b Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610054, China;
c College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Ji’nan 250014, China;
d Institute for Advanced Study, Chengdu University, Chengdu 610106, China;
e Department of Chemistry, College of Science, King Khalid University, P.O. Box 9004, 61413 Abha, Saudi Arabia
Nitrogen, essential in biogeochemistry, manifests in forms such as ammonia (NH3), hydrazine (N2H4), and nitrite/nitrate (NO2–/NO3–), vital for Earth's sustainability. NH3 stands out, widely used in agriculture and as a high-energy, zero-emission green energy source [1-4]. Traditionally, NH3 production has relied on the energy-intensive Haber-Bosch process, resulting in significant energy consumption and carbon emissions [5]. The electrochemical N2 reduction reaction (NRR) has gained considerable attention as a sustainable alternative for NH3 synthesis, operating at room temperature and driven by renewable energy sources. Nevertheless, NRR faces challenges related to the robustness of the N≡N triple bond, N2 solubility in water, and the simultaneous hydrogen evolution reaction (HER), limiting its broad applicability [6-15]. In contrast, nitrate (NO3–) features a lower bond energy, high solubility in aqueous solutions, and a superior theoretical reaction potential, rendering the electrochemical NO3– reduction reaction (NO3–RR) a more appealing option than NRR [16-20]. Moreover, excessive NO3– in wastewater from agricultural and factory wastewater sources poses health risks. Remediation techniques, like ion exchange and reverse osmosis, help, but often leave high NO3– level [18,21,22]. The NO3–RR process is thus promising, transforming NO3– into valuable NH3 and fostering global nitrogen equilibrium, embodying sustainability. However, NH3 production via NO3–RR involves an eight-electron transfer process, potentially leading to by-product formation. Given these considerations, the development of efficient and highly selective electrocatalysts for NO3–RR is an urgent research imperative.
Noble metal-based catalysts (e.g., Rh [23], Ru [24], Pt [25]) proficiently facilitate the conversion of NO3– to NH3, yet their prohibitive costs and scarce availability constrain their practical utilization. In recent years, many studies have focused on developing transition metal-based catalysts with abundant resources, low cost, and high electrocatalytic activity as efficient NO3–RR [26,27]. In this context, cobalt (Co) has emerged as a notable contender, with recent theoretical and empirical studies showing its efficacy in facilitating NH3 synthesis via NO3–RR among transition metals [28-30]. Co-based catalysts, specifically oxide [31], sulfide [32], and phosphide [33], have showcased exceptional selectivity and catalytic efficiency in this conversion process. Distinguished among its counterparts, Co-based selenide boasts near-metallic electrical conductivity, drawing considerable interest for its operational durability, and remarkable hydrogen adsorption capacity, which are pivotal for catalytic hydrogenation reactions [34-37]. Moreover, selenides have emerged as attractive and multi-functional electrocatalysts due to their high catalytic activity, tunable band gap, and good durability [38,39]. Shen et al. reported selenium vacancy-rich WSe2 (WSe2-x) nanosheets as electrocatalysts capable of electrochemically converting NO3– to NH3 with a high FE of 92.7% [40]. From a morphological perspective, the one-dimensional nanowire structure provides specific anti-aggregation ability, anisotropy, and an uninterrupted electron transfer pathway, reducing charge transfer resistance and enhancing catalytic performance [41,42]. Therefore, it is anticipated that Co-based selenide nanowire could facilitate a more efficient generation of NH3 through the NO3–RR process.
Herein, we present a CoSe2 nanowire array on carbon cloth (CoSe2/CC) as a highly efficient electrocatalyst for NH3 production via NO3–RR. Operational in a 0.1 mol/L NaOH electrolyte containing 0.1 mol/L NO3–, this CoSe2/CC catalyst delivered an impressive NH3 yield of 517.7 µmol h–1 cm–2 and achieved a Faradaic efficiency (FE) of 97.6% at –0.6 V versus the reversible hydrogen electrode (RHE). Notably, the CoSe2/CC maintained exceptional stability during continuous electrochemical operations. Additionally, insights into the reaction mechanism of NO3–RR on CoSe2 were gleaned through comprehensive density functional theory (DFT) calculations, further elucidating the catalytic process.
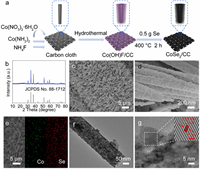
The CoSe2 nanowire array on CC was fabricated via straightforward hydrothermal and selenization processes (Fig. 1a), with details provided in Supporting information. The X-ray diffraction (XRD) analysis, shown in Fig. 1b, validates the characteristic peaks of CoSe2 (JCPDS No. 88–1712) [43], with the broad diffraction peak at ~26° corresponding to the CC substrate (Fig. S1 in Supporting information) [44]. As illustrated in Fig. S2 (Supporting information), CoSe2/CC can still be obtained by changing the annealing temperature or the heating rate during the selenization process, while the product becomes CoSe2@Co3Se4@CoO/CC when less Se powder was added. Scanning electron microscopy (SEM) images depicted in Fig. S3 (Supporting information) demonstrate the integral encapsulation of the CC by Co(OH)F nanowire. After selenization, the CoSe2/CC maintains its nanowire array with an enhanced thickness and roughness of the nanowires (Figs. 1c and d). Meanwhile, CoSe2/CC-500 and CoSe2/CC-10 acquired by annealing at different temperatures and heating rates, respectively, still maintained the nanowire array structure (Fig. S4 in Supporting information). The energy-dispersive X-ray (EDX) mapping, illustrated in Fig. 1e, confirms the uniform distribution of Co and Se elements within the nanowire arrays. Moreover, the EDX spectrum shows the absence of F element in the prepared CoSe2/CC (Fig. S5 in Supporting information). Transmission electron microscopy (TEM) image in Fig. 1f further show a nanowire characteristic. High-resolution TEM (HRTEM) image shows a lattice spacing of 0.26 nm, matching the (210) facet of CoSe2 (Fig. 1g). The surface elemental composition and valence states of CoSe2 were analyzed by X-ray photoelectron spectroscopy (XPS). As illustrated in Fig. S6 (Supporting information), the Co 2p spectrum can be roughly separated into Co 2p3/2 and Co 2p1/2 peaks. The peaks at 778.4 eV and 779.3 eV in Co 2p3/2 correspond to Co3+ and Co2+, respectively, while the Co 2p1/2 peaks are at 793.4 eV for Co3+ and 796.0 eV for Co2+ [45,46]. The presence of Co2+ indicates that the material is a CoSe2 crystal, while the formation of Co3+ corresponds to the partial surface oxidation of CoSe2 in air. In aspects of Se 3d (Fig. S7 in Supporting information), two peaks for Se 3d3/2 (55.6 eV) and Se 3d5/2 (54.6 eV) are attributed to Se2–. Moreover, the additional peaks at the interval 57–65 eV all belong to Co 3p1/2, Co 3p3/2, and SeO2 [47]. Using XPS analysis for element F, there was no characteristic peak of F detected in the F 1s spectrum, further verifying that no residual F element exists in CoSe2/CC (Fig. S8 in Supporting information). The above findings demonstrate that the preparation of CoSe2 loaded on carbon cloth was successful.
|
Download:
|
| Fig. 1. (a) Schematic diagram of the fabrication process of CoSe2/CC. (b) XRD pattern of CoSe2/CC. (c) Low and (d) high magnification SEM images of CoSe2/CC. (e) SEM and corresponding EDX mapping images of CoSe2/CC. (f) TEM and (g) HRTEM images of CoSe2. | |
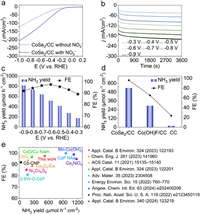
The electrocatalytic NO3–RR performance of CoSe2/CC was evaluated in both NO3–-free and NO3–-containing 0.1 mol/L alkaline electrolyte. The possible NO3–RR products, like NH3, N2H4, and NO2–, were quantified using distinct colorimetric methods, with their respective UV–vis spectra and calibration curves detailed in Figs. S9-S11 (Supporting information). Initial assessment of CoSe2/CC's NO3–RR electrocatalytic efficiency employed linear sweep voltammetry (LSV) curves. As depicted in Fig. 2a, the current density (j) of CoSe2/CC significantly increased upon the introduction of 0.1 mol/L NO3– into the 0.1 mol/L NaOH electrolyte. Comparative LSV tests of pure CC, the precursor Co(OH)F/CC, and CoSe2 powder/CC under identical conditions (Figs. S12 and S13 in Supporting information) revealed lower j for both pure CC (–71.2 mA/cm2), Co(OH)F/CC (–97.6 mA/cm2), and CoSe2 powder/CC (–128.0 mA/cm2), as compared to CoSe2/CC (–207.6 mA/cm2) at –1.0 V. We further compared the three catalysts (CoSe2/CC, CoSe2/CC-500, and CoSe2/CC-10) generated under different preparation conditions for NO3–RR. Preliminarily, it was found that the j of these three catalysts did not differ much within the alkaline electrolyte with and without NO3– (Fig. S14 in Supporting information). Chronoamperometry (CA) tests, conducted for 1 h at various potentials ranging from –0.3 V to –0.9 V (Fig. 2b), further probed CoSe2/CC's NO3–RR capabilities. The UV–vis spectra post-CA tests (Fig. S15 in Supporting information) exhibited progressively intensifying absorption peak intensities with increased negative potentials, indicating a preference for NH3 synthesis at higher negative potentials. Fig. 2c illustrates that the NH3 yield of CoSe2/CC increased consistently with more negative operating potentials, reaching a peak yield of 818.0 µmol h–1 cm–2 at –0.9 V. Additionally, the FE curve displayed a volcano-shaped profile, achieving a maximum FE of 97.6% at –0.6 V, correlating with an NH3 yield of 517.7 µmol h–1 cm–2, thereby confirming the superior NO3–RR performance for NH3 synthesis. The electrochemical NO3– reduction to NH3 capabilities of Co(OH)F/CC, CC, and CoSe2 powder/CC were compared under identical conditions at –0.6 V (Fig. 2d and Fig. S16 in Supporting information). The results showed that the NH3 yields and FEs for Co(OH)F/CC (292.6 µmol h–1 cm–2, 65.3%), CC (19.7 µmol h–1 cm–2, 23.6%), and CoSe2 powder/CC (150.1 µmol h–1 cm–2, 71.9%) were significantly lower than those of CoSe2/CC (517.7 µmol h–1 cm–2, 97.6%). Also, the NH3 yields and FEs of CoSe2–500 (498.0 µmol h–1 cm–2, 90.0%) and CoSe2/CC-10 (438.2 µmol h–1 cm–2, 83.4%), prepared under different conditions, were only slightly lower than that of CoSe2/CC (Fig. S17 in Supporting information). Remarkably, the highest NH3 yield and FE of CoSe2/CC surpass those of some recently reported NO3–RR electrocatalysts (Fig. 2e and Table S1 in Supporting information). This exceptional NO3–RR performance of CoSe2/CC substantiates the role of CoSe2 as the active phase for efficient and highly selective electrocatalytic conversion of NO3– to NH3.
|
Download:
|
| Fig. 2. (a) LSV curves of CoSe2/CC in 0.1 mol/L NaOH (contain or exclude 0.1 mol/L NO3–). (b) CA of CoSe2/CC at different given working potentials. (c) NH3 yields and FEs of CoSe2/CC at each operating potential. (d) Comparison of NH3 yields and FEs of CoSe2/CC, Co(OH)F/CC, and CC at –0.6 V. (e) Comparison of NH3 yields and FEs of CoSe2/CC with recently reported NO3–RR electrocatalysts. | |
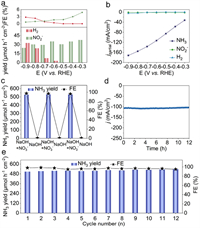
Since the electrochemical synthesis of NH3 from NO3– involves an eight-electron transfer process, the formation of by-products (e.g., NO2–, N2H4, and H2) is possible. Remarkably, our study observed no production of N2H4 across all tested potentials (Fig. S18 in Supporting information). Furthermore, the yields and FEs of NO2– and H2 generated during the electrocatalytic NO3–RR were minimal, almost negligible compared to NH3 (Fig. 3a). This low yield of H2 and its FEs indicate that the CoSe2/CC catalyst effectively suppresses the competing HER. The partial current densities (jpartial) of different products in the electrolyte are shown in Fig. 3b. At –0.9 V, the jpartial for NH3 (–172.1 mA/cm2) is significantly higher than that for NO2– (–1.3 mA/cm2) and H2 (–5.2 mA/cm2), further demonstrating CoSe2/CC's exceptional selectivity for the electroreduction of NO3– to NH3. To confirm the source of the detected NH3, control experiments were conducted on CoSe2/CC. The NH3 yield after 1-h electrolysis at open-circuit potential (OCP) was comparable to that in fresh electrolyte (Fig. S19 in Supporting information), ruling out contamination from experimental equipment or reagents. Alternating tests in NO3–-containing and NO3–-free alkaline electrolytes at –0.6 V (Fig. 3c and Fig. S20 in Supporting information) revealed high NH3 yields and FEs only in NO3–-containing solutions. Additionally, 15N isotope labeling experiments, analyzed via 1H nuclear magnetic resonance (1H NMR) spectra, validated the nitrogen origin in NH3. The 1H NMR spectra (Fig. 3d) showed distinct peaks for 15NH4+ and 14NH4+, confirming that the NH3 originated from the electrocatalytic reduction of NO3–, not external sources. Stability is crucial for assessing an electrocatalyst's performance. We conducted a 12-h continuous NO3–RR test on CoSe2/CC at –0.6 V. Fig. S21 (Supporting information) shows consistent j around –100 mA/cm2 throughout the test, indicating stable long-term operation. Additionally, 12 repeated cycles at the optimal NH3-producing potential (–0.6 V) demonstrated CoSe2/CC's excellent reproducibility. The NH3 yields and FEs, based on overlapping CA curves, accumulated charges, and corresponding UV–vis spectra, exhibited only minor variations (Fig. 3e and Fig. S22 in Supporting information). Significantly, XRD and SEM of CoSe2/CC after 12-h electrolysis (Figs. S23 and S24 in Supporting information) show that its crystalline phase and nanowire array structure have remained almost unchanged. Those conclusions demonstrate the durable electrocatalytic activity of CoSe2/CC in the NO3–RR.
|
Download:
|
| Fig. 3. (a) FEs and yields of NO2– and H2 of CoSe2/CC at each working potential. (b) jpartial of NH3, NO2–, and H2 of CoSe2/CC examined form the voltage range of –0.3 V to –0.9 V. (c) NH3 yields and FEs of CoSe2/CC during the alternating cycling tests. (d) 1H NMR spectra for the post-electrolysis electrolyte with Na15NO3 and Na14NO3 as the nitrogen resources. (e) NH3 yields and FEs under the 12 consecutive cycle tests for CoSe2/CC at −0.6 V. | |
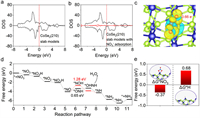
DFT computations were performed to elucidate the exceptional NO3–RR properties of CoSe2. The CoSe2(210) slab model in Fig. S25 (Supporting information) was constructed based on the HRTEM results. As indicated by the calculated electronic density of states (DOS) in Fig. 4a, CoSe2 exhibits a local density of states near the Fermi level, confirming its metallic behavior as established in prior studies [48]. Fig. 4b illustrates that the electronic state of CoSe2(210) with adsorbed NO3– is more pronounced around the Fermi level compared to the bare CoSe2(210), implying that NO3– adsorption boosts the electrical conductivity of CoSe2, thereby facilitating electron transfer during the electrocatalytic process. This is further corroborated by charge density differences and Bader charge analysis (Fig. 4c), which reveal that CoSe2(210) donates a charge of 0.66 e– to *NO3, enhancing the activation of N—O bonds and catalyzing the NO3–RR process. The free energy landscape of NO3–RR on CoSe2(210) surface was mapped out to gain deeper insights into the mechanism (Fig. 4d), with the optimized configurations of NO3–RR intermediates (Fig. S26 in Supporting information). The potential-determining step (PDS) of the NO3–RR involves the protonation of *NO to *ONH, exhibiting a relatively low energy barrier of 0.65 eV. This underscores the facilitation of NO3–RR on the CoSe2(210) surface. Additionally, the competitive HER was also investigated, given its prominence as a competing reaction in NO3–RR [49,50]. The free energy of *NO3 (–0.37 eV) on the CoSe2(210) surface, depicted in Fig. 4e, is significantly more negative than that of *H (0.68 eV), suggesting that the CoSe2(210) surface effectively suppresses the HER side reaction, thereby enhancing the overall efficiency and selectivity of NO3–RR to NH3 conversion.
|
Download:
|
| Fig. 4. (a, b) DOS of CoSe2(210) slab models without and with NO3– adsorption. (c) Differential charge density for NO3– adsorption configuration on CoSe2(210), the yellow and cyan representing electron accumulation and depletion, respectively. Isosurfaces are set to 0.003 e/Å3. (d) Free energy diagram of NO3–RR on CoSe2(210) surface. (e) Adsorption free energies of *NO3 and *H on CoSe2(210). | |
In summary, we have successfully synthesized CoSe2/CC and demonstrated its an effective electrocatalyst for NH3 synthesis via the NO3–RR process. The catalyst of CoSe2/CC achieves a substantial NH3 yield of 517.7 µmol h–1 cm–2 and a FE of 97.6% at –0.6 V. DFT calculations indicate that CoSe2 possesses a higher energy barrier for H2 evolution and a lower PDS energy barrier in NO3–RR. This research not only introduces a cost-effective and efficient catalyst for NH3 synthesis but showcases the potential for advancing metal selenides for NO3–RR applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementTing Xie: Writing – original draft, Data curation. Xun He: Data curation. Lang He: Data curation. Kai Dong: Data curation. Yongchao Yao: Data curation. Zhengwei Cai: Investigation. Xuwei Liu: Investigation. Xiaoya Fan: Investigation. Tengyue Li: Investigation. Dongdong Zheng: Investigation. Shengjun Sun: Validation. Luming Li: Validation. Wei Chu: Validation. Asmaa Farouk: Validation. Mohamed S. Hamdy: Validation. Chenggang Xu: Writing – review & editing, Conceptualization. Qingquan Kong: Writing – review & editing, Software. Xuping Sun: Writing – review & editing, Conceptualization.
AcknowledgmentThe authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding support through large group research project (No. RGP2/119/45).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110005.
| [1] |
N. Gruber, J.N. Galloway, Nature 451 (2008) 293-296. DOI:10.1038/nature06592 |
| [2] |
X. Zou, J. Xie, C. Wang, et al., Chin. Chem. Lett. 34 (2023) 107908. DOI:10.1016/j.cclet.2022.107908 |
| [3] |
D.E. Canfield, A.N. Glazer, P.G. Falkowski, Science 330 (2010) 192-196. DOI:10.1126/science.1186120 |
| [4] |
X. He, Z. Li, J. Yao, et al., iScience 26 (2023) 107100. DOI:10.1016/j.isci.2023.107100 |
| [5] |
I. Dybkjaer, Ammonia Production Processes, in: A. Nielsen (Ed.), Ammonia, Catalysis and Manufacture, Springer, Heidelberg, 1995, pp. 199–308.
|
| [6] |
W. Liao, K. Xie, L. Liu, et al., J. Energy Chem. 62 (2021) 359-366. DOI:10.1016/j.jechem.2021.03.043 |
| [7] |
Q. Liu, Y. Lin, S. Gu, et al., Nano Res. 15 (2022) 7134-7138. DOI:10.1007/s12274-022-4568-z |
| [8] |
Y. Wang, M. Batmunkh, H. Mao, et al., Chin. Chem. Lett. 33 (2022) 394-398. DOI:10.1016/j.cclet.2021.05.025 |
| [9] |
W. Liao, L. Qi, Y. Wang, et al., Adv. Funct. Mater. 31 (2021) 2009151. DOI:10.1002/adfm.202009151 |
| [10] |
L. Ouyang, J. Liang, Y. Luo, et al., Chin. J. Catal. 50 (2023) 6-44. DOI:10.1016/S1872-2067(23)64464-X |
| [11] |
L. Li, C. Tang, H. Jin, et al., Chem 7 (2021) 3232-3255. DOI:10.1016/j.chempr.2021.10.008 |
| [12] |
X. Li, P. Shen, Y. Luo, et al., Angew. Chem. Int. Ed. 134 (2022) e202205923. DOI:10.1002/ange.202205923 |
| [13] |
Z. Sun, J. Lin, S. Lu, et al., Langmuir 40 (2024) 5469-5478. DOI:10.1021/acs.langmuir.3c04025 |
| [14] |
X. Liu, Y. Jiao, Y. Zheng, et al., ACS Catal. 10 (2020) 1847-1854. DOI:10.1021/acscatal.9b04103 |
| [15] |
D. Liu, H. Yan, J. Lin, et al., Chem. Eng. Sci. 283 (2024) 119448. DOI:10.1016/j.ces.2023.119448 |
| [16] |
X. He, J. Li, R. Li, et al., Inorg. Chem. 62 (2022) 25-29. DOI:10.3847/psj/ac4793 |
| [17] |
Z. Wu, Y. Song, Y. Liu, et al., Chem. Catal. 3 (2023) 100786. DOI:10.1016/j.checat.2023.100786 |
| [18] |
X. He, T. Xie, K. Dong, et al., Sci. China Mater. (2024). DOI:10.1007/s40843-024-2798-5 |
| [19] |
X. Zhao, M. Chen, D. Wang, et al., Chin. Chem. Lett. 35 (2024) 109327. DOI:10.1016/j.cclet.2023.109327 |
| [20] |
Z. Wu, Y. Song, H. Guo, et al., Interdiscip. Mater. 3 (2024) e12152. |
| [21] |
S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski, et al., Appl. Catal. B: Environ. 236 (2018) 546-568. DOI:10.1016/j.apcatb.2018.05.041 |
| [22] |
H. Mao, G. Wang, F. Liao, et al., J. Hazard. Mater. 445 (2023) 130569. DOI:10.1016/j.jhazmat.2022.130569 |
| [23] |
H. Liu, J. Timoshenko, L. Bai, et al., ACS Catal. 13 (2023) 1513-1521. DOI:10.1021/acscatal.2c03004 |
| [24] |
M. Yang, B. Li, S. Li, et al., Nano Lett. 23 (2023) 7733-7742. DOI:10.1021/acs.nanolett.3c01978 |
| [25] |
G.A. Cerrón-Calle, A.S. Fajardo, C.M. Sánchez-Sánchez, et al., Appl. Catal. B: Environ. 302 (2022) 120844. DOI:10.1016/j.apcatb.2021.120844 |
| [26] |
H. Zhang, C. Wang, H. Luo, et al., Angew. Chem. Int. Ed. 62 (2023) e202217071. DOI:10.1002/anie.202217071 |
| [27] |
H. Luo, S. Li, Z. Wu, et al., Adv. Mater. 35 (2023) 2304695. DOI:10.1002/adma.202304695 |
| [28] |
X. Fan, D. Zhao, Z. Deng, et al., Small 19 (2023) 2208036. DOI:10.1002/smll.202208036 |
| [29] |
O.Q. Carvalho, R. Marks, H.K.K. Nguyen, et al., J. Am. Chem. Soc. 144 (2022) 14809-14818. DOI:10.1021/jacs.2c05673 |
| [30] |
B. Yang, Y. Zhou, Z. Huang, et al., Nano Energy 117 (2023) 108901. DOI:10.1016/j.nanoen.2023.108901 |
| [31] |
Z. Deng, C. Ma, Z. Li, et al., ACS Appl. Mater. Interfaces 14 (2022) 46595-46602. DOI:10.1021/acsami.2c12772 |
| [32] |
X.E. Zhao, Z. Li, S. Gao, et al., Chem. Commun. 58 (2022) 12995-12998. DOI:10.1039/d2cc05204k |
| [33] |
K. Fan, W. Xie, J. Li, et al., Nat. Commun. 13 (2022) 7958. DOI:10.1038/s41467-022-35664-w |
| [34] |
C.P. Lee, W.F. Chen, T. Billo, et al., J. Mater. Chem. A 4 (2016) 4553-4561. DOI:10.1039/C6TA00464D |
| [35] |
S.N. Hussain, Y. Men, Z. Li, et al., Chin. Chem. Lett. 34 (2023) 107364. DOI:10.1016/j.cclet.2022.03.087 |
| [36] |
L. Zhang, J. Liang, L. Yue, et al., Nano Res. 15 (2022) 304-309. DOI:10.1007/s12274-021-3474-0 |
| [37] |
Y.R. Zheng, S. Hu, X.L. Zhang, et al., Adv. Mater. 34 (2022) 2205414. DOI:10.1002/adma.202205414 |
| [38] |
C. Xia, Q. Jiang, C. Zhao, et al., Adv. Mater. 28 (2016) 77-85. DOI:10.1002/adma.201503906 |
| [39] |
Y. Shi, D. Zhang, H. Miao, et al., J. Mater. Chem. A 9 (2021) 24261-24267. DOI:10.1039/d1ta07644b |
| [40] |
P. Shen, G. Wang, K. Chen, et al., J. Colloid Interface Sci. 629 (2023) 563-570. DOI:10.1016/j.jcis.2022.09.012 |
| [41] |
S. Liu, W. Miao, K. Ma, et al., Appl. Catal. B: Environ. 350 (2024) 123919. DOI:10.1016/j.apcatb.2024.123919 |
| [42] |
X. Liu, C. Liu, X. He, et al., Nano Res. 17 (2024) 2276-2282. DOI:10.1007/s12274-023-6204-y |
| [43] |
W. Wei, L. Wang, C. Liang, et al., Chem. Eng. J. 474 (2023) 145788. DOI:10.1016/j.cej.2023.145788 |
| [44] |
R. Manjunatha, J. Yuan, L. Hongwei, et al., Carbon Energy 4 (2022) 762-775. DOI:10.1002/cey2.189 |
| [45] |
J. Tian, J. Li, Y. Zhang, et al., J. Mater. Chem. A 7 (2019) 21404-21409. DOI:10.1039/c9ta06273d |
| [46] |
Y. Yao, C. Yang, S. Sun, et al., Small 20 (2024) 2307294. DOI:10.1002/smll.202307294 |
| [47] |
S.H. Yang, S.K. Park, Y.C. Kang, Chem. Eng. J. 370 (2019) 1008-1018. DOI:10.1016/j.cej.2019.03.263 |
| [48] |
Y. Liu, H. Cheng, M. Lyu, et al., J. Am. Chem. Soc. 136 (2014) 15670-15675. DOI:10.1021/ja5085157 |
| [49] |
L. Xie, L. Hu, Q. Liu, et al., Inorg. Chem. Front. 9 (2022) 3392-3397. DOI:10.1039/d2qi00827k |
| [50] |
L. Xie, Q. Liu, S. Sun, et al., ACS Appl. Mater. Interfaces 14 (2022) 33242-33247. DOI:10.1021/acsami.2c07818 |
 2024, Vol. 35
2024, Vol. 35 

