b National Key Laboratory of Green Pesticide, College of Chemistry, Central China Normal University, Wuhan 430079, China;
c School of Environmental and Chemical Engineering, Jiangsu Ocean University, Lianyungang 222005, China
Amino acids and proteins are the essential substances used to maintain life-sustaining activity, and they are widely involved in various complex life activities in living organisms through transmembrane transportation [1-4]. It is more cost-effective for humans to take in essential amino acids and proteins from the outside than to produce them in the body [5-7]. Therefore, exploring and studying the transmembrane transportation of amino acids and proteins will promote healthy food choices, provide technical support in separating and purifying substances, and further deepen the understanding of life activities [8-10].
As a common mode of material transportation in a living body, transmembrane transportation is realized by the protein channels embedded in the cell membrane, which can identify and transmit various substances with high selectivity [11-13]. However, protein channels and biofilms with complex structures are unstable and easily damaged, limiting their wide-ranging applications. Recently, many nanofilms have been reported for selective separation of amino acids or proteins [14-18]. Supramolecular macrocycles, such as cucurbituril and pillararene, are selected as film-making materials because of their unique cavity structures and molecular recognition properties [19-25]. Through self-assembly of the macrocycles, these nanofilms with excellent separation and detection performance have simple preparation procedures. However, the size of the channels in the nanofilms cannot be easily adjusted to facilitate the passage of different-sized bioactive substances, leading to poor generalizability in selective separation [26].
Herein, AuNPs were layer-by-layer, self-assembled driven by CB[7], and fixed with the spin coating of polymethyl methacrylate (PMMA) to form a nanocomposite film (Scheme 1). In the nanocomposite film, CB[7] acted as a "glue" between gold nanoparticles, facilitating the connection between the layers of gold nanoparticles. Through the current-voltage (I-V) measurements using a picoammeter, the selective transmission of L-tryptophan in the nanocomposite film has been confirmed. Moreover, lysozyme can selectively pass through the nanocomposite film by adjusting the particle size of AuNPs in the assembly. The nanocomposite film exhibited wide versatility in the selective transport of amino acids and proteins by simply adjusting the size of AuNPs.

|
Download:
|
| Scheme 1. The construct of the film for selective transport of L-tryptophan and lysozyme. | |
In order to effectively assemble the AuNPs, circular silicon wafers with a diameter of 3 cm were selected as the substrates with some modifications. First, the surfaces of silicon wafers were subjected to NaOH (0.1 mol/L) and HNO3 (0.1 mol/L) solutions for surface hydroxylation [27]. Second, 3-mercaptopropyl trimethoxysilane was attached to silicon wafers by soaking them for 12 h. So, the contact angle values changed from 46.5° to 32.9° (Fig. S2 in Supporting information) due to the reduction of the hydrophilic hydroxyl groups, indicating the successful modification of 3-mercaptopropyl trimethoxysilane [28].
After a series of surface modifications, the prepared AuNPs with an average size of ~8 nm were modified firmly on the surface of the substrates through the Au-S bond [29-31]. The modification of AuNPs led to an increase in the hydrophilicity of the substrates and a corresponding decrease in the contact angle. Then, silicon wafers were submerged in 20 mL of CB[7] solution (1 mmol/L), and CB[7] was adsorbed onto the surface of AuNPs via the carbonyl oxygens at one end, causing a decrease in the contact angle. The carbonyl oxygens at the other end provide a large number of attachment sites for the assembly of AuNPs on the next layer. Repeating this process 3 more times, the 4-layer self-assembly of AuNPs was obtained with the alternate modification of AuNPs and CB[7]. From Fig. S2, a continuous decrease in the contact angle values clearly illustrated the assembly formation. Finally, polymethyl methacrylate (PMMA) was evenly applied to the substrate surfaces with a spin coating device (Fig. S1 in Supporting information). The nanocomposite film with layer-by-layer self-assembled AuNPs driven by CB[7] was successfully fabricated.
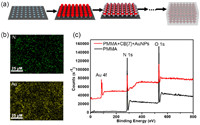
Compared to the PMMA film without the AuNPs, the nanocomposite film exhibited not only the elemental peaks of Au 4f (84.74 eV) in the X-ray photoelectron spectroscopic analysis (Fig. 1c) but also the new element peak of N 1s (399.52 eV) and S 2p (163.14 ev), which was derived from the nitrogen and sulfur elements in CB[7] and 3-mercaptopropyl trimethoxysilane respectively (Fig. S4 in Supporting information) [32,33]. Similarly, these three elements were shown on the elements energy dispersive X-ray spectroscopy (Fig. 1b). These results proved that the nanocomposite film did contain AuNPs and CB[7]. To gain further visual insight into the nanocomposite film, scanning electron microscope (SEM) measurements were carried out (Fig. S6 in Supporting information). The small-sized AuNPs stacked together and agglomerated, indicating the formation of assemblies driven by CB[7].
|
Download:
|
| Fig. 1. (a) Schematic representation of the synthesis of a nanocomposite film via combining layer-by-layer self-assembled AuNPs driven by CB[7] and PMMA film. (b) Energy dispersive spectroscopy (EDS) spectra of the nanocomposite film. (c) X-ray photoelectron spectroscopy (XPS) spectra of the nanocomposite film and PMMA film. | |
The current–voltage (I–V) characteristics were selected to detect the selectivity and transmission of amino acids in the nanocomposite film by a Keithley 6487 picoammeter. A solution of each amino acid (L-leucine, L-threonine, L-valine, L-proline, and L-tryptophan) was prepared with 1 mol/L KCl and 0.03 mol/L PBS (pH 7.0) on both sides of the nanocomposite film, which was fixed in the middle of an electrolytic cell. As amino acids in the electrolytic cell were transferred through the nanocomposite film, transmembrane currents changed accordingly. In Fig. 2a, only the transmembrane current of L-tryptophan has been significantly reduced at a concentration of 10 mmol/L. Considering the influence of amino acid concentrations, 10-fold greater concentrations were assayed (Fig. 2b). The same results demonstrated that the nanocomposite film was selective for L-tryptophan over other amino acids. Moreover, current change rates also illustrated the selective transport of L-tryptophan (Figs. 2c and d).

|
Download:
|
| Fig. 2. The I–V curves of L-proline (L-Pro), L-threonine (L-Thr), L-valine (L-Val), L-leucine (L-Leu), and L-tryptophane (L-Trp) at different concentrations of 10 mmol/L (a) and 100 mmol/L (b). The histogram of current change rates is at −2 V with different concentrations of 10 mmol/L (c) and 100 mmol/L (d). | |
The transmitted flux across the nanocomposite film has been investigated to investigate further this selective transport of the amino acids in the nanocomposite film. When 1.5 mL of each amino acid (0.5 mmol/L) was added to one side of the nanocomposite film separately, the other side without amino acid, served as a control group, was taken every 30 min and examined for fluorescence. Based on the standard curve for concentration vs. fluorescence intensity, the concentration of each amino acid was calculated from the fluorescence intensity in Fig. S7 (Supporting information). Then, the linear relationship between the amino acid transmission flux and time (Fig. S7) can be obtained by the transmission volume calculation formula (Eq. S1 in Supporting information). The transmitted fluxes of L-leucine, L-threonine, L-valine, L-proline, and L-tryptophan were 0.0116, 0.0131, 0.0112, 0.0107, and 0.0251 nmol cm−2 min−1, respectively. The selectivity coefficients of L-tryptophan relative to other amino acids in the nanocomposite film were α(Trp/Leu) = 2.16, α(Trp/Thr) = 1.92, α(Trp/Val) = 2.24, and α(Trp/Pro) = 2.35. Thus, this data visually illustrated the selective transmission of L-tryptophan in the nanocomposite film.
To account for the selectivity of the nanocomposite film, the host-guest interactions between CB[7] and these amino acids were considered. The formation of the host-guest complex was determined by the molar ratio method (Fig. S8 in Supporting information). As the concentration of CB[7] increased, the absorbance of the complex was constantly decreasing, indicating that L-tryptophan has entered into the cavity of CB[7] with a molar ratio of 1:1. In addition, amino acids with aromatic side chains, such as tryptophan, have a stronger binding with CB[7] among these amino acids [34]. These studies showed that CB[7] not only derived the self-assembly of the AuNPs, but also influenced the selectivity of the nanocomposite film through the host-guest interactions.
Based on the mechanism of the selective transmission of L-tryptophan in the nanocomposite film, proteins composed of multiple amino acids were introduced to transport experiments across the nanocomposite film. Unlike amino acids, proteins display a larger spatial structure, which puts elevated demand on the channels in the nanocomposite film. To obtain the greater gaps formed between the AuNPs, the bigger AuNPs with an average size of ~14 nm were pre-prepared to form a nanocomposite film following the same procedures. During the stacking process, these spherical AuNPs did not tightly bond together, but rather form loosely arranged gaps between them, which were correlated with the size of the particles. Therefore, under a loose arrangement, the larger the gold nanoparticles, the larger the gaps formed.
Using KCl (0.1 mol/L) and PBS (0.03 mol/L, pH 7.0) as an electrolyte solution, lysozyme, bovine serum albumin, and cytochrome c were chosen for the transport experiments. From Fig. 3, the transmembrane current of lysozyme exhibited a significant decrease at different concentrations of 10 mmol/L and 100 mmol/L, demonstrating that lysozyme was easier to pass the nanocomposite film. To better discuss the changes in the transmembrane current, current change rates were measured at an applied voltage of −2 V. The maximum current change rate of lysozyme further illustrated the selectivity of lysozyme in the nanocomposite film (Figs. 3c and d). Moreover, a substantial decrease in the transmembrane current of lysozyme was also observed in the transport experiments utilizing the nanocomposite film (Fig. 4). As a control, the transmembrane current of lysozyme, bovine serum albumin, and cytochrome c had no significant change when passing through the PMMA film. These results further illustrated the selective transport of lysozymes in the nanocomposite film. The transport selectivity of nanocomposite film did not arise from different interaction affinities between the film and amino acids/proteins.

|
Download:
|
| Fig. 3. The I–V curves of bovine serum albumin (BSA), cytochrome c (Cy C), and lysozyme (Lys) at different concentrations of 10 mmol/L (a) and 100 mmol/L (b). The histogram of the current rate of change is at −2 V with different concentrations of 10 mmol/L (c) and 100 mmol/L (d). | |

|
Download:
|
| Fig. 4. The I–V curves of bovine serum albumin (BSA), cytochrome c (Cy C), and lysozyme (Lys) at a concentrations of 10 µmol/L using an electrolyte solution (0.1 mol/L KCl and 0.01 mol/L PBS, pH 7.2) in the transport experiments utilizing the PMMA film (a) or the nanocomposite film (b). | |
In addition, the transmitted fluxes of these proteins were calculated in the transport experiments. From Fig. S9 (Supporting information), the ultraviolet absorbance of lysozyme increased rapidly over time. Relatively, the ultraviolet absorbances of bovine serum albumin and cytochrome c increased slowly. The transmitted fluxes of lysozyme, bovine serum albumin, and cytochrome c were 8.94, 5.11, and 4.99 nmol cm−2 h−1, respectively. Lysozyme has six tryptophan residues, while bovine serum albumin and cytochrome c have only two and one, respectively [35,36]. The presence of tryptophan residues greatly enhanced the selective transmission of lysozyme in the nanocomposite film through host-guest interactions. Similarly, the selectivity coefficients of lysozyme relative to bovine serum albumin and cytochrome c were α(Ly/BSA) = 1.74, α(Ly/Cy C) = 1.79, illustrating that the selectivity for lysozyme in the nanocomposite film was related to the tryptophan residues.
In conclusion, a nanocomposite film with layer-by-layer self-assembled AuNPs driven by CB[7] was successfully prepared. Due to the strong host-guest interaction between CB[7] and L-tryptophan, L-tryptophan was selectively transported across the nanocomposite film. The transmission rates of L-tryptophan were much greater than those of L-leucine, L-threonine, L-valine, and L-proline. Furthermore, the selective transport of lysozymes containing multiple tryptophan residues was also achieved by assembling the larger-sized AuNPs. This study provides a new strategy for the transmission and separation of bioactive substances and furthers our understanding of transmembrane transport in organisms.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by National Natural Science Foundation of China (Nos. 22371086, 22071074, 21772055), the National Key Research and Development Program (No. 2021YFA0716702), the 111 Project (No. B17019), the China Postdoctoral Science Foundation (No. 2020 M672388) and Self-determined research funds of CCNU from the colleges’ basic research and operation of MOE, National Natural Science Foundation of China (No. 22006055), the State Key Laboratory of Environmental Chemistry and Ecotoxicology, RCEES, CAS (No. KF2017-4).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109731.
| [1] |
D. Zhang, J. Wang, D. Xu, J. Control. Release 229 (2016) 130-139. DOI:10.1016/j.jconrel.2016.03.020 |
| [2] |
J. Velisek, R. Kubec, K. Cejpek, Czech J. Food Sci. 24 (2006) 93-109. DOI:10.17221/3304-cjfs |
| [3] |
A. Singh-Blom, R.A. Hughes, A.D. Ellington, J. Biotechnol. 178 (2014) 12-22. DOI:10.1016/j.jbiotec.2014.02.009 |
| [4] |
K.L. Wang, Z.N. Wen, F.S. Nie, M.L. Li, Chin. Chem. Lett. 16 (2005) 1133-1136. |
| [5] |
M. Lane, K. Hooper, D.K. Gardner, J. Assist. Reprod. Gen. 18 (2001) 519-525. DOI:10.1023/A:1016657228171 |
| [6] |
X. Lu, B. Xiao, R. Shang, L. Liu, Chin. Chem. Lett. 27 (2016) 305-311. DOI:10.1016/j.cclet.2015.12.021 |
| [7] |
S. Mitsuhashi, Curr. Opin. Biotech. 26 (2014) 38-44. DOI:10.1016/j.copbio.2013.08.020 |
| [8] |
W.M. Furuya, M. Michelato, A.L. Salaro, et al., Lat. Am. J. Aquat. Res. 43 (2015) 888-894. |
| [9] |
Q.Q. He, G.M. Fang, L. Liu, Chin. Chem. Lett. 24 (2013) 265-269. DOI:10.1016/j.cclet.2013.03.013 |
| [10] |
Y. Reynaud, C. Buffiere, B. Cohade, et al., Food Chem. 338 (2021) 128020. DOI:10.1016/j.foodchem.2020.128020 |
| [11] |
J.D. Xu, A. Lavan, Nat. Nanotechnol. 3 (2008) 666-670. DOI:10.1038/nnano.2008.274 |
| [12] |
E. Park, E.B. Campbell, R. MacKinnon, Nature 541 (2017) 500-505. DOI:10.1038/nature20812 |
| [13] |
J. Clatot, M. Hoshi, X.P. Wan, et al., Nat. Commun. 8 (2017) 2077. DOI:10.1038/s41467-017-02262-0 |
| [14] |
J.M. Rivera, M. Rivera, J. Porphyr. Phthalocya. 24 (2020) 577-588. DOI:10.1142/s1088424620500108 |
| [15] |
K. Ueno, T. Doi, B. Nanzai, M. Igawa, J. Membr. Sci. 537 (2017) 344-352. DOI:10.1016/j.memsci.2017.04.013 |
| [16] |
T. Tsuru, T. Shutou, S.I. Nakao, S. Kimura, Sep. Sci. Technol. 29 (1994) 971-984. DOI:10.1080/01496399408005611 |
| [17] |
Y.L. Ji, W.J. Qian, Q.F. An, et al., J. Ind. Eng. Chem. 66 (2018) 209-220. DOI:10.1016/j.jiec.2018.05.032 |
| [18] |
P.G. Ingole, H.C. Bajaj, K. Singh, Desalination 343 (2014) 75-81. DOI:10.1016/j.desal.2013.10.009 |
| [19] |
T. Ma, S. Chang, J. He, et al., Chem. Commun. 60 (2024) 150-167. DOI:10.1039/d3cc04851a |
| [20] |
T. Ma, L.L. Peng, Q.Y. Ran, et al., ACS Appl. Biol. Mater. 6 (2023) 5685-5694. DOI:10.1021/acsabm.3c00817 |
| [21] |
T. Ma, L.Z. Qiu, Y. Tao, et al., J. Alloys Compd. 979 (2024) 173477. DOI:10.1016/j.jallcom.2024.173477 |
| [22] |
T. Ogoshi, S. Takashima, T. Yamagishi, J. Am. Chem. Soc. 137 (2015) 10962-10964. DOI:10.1021/jacs.5b07415 |
| [23] |
J. Zhou, S.Z. Hou, J. Zhang, et al., Chin. Chem. Lett. 32 (2021) 725-728. DOI:10.1016/j.cclet.2020.07.039 |
| [24] |
S.M. Liu, H. Zhang, Y.Q. Wang, et al., Chin. Chem. Lett. 34 (2023) 107712. DOI:10.1016/j.cclet.2022.07.055 |
| [25] |
Q. Li, J.D. Sun, B. Yang, et al., Chin. Chem. Lett. 33 (2022) 1988-1992. DOI:10.1016/j.cclet.2021.10.017 |
| [26] |
R.C. Mutihac, A.A. Bunaciu, H.J. Buschmann, L. Mutihac, J. Incl. Phenom. Macro. 98 (2020) 137-148. DOI:10.1007/s10847-020-01019-5 |
| [27] |
M. Li, G. Qing, Y. Xiong, et al., Sci. Rep. 5 (2015) 15742. DOI:10.1038/srep15742 |
| [28] |
H. Zhang, X. Wu, Y. Yuan, et al., Food Chem. 265 (2018) 290-297. DOI:10.1016/j.foodchem.2018.05.090 |
| [29] |
B. de Luis, A. Morella-Aucejo, A. Llopis-Lorente, et al., Chem. Sci. 12 (2020) 1551-1559. |
| [30] |
Y.Q. Wang, L. Ding, H. Yu, et al., Chin. Chem. Lett. 33 (2022) 283-287. DOI:10.1016/j.cclet.2021.06.044 |
| [31] |
X.M. Zhou, X.J. Wang, L. Shang, Chin. Chem. Lett. 34 (2023) 108093. DOI:10.1016/j.cclet.2022.108093 |
| [32] |
L. Li, L. Liu, Z. Li, et al., Heliyon 5 (2019) e01714. DOI:10.1016/j.heliyon.2019.e01714 |
| [33] |
J. Liu, Y. Tang, W. Yang, et al., Biomater. Sci. 7 (2019) 1463-1476. DOI:10.1039/c8bm01611a |
| [34] |
Z.Z. Gao, J.L. Kan, L.X. Chen, et al., ACS Omega 2 (2017) 5633-5640. DOI:10.1021/acsomega.7b00429 |
| [35] |
A. Lamp, M. Kaltschmitt, O. Ludtke, Anal. Biochem. 543 (2018) 140-145. DOI:10.1016/j.ab.2017.12.009 |
| [36] |
S. Wakabayashi, H. Matsubara, C.H. Kim, et al., Biochem. Biophys. Res. Commun. 91 (1980) 1548-1554. |
 2025, Vol. 36
2025, Vol. 36 

