b School of Pharmacy, Fudan University, Shanghai 201203, China;
c Zhejiang Hongyuan Pharmaceutical Co., Ltd., Linhai 317016, China
Acute myeloid leukemia (AML) is the most common adult acute leukemia, which is a heterogeneous hematopoietic malignancy that arises from the dysregulation of cell differentiation, proliferation, and cell death [1,2]. The risk factors associated with the onset of AML include long-term exposure to radiation and chemicals such as benzene, smoking, genetic disorders, blood disorders, and others [3]. AML accounts for over 80,000 deaths globally per annum, with this number expected to double over the next two decades [1]. It is one of the worst survival rates of all cancers. The estimated 5-year overall survival is 31.7%, but only 9.4% for patients 65 years and older at diagnosis [1,4]. Strategies to treat AML, including a refinement of the conventional chemotherapy regimens, hypomethylating agents, and molecular targeted drugs (e.g., venetoclaxⓇ, midostaurinⓇ, enasidenibⓇ, and mylotargⓇ), have been developed in recent years. The advent of various small-molecule inhibitors and immune-targeted drugs has brought hope to patients who cannot tolerate intensive chemotherapy or with relapsed/refractory AML [5–7]. Nevertheless, AML remains a major area of unmet medical need among hematologic malignancies. Nature has been an important source of anti-cancer therapeutics, and nearly half of the currently marketed cancer drugs are derived from natural products (NPs) [8]. The management of AML with NPs remains a viable option.
Lindenane sesquiterpenoids (LDSs) and their oligomers, being the characteristic metabolites of the plants in the Chloranthaceae family (comprising the genera Chloranthus, Sarcandra, and Hedyosmum), have attracted considerable attention in recent decades due to their fascinating structures and interesting bioactivities [9–12]. So far, more than 230 LDS oligomers have been isolated and identified. Among them, only a dozen trimers have been reported hitherto, and the others were dimers [13–17]. Chloranthus holostegius var. trichoneurus is a rare Chloranthus species only distributed in Yunnan and Guizhou Provinces of China [18]. To date, only two LDS dimers have been reported from this plant [19]. In this study, as part of our ongoing search for novel bioactive compounds from the Chloranthus plants endemic to China [20–25], two LDS trimers [holotrichones A (1) and B (2)] equipped with intriguing skeletons were identified from the whole plant of C. holostegius var. trichoneurus.
An ultra performance liquid chromatography-photodiode array detector-mass spectrometry (UPLC-PDA-MS)-guided isolation strategy was introduced to investigate the potential novel LDS oligomers in our research. This, in combination with the application of selected databases and previously purified LDS oligomers on hand as authentic samples was employed for the dereplication in the crude fractions. In general, the EtOAc portion of the 90% MeOH extract of the whole plant of C. holostegius var. trichoneurus was subjected to silica gel column chromatography to yield ten fractions (Fr.1–Fr.10). Then all fractions were profiled by UPLC-PDA-MS (positive-ion mode) with a full scan and this analysis revealed that Fr.6 was rich in LDS oligomers (Fig. S1 in Supporting information). Fr. 6 was then further split into 10 subfractions by chromatography on middle chromatogram isolated (MCI) gel followed by extensive UPLC-PDA-MS analyses. As a result, two peaks related to the LDS trimers featuring previously undescribed molecular weights (MWs) (i.e., MW = 950 and 806) were recognized. Subsequently, target purification of the aforementioned peaks afforded compounds 1 (4.1 mg) and 2 (5.0 mg), respectively (Fig. 1). This is Part XXXII of the “Phytochemical and biological studies on rare and endangered plants endemic to China” series; for Part XXXI and XXX, see Zhou et al., 2024 [26] and Chen et al., 2024 [27], respectively.
|
Download:
|
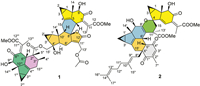
| Fig. 1. Chemical structures of holotrichones A (1) and B (2). | |
Holotrichone A (1), colorless crystals, possesses a molecular formula C54H62O15 as determined by its 13C nuclear magnetic resonance spectroscopy (NMR) data and high resolution electrospray ionization mass spectroscopy (HRESIMS) at m/z 973.3988 [M + Na]+ (calcd. 973.3981), requiring 24 indices of hydrogen deficiency (IHDs). The 13C NMR spectrum resolved 54 carbon resonances corresponding to two ketocarbonyls, five ester carbonyls, ten olefinic carbons, six sp3 quaternary carbons, 13 sp3 methines, eight sp3 methylenes, and ten methyls (two methoxys) (Table S1 in Supporting information) as distinguished by the distortionless enhancement by polarization transfer (DEPT) and heteronuclear singular quantum correlation (HSQC) NMR experiments. In the upfield region of its 1H NMR spectrum, three pairs of cyclopropane methylenes [δH 0.27 (ddd, J = 4.4, 4.3, 3.7 Hz, H-2β), 1.01 (ddd, J = 8.0, 7.7, 4.3 Hz, H-2α); 0.70 (ddd, J = 8.5, 8.3, 5.6 Hz, H-2′α), 1.18 (ddd, J = 5.6, 5.4, 5.3 Hz, H-2′β); 0.11 (ddd, J = 4.4, 4.2, 3.9 Hz, H-2′′′β), 0.88 (ddd, J = 8.0, 7.7, 4.2 Hz, H-2′′′α)] characteristic for the LDS [13] were observed. This, combining analysis of the molecular formula and 13C NMR data, showed that 1 is an LDS trimer, comprising three lindenane units (units A–C, Fig. 2).
|
Download:
|
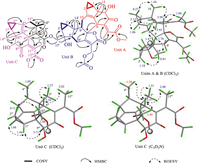
| Fig. 2. Key COSY, HMBC, and ROESY correlations of 1. | |
A comprehensive investigation of the 2D NMR spectra of 1 delineated its planar structure (Fig. 2). The data attribution of HSQC and heteronuclear multiple bond correlation (HMBC) unveiled that the structures and linkage of units A and B were identical to those of chlorahollide D, an LDS dimer often encountered from Chloranthus species [21,28–30]. The remaining 16 carbons (including one methoxy group) were then assigned to the third LDS moiety in unit C, which was fused with the 2-methylbutyryl chain (C-1′′ to C-5′′) of chlorahololide D. Based on the HMBC correlations from H-3′′ to C-4′′′/C-6′′′, from H3–4′′ to C-15′′′/C-2′′/C-3′′, and from H3–5′′ to C-1′′/C-2′′/C-3′′/C-6′′′, the 2-methylbutyryl fragment was linked to the third LDS moiety through the newly formed C-3′′–C-15′′′ and C-2′′–C-6′′′ bonds. A unique 3/5/6/6-fused unit C was thus constructed. Finally, the connection between units B and C was evidenced by the HMBC cross-peaks between H2–15′ and C-1′′.
The relative configuration of 1 was resolved via the interpretation of its rotating frame Overhauser effect spectroscopy (ROESY) spectrum (Fig. 2). For units A and B, the relative configuration was congruent with that of chlorahololide D, as judged from the ROE correlations of H-2β/H3–14, H3–14/H-6, H-1/H-3, H-1/H-9, H-9/H-5′, H-5′/H2–15′, H-2′β/H3–14′, H3–14′/H-9′. As for unit C, the correlations of H-1′′′/H-3′′′ and H-1′′′/H-9′′′ implied that these protons adopted α-orientations, whereas the ROE between H-2′′′β and H3–14′′′ designated their β-orientations. To distinguish the overlapped proton signals (H3–14′′′: δH 1.04 and H3–4′′: δH 1.05, in CDCl3) by which the configuration at C-2′′, C-3′′, and C-6′′′ cannot be determined, the 1H NMR and ROESY spectra were thereby re-acquired in pyridine-d5. As expected, the chemical shifts of H3–14′′′, H3–4′′, and H-6′′′ were downfield shifted to δH 1.44, 1.28, and 3.57 ppm, respectively. Strong ROE correlations of H-6′′′ with H3–4′′, H3–5′′, and H3–14′′′ were then discerned, which identified these protons as co-facial and β-orientated. The Z-geometry of Δ7′′′(11′′′) was confirmed by the ROE correlation between H-6′′′ and H3–13′′′. To further consolidate the relative configuration assignments for the newly formed chiral carbons of C-2′′, C-3′′ and C-6′′′ in unit C, GIAO (the Gauge Independent Atomic Orbital method) 1H and 13C NMR chemical shift calculations were performed using the corrected mean absolute error (CMAE) and DP4+ probability analyses (Tables S6, S7, and S10 in Supporting information) [31–34]. The PCM/mPW1PW91/6–311+G(d,p) level of theory was used for the analysis. The results of DP4+ probability analysis indicated that, among the eight diastereoisomers, (2″R*,3″S*,6′′′R*)−1a best matches with the experimental data of 1 with 100% probability (all DP4+ data, Table S4 in Supporting information). The carbon- and proton-CMAE analysis was also consistent with the DP4+ results.
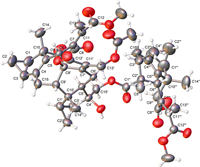
The absolute configuration of 1 was first corroborated by the electronic circular dichroism (ECD) calculation (Fig. 3) using time-dependent density functional theory (TDDFT) [35]. Finally, the whole structure of 1 with its absolute configuration (1R,3S,6R,9R,10S,1′R,3′S,4′S,5′R,8′S,9′S,10′S,2″R,3″S,1′′′R,3′′′S,6′′′R,9′′′R,10′′′S) was unequivocally established as shown by a single-crystal X-ray diffraction study using Ga Kα radiation through a Flack parameter of 0.00(6) (Fig. 4 and Table S3 in Supporting information, CCDC 2260115).

|
Download:
|
| Fig. 3. Experimental and calculated ECD spectra of 1 and 2. | |
|
Download:
|
| Fig. 4. Olex2 drawing of 1. | |
Holotrichone B (2) was obtained as a yellow oil and presented a molecular formula of C49H58O10, according to the protonated HRESIMS ion at m/z 807.4104 (calcd. for C49H59O10, 807.4103) and 13C NMR data (Table S2 in Supporting information), indicating 21 IHDs. The 1H NMR spectrum showed signals for two sets of cyclopropane methylenes [δH 0.43 (ddd, J = 6.3, 4.4, 4.2 Hz, H-2β), 0.78 (ddd, J = 9.0, 8.8, 6.3 Hz, H-2α); 0.24 (ddd, J = 4.0, 3.9, 3.7 Hz, H-2′β), 0.83 (ddd, J = 7.9, 7.8, 4.0 Hz, H-2′α)], indicating the presence of two LDS units (A and B, Fig. S2 in Supporting information) in 2. Comparison of the 1H and 13C NMR data between 1 and 2 indicated they both contain a similar LDS-type unit A (Tables S1 and S2). Further analysis disclosed that the dimeric LDS moiety (i.e., units A and B) of 2, were closely related to spirolindemer A (an LDS dimer obtained from C. Henryi, Fig. S2) [16]. Unlike the known structure with a ketone at C-9′, a hydroxy substituent was attached to C-9′ (δH 4.35 for H-9′ and 2.98 for OH-9′, δC 73.5 for C-9′) in 2, which was evidenced by the HMBC correlations of H3–14′/C-9′, H-9′/C-7′, and 9′-OH/C-8′ (Fig. S2). Meanwhile, the HMBC correlations from H2–15 to C-7′/C-11′/C-12′ and from H3–13′ to C-15/C-7′/C-11′/C-12′, confirmed the unique linkage of C-15–C-11′. This, along with the similar chemical shifts of C-4 (δC ~84) and C-8′ (δC ~147) to those of spirolindemer A, suggesting that the units A and B in 2 feature the same dimeric LDS skeleton equipped with an oxaspiro[4,5]-decane unit.
Differing from spirolindemer A (Fig. S2), neither the exocyclic double bond of Δ4′(15′) nor the vinyl methyl group of Me-15′ was observed in the unit B of 2. This implied another polymerization of unit C occurring here. The remaining 17 carbons (from C-1′′ to C-17′′, Table S2) ascribed to unit C comprises two keto-carbonyls, six olefinic carbons, one sp3 quaternary carbon, one sp3 methine, three sp3 methylenes, and four vinyl methyls. The presence of a 2-methylcyclohex-2-en-1,4–dione unit [36] was then delineated by 2D NMR data (Fig. S2). The successive 1H–1H correlation spectroscopy (COSY) correlations of H2–8′′–H-9′′–H3–15′′–H2–11′′–H2–12′′–H-13′′–H3–16′′/H3–17′′, and the HMBC correlations of H3–17′′ to C-13′′/C-14′′/C-16′′ and H3–15′′ to C-9′′/C-10′′/C-11′′, allowed the construction of the geranyl sidechain. Meanwhile, the 2-methylcyclohex-2-en-1,4–dione moiety was substantiated by the two carbonyls resonating at δC 200.0 and 202.0 typical for enones, and the HMBC correlations from H3–7′′ to C-1′′/C-2′′/C-3′′, from H-3′′ to C-1′′/C-5′′, and from H-5′′ to C-4′′/C-6′′. The geranyl sidechain was linked to C-6′′ as confirmed by the HMBC cross-peaks from H2–8′′ to C-1′′ and C-6′′. The geranylated 1,4-benzoquinone fragment was then connected to unit B by the two linkage bonds of C-6′–C-5′′ and C-15′–C-6′′, furnishing the formation of an additional hexene ring, based on the COSY correlation of H-5′′/H-6′ and the key HMBC correlations from H-15′ to C-1′′ and from H-5′′ to C-5′/C-6′/C-15′. Taken together, the structure of 2 was established as a 3/5/6/6/3/5/6/6/6-fused nonacyclic conjugate of a unique spirocyclic LDS dimer and a geranylated 1,4-benzoquinone derivative.
The strong ROE correlations (Fig. S2) of H-1/H-3, H-1/H-9, H-2β/H3–14, OH-9/H3–14, H-15β/H3–14, H-6/H3–13, H-1′/H-3′, and H-2′β/H3–14′ verified that the relative configuration of the spirocyclic LDS moiety in 2 was generally coincident with spirolindemer A. The 9′-OH group was assigned to be β-oriented based on the ROE correlation of H-9′ with H-1′. For unit C and the newly formed chiral center C-6′, their relative configurations were resolved on account of ROESY data. The strong ROE correlations of H-6′ with H3–14′, H-5′′, and H-8′′b denoted their β-orientations, while that between H2–8′′ and H3–15′′ demonstrated that Δ9′′ adopted the E-geometry. Nevertheless, how to determine the stereochemistry at C-11′ remained to be a challenge. Due to the flexibility of the spiroring, the ROESY data could not afford convincible evidence. After many attempts, the single crystal of 2 was still unavailable. Therefore, the quantum chemical calculations of 1H and 13C NMR chemical shifts [31–34] of 2 were applied to corroborate the configuration of C-11′. The calculation results confirmed that the relative configuration at C-11′ in 2 as R* with a full DP4+ probability of 100% (Table S5 in Supporting information). Subsequently, the application of TDDFT-ECD [35] calculations (Fig. 3, Fig. S4 and Table S12 in Supporting information) of compound 2 assigned the absolute configuration. The tendencies of the calculated ECD curve of (1R,3S,4R,9R,10S,1′R,3′S,6′R,9′R,10′S,11′R,5″S,6″R)−2 roughly matched the experimental one (Fig. 3). It is worth to note that C-9 and C-10 in the similar LDS-type unit A in both compounds 1 and 2 have the same absolute configurations, which are consistent with our previous findings from other Chloranthus plants [20,21].
Notably, compound 1 is the first example of an LDS trimer incorporating a unique 3/5/6/6-fused carbon skeleton. Compound 2 is an unprecedented hybrid of an LDS dimer and a p-benzoquinone-meroterpenoid, featuring an unusual 3/5/6/6/3/5/6/6/6 ring system. To our knowledge, so far only five LDS hetero-trimers have been previously obtained from the Lindera genus [9]. This is the first representative of LDS hetero-trimers being isolated from the whole Chloranthaceae family. Also, compound 2 features an unusual oxaspiro[4.5]decane core for the LDS dimer moiety, and only two examples with the same unit were previously reported from the Chloranthus genus [16].
A putative biogenetic pathway for 1 and 2 is proposed (Scheme 1). Given the abundant sesquiterpenoid monomer in this genus, chloranthalactone A could serve as the monomeric precursor [16,37]. The intermediate i, which was suggested to originate from chloranthalactone A by oxidation, would give the vital intermediate ii by lactone opening and olefination. The intermediate chlorahololide D would be then produced by a homo-Diels-Alder addition between ii and chloranthalactone A followed by diacylation with a 2-methylbutyroxyl group attached to C-15′ and an acetoxyl at C-13′. Subsequently, the homo-Diels-Alder addition between the 2-methylbutyryl substituent of chlorahololide D and the conjugated diene moiety of ii would furnish 1. Two molecules of ii underwent a key hetero-Diels-Alder reaction to construct the oxspiro[4.5]decane skeleton (iii). A further [4 + 2] addition of iii with the geranylated 1,4-benzoquinone could afford the unique hybrid 2.

|
Download:
|
| Scheme 1. Proposed biogenetic pathway of compounds 1 and 2. | |
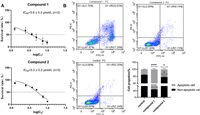
Structurally diverse naturally occurring sesquiterpenes [3,38–41] and their synthesized dimeric derivatives [42,43] have been extensively reported to show cytotoxic activities against different AML cell lines. Considering the complexity of AML, it is impossible to identify a single cell line to establish an ideal screening model [44]. Herein, the cytotoxic activities of compounds 1 and 2 against two human AML cell lines MV-4–11 and HL-60 were evaluated. MV-4–11 is a cell line established from the blast cells of a 10-year-old male with biphenotypic B-myelomonocytic leukemia, while HL-60 is a leukemic promyelocytic cell line [44]. The well-known anti-leukemia sesquiterpenoid, dimethylaminoparthenolide (DMAPT) was used as the positive control [45]. Compounds 1 and 2 displayed noticeable cytotoxicities against the MV-4–11 cells, with half-maximal inhibitory concentration (IC50) values of 3.6 and 5.2 µmol/L, respectively (Fig. 5A), while DMAPT possessed an IC50 vaule of 4.8 µmol/L. In contrast, they were judged inactive on the HL-60 cell line (cell viability >50% at the concentration of 10 µmol/L, data not shown). To investigate the potential cytotoxic mechanism, a cell apoptosis assay was performed with an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay at 4 µmol/L. The results showed that both compounds 1 and 2 significantly induced apoptosis in MV-4–11 cells compared with untreated cells (P < 0.0001 and P < 0.05 vs. control, respectively), while cell necrosis was rarely seen. Among them, the apoptosis rate of compound 1 (with better apoptotic activity) was significantly higher than that of compound 2 (Fig. 5B). The bars represent the percentage of apoptotic and non-apoptotic cells as described above (n = 3). The different responses of the two cell lines to the holotrichones have provided hints of a potential unknown mechanism which needs to be further explored.
|
Download:
|
| Fig. 5. Cytotoxicity IC50 values (A) and cell apoptosis assays (B) for 1 and 2 in MV-4–11 cells. Each bar represents the mean ± standard deviation (SD) (n = 3). P < 0.05, ****P < 0.0001 compared with the control group. | |
In summary, two undescribed LDS trimers, holotrichones A (1) and B (2), were isolated from the rare Chloranthus plant C. holostegius var. trichoneurus. Compound 1 represents the first LDS trimer incorporating a unique 3/5/6/6-fused carbon skeleton of an LDS monomer and the 2-methylbutyryl substituent bridged by a six-membered ring system. Compound 2, possessing an uncommon oxaspiro[4.5]decane ring system, is the first hybrid of an LDS dimer and a benzoquinone-meroterpenoid. Biogenetically, homo- and/or hetero-Diels-Alder additional reactions were proposed to be involved in the formation of the two unique LDS trimers. Compounds 1 and 2 displayed potent cytotoxic effects against MV-4–11 leukemia cells, likely associated with inducing celluar apoptosis. In this study, the chemically distinctive findings extend the diversity of sesquiterpenoid oligomers and would provide remarkable potential for the further investigation of Chloranthus-originated LDS as novel anti-leukemic agents.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by grants from the National Natural Science Foundation of China (Nos. 21772025, 21937002). We also would like to express our sincere gratitude to Dr. Mark T. Hamann (Colleges of Pharmacy and Medicine at Medical University of South Carolina) and Dr. Yeun-Mun Choo (Chemistry Department at University of Malaya) for their valuable comments, which have greatly improved this paper.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109682.
| [1] |
C.D. DiNardo, H.P. Erba, S.D. Freeman, A.H. Wei, Lancet 401 (2023) 2073-2086. DOI:10.1016/S0140-6736(23)00108-3 |
| [2] |
S. Shimony, M. Stahl, R.M. Stone, Am. J. Hematol. 98 (2023) 502-526. DOI:10.1002/ajh.26822 |
| [3] |
C. Egbuna, K.C. Patrick-Iwuanyanwu, E.N. Onyeike, et al., Food Sci. Nutr. 11 (2023) 4191-4210. DOI:10.1002/fsn3.3420 |
| [4] |
SEER Program, “Cancer stat facts: leukemia—acute myeloid leu-kemia (AML)”, https://seer.cancer.gov/statfacts/html/amyl.html, 2023 (accessed: Dec 10th 2023).
|
| [5] |
P. Stelmach, A. Trumpp, Haematologica 108 (2023) 353-366. DOI:10.3324/haematol.2022.280800 |
| [6] |
H. Kantarjian, T. Kadia, C. DiNardo, et al., Blood Cancer J. 11 (2021) 41. DOI:10.1038/s41408-021-00425-3 |
| [7] |
S.R. Bohl, L. Bullinger, F.G. Rücker, Int. J. Mol. Sci. 20 (2019) 1983. DOI:10.3390/ijms20081983 |
| [8] |
D.J. Newman, G.M. Cragg, J. Nat. Prod. 83 (2020) 770-803. DOI:10.1021/acs.jnatprod.9b01285 |
| [9] |
J. Luo, D. Zhang, P. Tang, et al., Nat. Prod. Rep. 41 (2024) 25-58. DOI:10.1039/D3NP00022B |
| [10] |
M. Zhang, D. Liu, G. Fan, et al., Heterocycl. Commun. 22 (2016) 175-220. DOI:10.1515/hc-2016-0084 |
| [11] |
A.R. Wang, H.C. Song, H.M. An, et al., Chem. Biodivers. 12 (2015) 451-473. DOI:10.1002/cbdv.201300376 |
| [12] |
J.X. Li, Z.R. Cui, Y.Y. Li, et al., Chin. Chem. Lett. 33 (2022) 4257-4260. DOI:10.1016/j.cclet.2022.01.084 |
| [13] |
J. Kawabata, E. Fukushi, J. Mizutani, Phytochemistry 47 (1998) 231-235. DOI:10.1016/S0031-9422(97)00419-6 |
| [14] |
J. Chi, S. Wei, H. Gao, et al., J. Org. Chem. 84 (2019) 9117-9126. DOI:10.1021/acs.joc.9b00986 |
| [15] |
J.S. Zhou, Q.F. Liu, F.M. Zimbres, et al., Org. Chem. Front. 8 (2021) 1795-1801. DOI:10.1039/D1QO00089F |
| [16] |
J. Li, J. Chi, P. Tang, et al., Chin. J. Chem. 40 (2022) 603-608. DOI:10.1002/cjoc.202100743 |
| [17] |
S. Wang, Y. Sun, Y. Li, et al., J. Org. Chem. 88 (2023) 347-354. DOI:10.1021/acs.joc.2c02372 |
| [18] |
N. Xia, J. Joël, Chloranthaceae, in: Z. Y. Wu, P. H. Raven (Eds.), Flora of China, Vol. 4, Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, 1999, pp. 132–138.
|
| [19] |
J. Chi, W. Xu, S. Wei, et al., Org. Lett. 21 (2019) 789-792. DOI:10.1021/acs.orglett.8b04046 |
| [20] |
X.J. Wang, J.L. Xin, S.Z. Yu, et al., Nat. Prod. Res. 37 (2023) 882-890. DOI:10.1080/14786419.2022.2095633 |
| [21] |
X.J. Wang, S.Z. Yu, J.L. Xin, et al., Fitoterapia 156 (2022) 105068. DOI:10.1016/j.fitote.2021.105068 |
| [22] |
J. Xiong, Z.L. Hong, P. Xu, et al., Org. Biomol. Chem. 14 (2016) 4678-4689. DOI:10.1039/C6OB00731G |
| [23] |
J. Xiong, Z.L. Hong, J. Shen, et al., J. Org. Chem. 80 (2015) 11080-11085. DOI:10.1021/acs.joc.5b01658 |
| [24] |
L.J. Wang, J. Xiong, S.T. Liu, et al., J. Nat. Prod. 78 (2015) 1635-1646. DOI:10.1021/acs.jnatprod.5b00195 |
| [25] |
S.T. Liu, J. Xiong, Y. Tang, et al., Chem. Biodivers. 11 (2014) 904-909. DOI:10.1002/cbdv.201300247 |
| [26] |
P.J. Zhou, T. Huang, G.L. Ma, et al., J. Mol. Struct. 1305 (2024) 137754. DOI:10.1016/j.molstruc.2024.137754 |
| [27] |
H.W. Chen, X.Y. Wu, Z.Y. Zhao, et al., Phytochemistry 219 (2024) 113963. DOI:10.1016/j.phytochem.2023.113963 |
| [28] |
B. Bai, S.X. Ye, D.P. Yang, et al., J. Nat. Prod. 82 (2019) 407-411. DOI:10.1021/acs.jnatprod.8b00418 |
| [29] |
S.Y. Kim, Y. Kashiwada, K. Kawazoe, et al., Chem. Pharm. Bull. 59 (2011) 1281-1284. DOI:10.1248/cpb.59.1281 |
| [30] |
S.P. Yang, Z.B. Gao, Y. Wu, G.Y. Hu, J.M. Yue, Tetrahedron 64 (2008) 2027-2034. DOI:10.1016/j.tet.2007.12.057 |
| [31] |
A. Sikandar, A. Popoff, R.P. Jumde, et al., Angew. Chem. Int. Ed. 62 (2023) e202306437. DOI:10.1002/anie.202306437 |
| [32] |
J. Xiong, P.J. Zhou, H.W. Jiang, et al., Angew. Chem. Int. Ed. 60 (2021) 22270-22275. DOI:10.1002/anie.202109082 |
| [33] |
P.J. Zhou, Y. Zang, C. Li, et al., Chin. Chem. Lett. 33 (2022) 4264-4268. DOI:10.1016/j.cclet.2021.12.009 |
| [34] |
N. Grimblat, M.M. Zanardi, A.M. Sarotti, J. Org. Chem. 80 (2015) 12526-12534. DOI:10.1021/acs.joc.5b02396 |
| [35] |
T. Bruhn, A. Schaumloffel, Y. Hemberger, G. Bringmann, Chirality 25 (2013) 243-249. DOI:10.1002/chir.22138 |
| [36] |
M. Resch, A. Steigel, Z.L. Chen, R. Bauer, J. Nat. Prod. 61 (1998) 347-350. DOI:10.1021/np970430b |
| [37] |
W.Y. Tsui, G.D. Brown, Phytochemistry 43 (1996) 819-821. DOI:10.1016/0031-9422(96)00352-4 |
| [38] |
Y. Feng, Y. Han, A. Hu, et al., Acta Pharm. Sin. B 13 (2023) 598-617. DOI:10.1016/j.apsb.2022.10.018 |
| [39] |
L.M.R. de Novais, L.F. Ferreira, P.T. de Sousa Jr, et al., Planta Med. 86 (2020) 55-60. DOI:10.1055/a-1021-0611 |
| [40] |
I. Nepstad, K.J. Hatfield, I.S. Grønningsæter, H. Reikvam, Int. J. Mol. Sci. 21 (2020) 2907. DOI:10.3390/ijms21082907 |
| [41] |
P.E. Ordóñez, K.K. Sharma, L.M. Bystrom, et al., J. Nat. Prod. 79 (2016) 691-696. DOI:10.1021/acs.jnatprod.5b00383 |
| [42] |
V. Janganati, J. Ponder, C.T. Jordan, et al., J. Med. Chem. 58 (2015) 8896-8906. DOI:10.1021/acs.jmedchem.5b01187 |
| [43] |
X.H. Lu, T. Efferth, Semin. Cancer Biol. 68 (2021) 291-312. DOI:10.1016/j.semcancer.2020.05.016 |
| [44] |
R. Skopek, M. Palusińska, K. Kaczor-Keller, et al., Int. J. Mol. Sci. 24 (2023) 5377. DOI:10.3390/ijms24065377 |
| [45] |
Y.H. He, Q.X. Li, Y.F. Wu, et al., J. Org. Chem. 87 (2022) 6927-6933. DOI:10.1021/acs.joc.2c00318 |
 2024, Vol. 35
2024, Vol. 35 

