b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China;
c School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China;
d Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, Zhejiang Normal University, Jinhua 321004, China
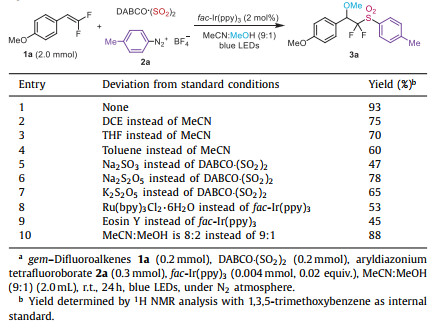
Sulfonyl groups are privileged building blocks in pharmaceuticals and natural products [1,2]. Among them, organic sulfones possessing difluoromethyl moiety have drawn condiserable interest due to the presence of this moiety in broad bioactive compounds (Scheme 1a). For instance, compound A exhibits potent antifungal activity against Candida albicans in vivo [3]. Compounds B is demonstrated as hypoxia inducible factor 2α (HIF-2α) inhibitors [4]. Compounds C is able to control animal parasites [5]. With such a plethora of bioactivities, protocols addressing the rapid preparation of α,α-difluoromethyl sulfones have attracted great attention in the past few years. Strategies for the synthesis of α,α-difluoromethyl sulfones with various fluoroalkylation reagents have been developed [6-22]. However, there are few reports for the direct preparation of α,α-difluoromethyl sulfones without fluoroalkylation reagents.
Multicomponent reactions are considered as a reliable and powerful tools for rapid construction of complex molecules due to their efficiency and simplicity [23-30]. Thus, developing multicomponent reactions in both academic and industry communities is highly desirable. On the other hand, radical sulfonylation has been recognized as a useful approach to sulfonyl compounds [31-39]. In particular, the insertion of sulfur dioxide with readily available surrogates such as DABCO·(SO2)2 and inorganic metabisulfites has been widely explored for the synthesis of various sulfonyl compounds [40-55]. In 2020 and 2022, our group developed photocatalyzed three-component reactions of 2,2-difluoro enol silyl ethers, sulfur dioxide surrogates and aryldiazonium tetrafluoroborates [56] or thianthrenium salts [57] to access α,α-difluoromethyl sulfones (Scheme 1b). However, only α,α-difluoromethyl-β-sulfones were produced.
Recently, gem–difluoroalkenes have emerged as a class of important fluoro synthons [58-60]. An impressive number of transformations have been achieved with gem–difluoroalkenes, such as defluorination reactions [61-69] and trifluoromethylation [70-74]. In 2022, Wu's group developed the alkoxysulfonylation of gem–difluoroalkenes with sulfonyl chlorides [75]. Encouraged by these advances and the importance of α,α-difluoromethyl sulfones, as well as our continuing interest in sulfur chemistry [76-88], we envisioned that the difunctionalizaiton of gem–difluoroalkenes via sulfur dioxide insertion would be feasible for the construction of significant α,α-difluoromethyl sulfones (Scheme 1c).
|
Download:
|
| Scheme 1. Bioactive compounds containing α,α-difluoromethyl sulfones and synthesis of α,α-difluoromethyl sulfones. | |
We started our investigations by evaluating the four-component reaction of 1-(2,2-difluorovinyl)−4-methoxybenzene 1a, p-methylphenyldiazonium tetrafluoroborate 2a, DABCO·(SO2)2 and methanol. Initially, this reaction was performed in MeCN with fac-Ir(ppy)3 as the photocatalyst under the irradiation of blue LEDs at room temperature. To our delight, high efficiency of this transformation was observed, and the corresponding α,α-difluoromethyl sulfone 3a was afforded in 93% NMR yield (Table 1, entry 1). Lower yields were obtained when the reactions were performed in DCE, THF and toluene (Table 1, entries 2–4). Other sulfur dioxide surrogates, such as Na2SO3, Na2S2O5 and K2S2O5 gave inferior results (Table 1, entries 5–7). Examination of different photocatalyst showed lower yields (Table 1, entries 8 and 9). No higher yield was observed when the reaction was carried out in MeCN:MeOH (8:2) (Table 1, entry 10).
|
|
Table 1 Optimization of the reaction conditions.a |
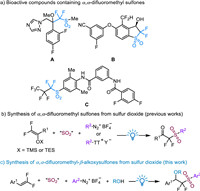
Having the optimized conditions in hand, the generality of this four-component reaction with respect to gem–difluoroalkenes 1 was then investigated. The results are summarized in Scheme 2. Aromatic gem–difluoroalkenes with electron-donating groups afforded the desired products in moderate to excellent yields (3a-3k). Next, the scope of aryldiazonium tetrafluoroborate 2 was then explored. Aromatic rings with electron-donating groups and electron-withdrawing groups, such as methoxyl, tert–butyl, phenyl, fluoro, bromo, dihydrobenzofuran and 4-methyl-benzopyranone, were suitable for this transformation, leading to the target α,α-difluoromethyl sulfones 3l-3s in 59%−90% yields. Finally, a range of alcohols with a bulky group or a long chain were explored. Both primary (ethanol, benzyl alcohol) and tertiary alcohols (tert–butanol) underwent this reaction smoothly, affording the corresponding products (3t-3v) in 49%−90% yields. Unfortunately, aromatic gem–difluoroalkenes with only electron-withdrawing group and alkyl gem–difluoroalkenes could not afford the desired products.
|
Download:
|
| Scheme 2. Substrate Scope for the synthesis of α,α-difluoromethyl-β-alkoxysulfones. Conditions: gem–difluoroalkenes 1 (0.2 mmol), DABCO·(SO2)2 (0.2 mmol), aryldiazonium tetrafluoroborate 2 (0.3 mmol), fac-Ir(ppy)3 (0.004 mmol, 0.02 equiv.), MeCN:MeOH (9:1) (2.0 mL), r.t., 24 h, blue LEDs, under N2 atmosphere. Isolated yield based on gem–difluoroalkenes 1. | |
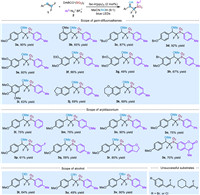
Recently, thianthrenium salts have been developed as valuable radical precursors for various photoredox-catalyzed reactions [89-97]. Thus, we also examined thianthrenium salts as radical precursors in this reaction. To our delight, the desired product 3a was successfully furnished, though with only 30% yield (Scheme 3a). However, the alkyl substituted thianthrenium salt 4b could not provide the desired product (Scheme 3a). Furthermore, the benzyl mercaptane as a nucleophilic trapper was also examined in this reaction. Unfortunately, the corresponding product could not be furnished (Scheme 3b).
|
Download:
|
| Scheme 3. Thianthrenium salts as radical precursors and BnSH as a nucleophilic trapper. | |
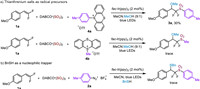
To verify the practicability of this photoredox-catalyzed synthesis of α,α-difluoromethyl sulfones from sulfur dioxide, a scale-up (2.0 mmol) experiment was performed. Gratifyingly, a similar result was smoothly afforded (Scheme 4a). To examine the utility of the obtained α,α-difluoromethyl sulfones, some synthetic transformations were realized (Scheme 4b). First, the methoxyl group of 3a could be readily removed with triethylsilyl hydride in the presence of aluminum chloride, furnishing product 5 in 74% yield. Furthermore, the methoxyl group could also be replaced by allyl moiety with allylsilane and aluminum chloride, delivering the α,α-difluoromethyl β-allylsulfones 6 in good yields.
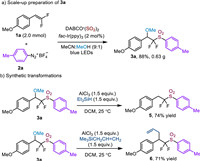
|
Download:
|
| Scheme 4. Scale-up preparation of compound 3a and synthetic transformations. | |
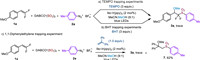
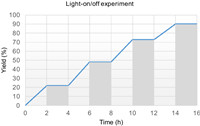
To gain mechanistic insights into this photoredox-catalyzed synthesis of α,α-difluoromethyl sulfones from sulfur dioxide, several control experiments were conducted, as shown in Scheme 5. The reaction was completely suppressed when the radical scavenger 2,2,6,6-tetramethylpiperidinooxy (TEMPO) or butylated hydroxytoluene (BHT) was added under the standard conditions (Schemes 5a and b). When 1,1-diphenylethylene was added to the standard conditions, trace amount of 3a was detected along with 62% yield of radical addition product 7 (Scheme 5c). These results confirmed that the arylsulfonyl radical was generated during this transformation. Furthermore, light-on/off experiment was conducted and the results indicated that this reaction was exclusive light dependence (Fig. 1).
|
Download:
|
| Scheme 5. Control experiments. | |
|
Download:
|
| Fig. 1. Light-on/off experiment. | |
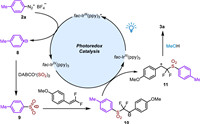
On the basis of control experiments and previous studies, a possible mechanism for this photoredox-catalyzed α,α-difluoromethyl sulfones from sulfur dioxide is outlined in Scheme 6. Initially, the excitation of fac-Ir(ppy)3 under visible light irradiation produced the excited state of fac-Ir(ppy)3*, which reduced aryldiazonium tetrafluoroborate 2a to aryl radical 8 along with oxidized fac-IrIV(ppy)3. Subsequently, the aryl radical 8 was trapped by sulfur dioxide from DABCO·(SO2)2 to furnish an arylsulfonyl radical 9, which added to the double bond of gem–difluoroalkenes to form difluoromethylated radical 10. Then, the radical intermediate 10 would be oxidized by fac-IrIV(ppy)3, leading to carbocation intermediate 11 along with the regeneration of fac-Ir(ppy)3. Finally, the carbocation 11 would react with a nucleophilic methanol to give the final product 3a.
|
Download:
|
| Scheme 6. Proposed mechanism. | |
In summary, we have developed a photoredox-catalyzed synthesis of α,α-difluoromethyl sulfones from sulfur dioxide with readily available gem–difluoroalkenes. This reaction features mild reaction conditions, broad substrate scope and good functional group tolerance. Furthermore, this transformation provides an efficient method for the synthesis of α,α-difluoromethyl sulfones from readily avaiable starting materials. Based on the results of control experiments and previous studies, a plausible mechanism involving the aryl radical-trigged insertion of sulfur dioxide is proposed.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementMinjun Yin: Methodology, Investigation. Yuhui Lin: Methodology, Investigation. Manli Zhuang: Investigation, Data curation. Wei Xiao: Writing – review & editing, Writing – original draft, Project administration. Jie Wu: Writing – original draft, Project administration.
AcknowledgmentsFinancial support from National Natural Science Foundation of China (Nos. 22201202, 22171206 and 22371201), Natural Science Foundation of Zhejiang Province (No. LZ23B020001), Open Foundation of Hunan Provincial Key Laboratory of Controllable Preparation and Functional Application of Fine Polymers (No. E22307), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (No. 2020ZD04), and Open Research Fund of Key Laboratory of the Ministry of Education for Advanced Catalysis Materials and Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Zhejiang Normal University is gratefully acknowledged.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109926.
| [1] |
M. Feng, B. Tang, H.S. Liang, X. Jiang, Curr. Top. Med. Chem. 16 (2016) 1200-1216. DOI:10.2174/1568026615666150915111741 |
| [2] |
K.A. Scott, J.T. Njardarson, Top. Curr. Chem. 376 (2018) 5. DOI:10.1007/s41061-018-0184-5 |
| [3] |
H. Eto, Y. Kaneko, S. Takeda, et al., Chem. Pharm. Bull. 49 (2001) 173-182. DOI:10.1248/cpb.49.173 |
| [4] |
R.K. Bruick, Y. Chen, J.C.F. Ruiz, Patent, WO2016057242A1, 2016.
|
| [5] |
C. A.G. Bayer, Patent, WO2009080203, 2009.
|
| [6] |
W. Zhang, W. Huang, J. Hu, Angew. Chem. Int. Ed. 48 (2009) 9858-9861. DOI:10.1002/anie.200905077 |
| [7] |
L. Zhu, Y. Li, Y. Zhao, J. Hu, Tetrahedron Lett. 51 (2010) 6150-6152. DOI:10.1016/j.tetlet.2010.09.068 |
| [8] |
G.K.S. Prakash, C. Ni, F. Wang, J. Hu, G.A. Olah, Angew. Chem. Int. Ed. 50 (2011) 2559-2563. DOI:10.1002/anie.201007594 |
| [9] |
W. Huang, C. Ni, Y. Zhao, B. Gao, J. Hu, J. Fluorine Chem. 143 (2012) 161-166. DOI:10.1016/j.jfluchem.2012.05.018 |
| [10] |
W. Huang, C. Ni, Y. Zhao, et al., Tetrahedron 68 (2012) 5137-5144. DOI:10.1016/j.tet.2012.04.039 |
| [11] |
H. Jia, A.P. Häring, F. Berger, L. Zhang, T. Ritter, J. Am. Chem. Soc. 143 (2021) 7623-7628. DOI:10.1021/jacs.1c02606 |
| [12] |
Y. Li, X. Liang, K. Niu, et al., Org. Lett. 24 (2022) 5918-5923. DOI:10.1021/acs.orglett.2c02150 |
| [13] |
Y.M. Su, Y. Hou, F. Yin, et al., Org. Lett. 24 (2022) 2958-2961. |
| [14] |
W. Miao, C. Ni, Y. Zhao, J. Hu, Org. Lett. 18 (2016) 2766-2769. DOI:10.1021/acs.orglett.6b01258 |
| [15] |
J. Rong, L. Deng, P. Tan, et al., Angew. Chem. Int. Ed. 55 (2016) 2743-2747. DOI:10.1002/anie.201510533 |
| [16] |
J. Xie, T. Zhang, F. Chen, et al., Angew. Chem. Int. Ed. 55 (2016) 2934-2938. DOI:10.1002/anie.201508622 |
| [17] |
J. Chen, J.H. Lin, J.C. Xiao, Tetrahedron 74 (2018) 4295-4297. DOI:10.1016/j.tet.2018.06.062 |
| [18] |
Y.J. Zhu, Z.L. Lei, D.K. Huang, et al., Tetrahedron Lett. 59 (2018) 3184-3187. DOI:10.1016/j.tetlet.2018.07.021 |
| [19] |
P. Xiao, C. Ni, W. Miao, et al., J. Org. Chem. 84 (2019) 8345-8359. DOI:10.1021/acs.joc.9b00419 |
| [20] |
E. Nobile, T. Castanheiro, T. Besset, Chem. Commun. 57 (2021) 12337-12340. DOI:10.1039/d1cc04737j |
| [21] |
H. Uno, K. Kawai, T. Araki, M. Shiro, N. Shibata, Angew. Chem. Int. Ed. 61 (2022) e202117635. DOI:10.1002/anie.202117635 |
| [22] |
E. Nobile, F. Doche, T. Castanheiro, D.G. Musaev, T. Besset, Chem. Eur. J. 30 (2024) e202303362. DOI:10.1002/chem.202303362 |
| [23] |
W.B. He, S.J. Zhao, J.Y. Chen, et al., Chin. Chem. Lett. 34 (2023) 107640. DOI:10.1016/j.cclet.2022.06.063 |
| [24] |
H.T. Ji, K.L. Wang, W.T. Ouyang, et al., Green Chem. 25 (2023) 7983-7987. DOI:10.1039/d3gc02575f |
| [25] |
J. Chen, G. Zhu, J. Wu, Acta Chim. Sin. 81 (2023) 1609-1623. DOI:10.6023/a23070339 |
| [26] |
Y.H. Lu, C. Wu, J.C. Hou, et al., ACS Catal. 13 (2023) 13071-13076. DOI:10.1021/acscatal.3c02268 |
| [27] |
W.T. Ouyang, H.T. Ji, J. Jiang, et al., Chem. Commun. 59 (2023) 14029-14032. DOI:10.1039/d3cc04020h |
| [28] |
J. Huang, J. Wu, Acta Chim. Sin. 81 (2023) 520-532. DOI:10.6023/A23030088 |
| [29] |
Z. Wang, N. Meng, Y. Lv, et al., Chin. Chem. Lett. 34 (2023) 107599. DOI:10.1016/j.cclet.2022.06.022 |
| [30] |
H.Y. Song, J. Jiang, Y.H. Song, et al., Chin. Chem. Lett. 35 (2024) 109246. DOI:10.1016/j.cclet.2023.109246 |
| [31] |
G. Qiu, L. Lai, J. Cheng, J. Wu, Chem. Commun. 54 (2018) 10405-10414. DOI:10.1039/c8cc05847d |
| [32] |
D.Q. Dong, Q.Q. Han, et al., ChemistrySelect 5 (2020) 13103-10134. DOI:10.1002/slct.202003650 |
| [33] |
S. Ye, X. Li, W. Xie, J. Wu, Eur. J. Org. Chem. 2020 (2020) 1274-1287. DOI:10.1002/ejoc.201900396 |
| [34] |
K. Hofman, N. Liu, G. Manolikakes, Chem. Eur. J. 24 (2018) 11852-11863. DOI:10.1002/chem.201705470 |
| [35] |
G. Qiu, L. Lai, J. Cheng, J. Wu, Chem. Commun. 54 (2018) 10405-10414. DOI:10.1039/c8cc05847d |
| [36] |
Y. Li, D. Huang, D. Deng, S.R. Guo, Cur. Org. Chem. 26 (2022) 369-381. DOI:10.2174/1385272826666220222110614 |
| [37] |
Y. Wu, Y. Yan, W. Liao, Chin. J. Org. Chem. 43 (2023) 3713-3727. DOI:10.6023/cjoc202305014 |
| [38] |
J. Zhang, P. Wang, Y. Li, J. Wu, Chem. Commun. 59 (2023) 3821-3826. DOI:10.1039/d2cc06339e |
| [39] |
G. Chen, Z. Lian, Eur. J. Org. Chem. 26 (2023) e202300217. DOI:10.1002/ejoc.202300217 |
| [40] |
S. Chen, Y. Li, M. Wang, X. Jiang, Green Chem. 22 (2020) 322-326. DOI:10.1039/C9GC03841H |
| [41] |
X. Gong, M. Yang, J.B. Liu, et al., Green Chem. 22 (2020) 1906-1910. DOI:10.1039/d0gc00332h |
| [42] |
Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 8907-8911. DOI:10.1002/anie.202001589 |
| [43] |
X. Jia, S. Kramer, T. Skrydstrup, Z. Lian, Angew. Chem. Int. Ed. 60 (2021) 7353-7359. DOI:10.1002/anie.202014111 |
| [44] |
Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 8907-8911. DOI:10.1002/anie.202001589 |
| [45] |
Y. Meng, M. Wang, X. Jiang, Angew. Chem. Int. Ed. 59 (2020) 1346-1353. DOI:10.1002/anie.201911449 |
| [46] |
H. Zhang, M. Wang, X. Jiang, Green Chem. 22 (2020) 8238-8242. DOI:10.1039/d0gc03135f |
| [47] |
S. Jin, G.C. Haug, R. Trevino, et al., Chem. Sci. 12 (2021) 13914-13921. DOI:10.1039/d1sc04245a |
| [48] |
T. Zhong, J.T. Yi, Z.D. Chen, et al., Chem. Sci. 12 (2021) 9359-9365. DOI:10.1039/d1sc02503a |
| [49] |
L. Chen, X. Zhang, M. Zhou, et al., ACS Catal. 12 (2022) 10764-10770. DOI:10.1021/acscatal.2c02297 |
| [50] |
F.S. He, C. Zhang, M. Jiang, et al., Chem. Sci. 13 (2022) 8834-8839. DOI:10.1039/d2sc02497g |
| [51] |
J. Huang, F. Liu, L.H. Zeng, et al., Nat. Commun. 13 (2022) 7081. DOI:10.1038/s41467-022-34836-y |
| [52] |
T.S.B. Lou, Y. Kawamata, T. Ewing, et al., Angew. Chem. Int. Ed. 61 (2022) e202208080. DOI:10.1002/anie.202208080 |
| [53] |
C. Zhang, M. Yang, Y. Qiu, et al., Chem. Sci. 13 (2022) 11785-11791. DOI:10.1039/d2sc04027a |
| [54] |
M. Chen, W. Sun, J. Yang, et al., Green Chem. 25 (2023) 3857-3863. DOI:10.1039/d3gc01059g |
| [55] |
M. Zhang, L. Liu, B. Wang, et al., ACS Catal. 13 (2023) 11580-11588. DOI:10.1021/acscatal.3c03096 |
| [56] |
F.S. He, Y. Yao, W. Xie, J. Wu, Chem. Commun. 56 (2020) 9469-9472. DOI:10.1039/d0cc03591b |
| [57] |
F.S. He, P. Bao, Z. Tang, et al., Org. Lett. 24 (2022) 2955-2960. DOI:10.1021/acs.orglett.2c01132 |
| [58] |
Z. Li, X. Qiu, J. Lou, Q. Wang, Chin. J. Org. Chem. 41 (2021) 4192-4207. DOI:10.6023/cjoc202106013 |
| [59] |
P. Sorrentino, R.A. Altman, Synthesis 53 (2021) 3935-3950. DOI:10.1055/a-1547-9270 |
| [60] |
M.O. Zubkov, M.D. Kosobokov, A.D. Dilman, Russ. J. Org. Chem. 57 (2021) 1017-1035. DOI:10.1134/s1070428021070010 |
| [61] |
S.S. Yan, D.S. Wu, J.H. Ye, et al., ACS Catal. 9 (2019) 6987-6992. DOI:10.1021/acscatal.9b02351 |
| [62] |
C.J. Lu, X. Yu, Y.T. Chen, Q.B. Song, H. Wang, Org. Chem. Front. 7 (2020) 2313-2318. DOI:10.1039/d0qo00553c |
| [63] |
Y. Wang, Q. Ma, G.C. Tsui, Org. Lett. 23 (2021) 5241-5245. DOI:10.1021/acs.orglett.1c01768 |
| [64] |
L. Ge, C. Zhang, C. Pan, et al., Nat. Commun. 13 (2022) 5938. DOI:10.1038/s41467-022-33602-4 |
| [65] |
F. Liu, Z. Zhuang, Q. Qian, X. Zhang, C. Yang, J. Org. Chem. 87 (2022) 2730-2739. DOI:10.1021/acs.joc.1c02662 |
| [66] |
X. Liu, J. Wu, C. Zhang, Org. Lett. 25 (2023) 1564-1568. DOI:10.1021/acs.orglett.3c00347 |
| [67] |
Y.T. Liu, Y.H. Fan, Y. Mei, et al., Org. Lett. 25 (2023) 549-554. DOI:10.1021/acs.orglett.3c00016 |
| [68] |
H. Tan, Y. Zong, Y. Tang, G.C. Tsui, Org. Lett. 25 (2023) 877-882. DOI:10.1021/acs.orglett.3c00108 |
| [69] |
Y. Zhang, J. Wang, Y. Guo, S. Liu, X. Shen, Angew. Chem. Int. Ed. 63 (2024) e202315269. DOI:10.1002/anie.202315269 |
| [70] |
H. Liu, L. Ge, D.X. Wang, N. Chen, C. Feng, Angew. Chem. Int. Ed. 58 (2019) 3918-3922. DOI:10.1002/anie.201814308 |
| [71] |
W.J. Yoo, J. Kondo, J.A. Angew, Chem. Int. Ed 58 (2019) 6772-6775. DOI:10.1002/anie.201902779 |
| [72] |
T.Y. Lin, Z. Pan, Y. Tu, et al., Angew. Chem. Int. Ed. 59 (2020) 22957-22962. DOI:10.1002/anie.202008262 |
| [73] |
R. Chen, D. Yin, L. Lu, et al., Org. Lett 25 (2023) 7293-7297. DOI:10.1021/acs.orglett.3c02512 |
| [74] |
X. Yu, A. Maity, A. Studer, Angew. Chem. Int. Ed. 62 (2023) e202310288. DOI:10.1002/anie.202310288 |
| [75] |
W. Zhu, H. Xi, W. Jiao, et al., Org. Lett. 24 (2022) 720-725. DOI:10.1021/acs.orglett.1c04165 |
| [76] |
P. Bao, F. Yu, F.S. He, et al., Org. Chem. Front. 8 (2021) 4820-4825. DOI:10.1039/d1qo00732g |
| [77] |
J.Q. Chen, N. Liu, Q. Hu, et al., Org. Chem. Front. 8 (2021) 5316-5321. DOI:10.1039/d1qo00957e |
| [78] |
F.S. He, P. Bao, F. Yu, et al., Org. Lett. 23 (2021) 7472-7476. DOI:10.1021/acs.orglett.1c02665 |
| [79] |
F.S. He, Y. Yao, Z. Tang, et al., Chem. Commun. 57 (2021) 12603-12606. DOI:10.1039/d1cc05690e |
| [80] |
F.S. He, M. Zhang, M. Zhang, X. Luo, J. Wu, Org. Chem. Front. 8 (2021) 3746-3751. DOI:10.1039/d1qo00556a |
| [81] |
J. Huang, F. Ding, Z. Chen, G. Yang, J. Wu, Org. Chem. Front. 8 (2021) 1461-1465. DOI:10.1039/d0qo01546f |
| [82] |
X. Tu, J. Huang, W. Xie, T. Zhu, J. Wu, Org. Chem. Front. 8 (2021) 1789-1794. DOI:10.1039/d0qo01551b |
| [83] |
M. Yang, X. Chang, S. Ye, Q. Ding, J. Wu, J. Org. Chem. 86 (2021) 15177-15184. DOI:10.1021/acs.joc.1c01778 |
| [84] |
Y. Yao, Z. Yin, F.S. He, et al., Chem. Commun. 57 (2021) 2883-2886. DOI:10.1039/d0cc07927h |
| [85] |
C. Zhang, C. Zhang, J. Tang, et al., Adv. Synth. Catal. 363 (2021) 3109-3114. DOI:10.1002/adsc.202100066 |
| [86] |
T. Zhu, J. Shen, Y. Sun, J. Wu, Chem. Commun. 57 (2021) 915-918. DOI:10.1039/d0cc07632e |
| [87] |
F. Liu, J. Huang, X. Wu, et al., J. Org. Chem. 87 (2022) 6137-6145. DOI:10.1021/acs.joc.2c00381 |
| [88] |
Y. Qiu, J. Yao, H. Xia, et al., Adv. Synth. Catal. 365 (2023) 3392-3396. DOI:10.1002/adsc.202300839 |
| [89] |
C. Chen, Z.J. Wang, H. Lu, Y. Zhao, Z. Shi, Nat. Commun. 12 (2021) 4526. DOI:10.1038/s41467-021-24716-2 |
| [90] |
Y. Zhao, C. Yu, W. Liang, F.W. Patureau, Org. Lett. 23 (2021) 6232-6236. DOI:10.1021/acs.orglett.1c01904 |
| [91] |
M.J. Cabrera-Afonso, A. Granados, G.A. Molander, Angew. Chem. Int. Ed. 61 (2022) e202202706. DOI:10.1002/anie.202202706 |
| [92] |
X. Li, W. Si, Z. Liu, et al., Org. Lett. 24 (2022) 4070-4074. DOI:10.1021/acs.orglett.2c01525 |
| [93] |
M. Liu, Y. Qian, Y. Wu, F. Zhang, Green Chem. 25 (2023) 3852-3856. DOI:10.1039/d3gc00336a |
| [94] |
W. Liu, H. Hou, H. Jing, et al., Org. Lett. 25 (2023) 8350-8355. DOI:10.1021/acs.orglett.3c03473 |
| [95] |
H. Xu, X. Li, Y. Dong, et al., Org. Lett. 25 (2023) 3784-3789. DOI:10.1021/acs.orglett.3c01303 |
| [96] |
W. Qi, S. Gu, L.G. Xie, Org. Lett. 26 (2024) 728-733. DOI:10.1021/acs.orglett.3c04183 |
| [97] |
H. Xu, X. Li, Y. Wang, et al., Org. Lett. 26 (2024) 1845-1850. DOI:10.1021/acs.orglett.4c00017 |
 2025, Vol. 36
2025, Vol. 36