4qo00636d |
| [96] |
J. Zou, Z. Ying, P. Zhang, Z. Tao, J. Li, Patent, CN111039978B, 2017.
|
| [97] |
J. Shen, B. Xiao, Y. Hou, et al., Adv. Synth. Catal. 361 (2019) 5198-5209. DOI:10.1002/adsc.201900873 |
| [98] |
D. Leifert, A. Studer, Angew. Chem. Int. Ed. 59 (2020) 74-108. DOI:10.1002/anie.201903726 |
 2025, Vol. 36
2025, Vol. 36