b Engineering Center of Catalysis and Synthesis for Chiral Molecules, Fudan University, Shanghai 200433, China
As attractive targets in organic synthesis, the chromones keep receiving extensive attention in synthetic method development [1–3]. In order to enhance the structural diversity of compounds featuring a chromone core, a plethora of synthetic protocols consisting of chromone ring construction and different other cascade chemical transformations have been established over the recent decade [4–11]. The notable advances in chromone synthesis, as expected, also have promoted the discovery of chromones possessing fantastic functions, including but not limited in biologically relevant lead compounds [12–14] and organic materials etc. [14–17]. Among the currently available protocols, the domino reactions consisting of a featured chromone annulation of o-hydroxyaryl enaminones and other different C—H functionalization transformation have been identified as the most prevalently investigated and employed tactic [18]. Especially over the last decade, the o-hydroxyphenyl enaminone-based chromone synthesis has received booming advances in terms of both reaction pathways and product diversity. By means of proper catalytic conditions, this typical chromone annulation takes place together with the formation of various other new chemical bonds in form of domino reactions, leading to the synthesis of chromones with C3-substituents such as alkyl and functionalized alkyl [19–26], perfluoroalkyl [27–29], aryl [30–31], alkenyl [32], alkynyl [33], chalcogen atoms [34–39], nitrogen [40–41] and various other carbon- and heteroatom-based functional structures [42–45].
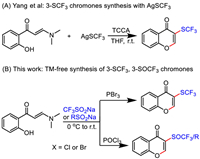
On the other hand, due to the significant role of trifluoromethylthio group in determining the functionality of organic molecules [46–49], the synthesis of trifluoromethylthiolated molecules has won highly extensive attention [50–53]. Unsurprisingly, the synthesis of trifluoromethylthiolated chromones has gained high interest of chemists. In 2014, Yang and Xiang [54] reported the synthesis of 3-trifluoromethylthiolated (3-CF3S) chromones by employing AgSCF3 as the source of SCF3 group (Scheme 1A). To date, no other catalytic method enabling the synthesis of 3-(trifluoromethylthio) chromones with o-hydroxyphenyl enaminones is available. Accordingly, developing new methods, especially the ones without relying on expensive transition metal-catalyst or reagent for the synthesis of such chromone derivatives is currently of high demand [55–64]. Herein, upon our sustaining efforts in developing enaminone-based synthesis [65–71], we report the first method on the o-hydroxyphenyl enaminone-based synthesis of 3-(trifluoromethylthio) chromones by employing CF3SO2Na as a cheap CF3S source. Besides, we also have realized the chemo-selective synthesis of 3-trifluoromethylsulfinyl chromones with identical substrates by simply modifying reaction conditions. Moreover, the 3-sulfinyl chromones which are not accessible by any previous synthetic efforts, have been easily furnished by our method with simple sodium sulfinates as the reaction partners (Scheme 1B).
|
Download:
|
| Scheme 1. Known method for the synthesis of 3-(trifluoromethylthio) chromones with AgSCF3 and this work. | |
Originally, the o-hydroxyphenyl enaminone 1a and CF3SO2Na (2a) were selected as starting materials for the potential synthesis of 3-(trifluoromethylthio) chromone 3a. By screening different reagents, the PBr3 was found to be an applicable reagent for the synthesis of the target product 3a by acting as both coupling and reducing agent. To explore the proper conditions enabling the efficient synthesis of 3a, the reaction parameters, including the loading of 2a and PBr3, the reaction medium, concentration, as well as the reaction temperature were optimized, respectively (Tables S1–S3 in Support information). The results indicated that the optimal result of 70% 3a was afforded by running the reaction in DMF at 0 ℃ to room temperature. The GC–MS analysis on the reaction residue from the optimal entry indicated no presence of CF3SO- or CF3SO2-substituted chromone, documenting the fine stability of the 3a against aerobic oxidation.
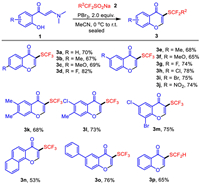
By employing the optimized conditions, the scope on the synthesis of CF3S-functionalized chromones 3 was then investigated with various enaminones 1 as starting materials to react with 2. As outlined in Scheme 2, the synthesis of such functionalized chromones exhibited general tolerance to the functional group in the aryl ring of 1. The products 3a-3o were obtained with generally good to excellent yields. While electron withdrawing group (EWG) substituted substrates gave halo-atom or nitrophenyl-based products 3d, 3g-3j and 3n with 74%–82% yield, the equivalent electron donating group (EDG), such as alkyl or alkoxyl functionalized products were given with slightly lower yield (3b-3c and 3e-3f, Scheme 2). Additionally, fused biaryl (3n, Scheme 2) and linear biaryl (3o, Scheme 2) derived products were also afforded with practical results. When HCF2SO2Na was used as the reaction partner, the HCF2S-functionalized product 3p was acquired with 65% yield. Further efforts by employing N-Boc-o-aminophenyl enaminone for the synthesis of corresponding CF3S-functionalized quinoline-4-one was not succeeded under current conditions.
|
Download:
|
| Scheme 2. Scope on the synthesis of 3-(trifluoromethylthio) chromones. | |
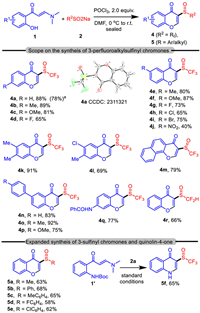
Subsequently, considering the variable chemical valence of sulfur atom, we assumed that it would be applicable to switch the chemo-selectivity of the reaction by providing trifluoromethyl-sulfinyl (SOCF3) chromones with identical substrates. To avoid the over reduction to the sulfur center, simple POCl3 was selected as the coupling reagent. Delightfully, we successfully realized the selective synthesis of 3-trifluoromethylsulfinyl chromone 4a via the reaction of 1a and 2. Analogously, the conditions for this transformation were optimized by comparing the effect of different parameters, by which the satisfactory result was acquired by running the reaction in DMF in the presence of 2.0 equiv. POCl3 by stirring at 0 ℃ to room temperature (see Support information for details on condition optimization). On the basis of optimization, the scope for the selective 3-trifluoromethylsulfinyl chromones synthesis was also studied. The results provided by the experiments inarguably verified the high applicability of this method in the synthesis of such unprecedented 3-substituted chromones (Scheme 3).
|
Download:
|
| Scheme 3. Scope on the synthesis of 3-perfluoroalkylsulfinyl chromones and 3-sulfinyl chromones. a Yield from 1 mmol-scale reaction. | |
As shown by the results, the synthesis of 3-trifluorometylsulfinyl chromones 4a-4q were provided with good to excellent yields, and the single crystal analysis on 4a (CCDC: 2311321) confirmed the product structure. Specifically, the enaminones bearing no substitution or with EDG in the phenyl ring reacted with 2 to give the products with excellent yield (4a-4c, 4e-4f and 4k, Scheme 3). Equivalent reactions with enaminones containing EWG in the phenyl, on the contrary, provided product with lower yield (4d, 4g and 4h-4j, Scheme 3). Moreover, products with disubstituted phenyl (4l, Scheme 3), fused aryl (4m, Scheme 3) and biaryl (4n-4o, Scheme 3) were also synthesized with satisfactory results. The method, notably, was also proven to be applicable for the synthesis of difluoromethylsulfinyl chromone with fine efficiency (4r, Scheme 3). More interestingly, the method was successfully expanded to the synthesis of aryl/alkyl-based 3-sulfinyl chromones 5. While such sulfur group-functionalized chromones had not been previously synthesized, the present method displayed attractive synthetic application by donating products 5a-5e with generally good yield (Scheme 3). Moreover, the CF3SO-functionalized quinoline-4-one 5f could be efficiently synthesized by using N-Boc-o-aminophenyl enamione 1′ as substrate, demonstrating the highly general tolerance of this method in the synthesis of novel sulfur groups functionalized chromones in the C3 site. Notably, GC–MS analysis on the reactions both for the synthesis of both 3a and 4a showed no formation of 3-trifluoromethyl chromone [27].
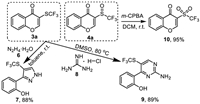
Following the examination on the switchable synthesis of both products 3 and 4, the synthetic application of these compounds was also primarily investigated (Scheme 4). First, employing the hydrazine hydrate 6 or guanidine hydrochloride salt 8 as reaction partner of compound 3a enabled the efficient synthesis of CF3S-funcitonalized pyrazole 7 and pyrimidine 9 by simple heating, respectively. On the other hand, subjecting compound 4a to m-CPBA allowed the formation of trifluoromethylsulfonyl chromone 10 with near quantitative yield. However, employing m-CPBA with enaminone 1a and CF3SO2Na in DMF in one-step operation did not provide compound 10.
|
Download:
|
| Scheme 4. Transformation of the synthesized products 3 and 4. | |
To probe the reaction mechanism, some control experiments were executed. First, for both the synthesis of CF3S- and CF3SO-substituted chromones, the parallel entries in the presence of free radical scavenger (FRS) were performed. The results showed that FRS such as BHT or DPE did not inhibit the formation of product 3a or 4a (Scheme 5a). Successively, the reaction sequence was probed by employing chromone 11 as substrate to react with 2, but neither 3a nor 4a was formed under corresponding conditions (Scheme 5b), suggesting that the chromone annulation took place after the C—H functionalization.

|
Download:
|
| Scheme 5. Control experiments. | |
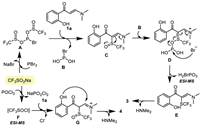
Based on these results and known examples involving phosphanes as deoxygenating reagents [72,73], the mechanisms for the reactions providing different chromone products are proposed (Scheme 6). For the reactions mediated by PBr3, the CF3SONa first couples PBr3 to generate species A. The nucleophilic substitution of the enaminone's α-carbon to the S-site in A gives sulfinylated enaminone C and releases compound B. Further, the nucleophilic substitution of the O-site in C to the P—Br bond in B affords intermediate D with the assistance of the electron donating effect from the amino group. Subsequently, the P to O electron pair transfer in D takes place to enable the break of the S—O bond to provide the trifluoromethylthiolated enaminone E. After that, the classical chromone annulation in the o-hydroxyphenyl enaminone yields the CF3S-functionalized chromones 3 by eliminating HNMe2. On the other hand, for the POCl3 mediated formation of sulfinyl chromones, the incorporation of POCl3 and CF3SONa happens initially to give sulfinyl chloride F. This species couples enaminone 1 in the α-site to generate sulfinylated iminium G by acting as an electrophile. The nucleophilic annulation and a subsequent amine elimination would then provide 3-trifluoromethylsulfinyl chromones 4. The sulfinyl chromones 5 are formed following similar mechanism.
|
Download:
|
| Scheme 6. The proposed reaction mechanisms. | |
In conclusion, by means of the featured C—H functionalization and chromone annulation cascade on o-hydroxyaryl enaminones, we have attained the first transition metal-free synthesis of 3-(trifluoromethylthio) chromones. The main tactic is the successful application of trivalent phosphine PBr3 as reductive coupling reagent. More interestingly, the chemo-selectivity could be switched for the synthesis of 3-trifluoromethylsulfinyl chromones by using pentavalent POCl3 as coupling reagent. Besides the synthesis of 3-trifluoromethylsulfinyl chromones, the synthesis of 3-aryl/alkylsulfinyl chromones with corresponding aryl/alkyl sulfinate as reaction partners has also been achieved. These results significantly expanded the application scope of enaminones in the synthesis of chromone derivatives. The transition metal-free conditions as well as the sulfur-group diversity in the C3-site of the chromone products consists of the major advantages of the work.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work is financially supported by National Natural Science Foundation of China (No. 22161022).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109683.
| [1] |
S. Tian, T. Luo, Y. Zhu, et al., Chin. Chem. Lett. 31 (2020) 307-3082. DOI:10.1016/j.cclet.2020.07.042 |
| [2] |
M. Mohadeszadeh, M. Iranshahi, Mini-Rev. Med. Chem. 17 (2017) 1377-1397. |
| [3] |
M. Fan, W. Yang, M. He, et al., Eur. J. Med. Chem. 237 (2022) 114397. DOI:10.1016/j.ejmech.2022.114397 |
| [4] |
L. Cai, X. Zhu, J. Chen, et al., Org. Chem. Front. 6 (2019) 3688-3692. DOI:10.1039/C9QO00830F |
| [5] |
J. Xiao, Z. Ai, X. Li, et al., Green Synth. Catal. 3 (2022) 198-201. DOI:10.1016/j.gresc.2021.12.003 |
| [6] |
J.R. Dhanaji, P. Samtha, S. Raju, Chem. Commun. 59 (2023) 2648-2651. DOI:10.1039/D2CC05992D |
| [7] |
B. Hasimujiang, J. Zhu, W. Xu, et al., Adv. Synth. Catal. 365 (2023) 2929-2935. DOI:10.1002/adsc.202300512 |
| [8] |
X. Li, Y. Li, J. Yang, et al., Org. Lett. 24 (2022) 7216-7221. DOI:10.1021/acs.orglett.2c03017 |
| [9] |
J. Liu, D. Ba, Y. Chen, et al., Chem. Commun. 56 (2020) 4078-4081. DOI:10.1039/C9CC09460A |
| [10] |
A. St-Gelais, J. Alsarraf, J. Legault, Org. Lett. 20 (2018) 7424-7428. DOI:10.1021/acs.orglett.8b03148 |
| [11] |
Y.F. Ye, F. Li, J.L. Chen, et al., J. Org. Chem. 87 (2022) 14005-14015. DOI:10.1021/acs.joc.2c01637 |
| [12] |
M. Kim, M. Truss, P.P. Paggare, et al., Bioorg. Med. Chem. Lett. 30 (2020) 127511. DOI:10.1016/j.bmcl.2020.127511 |
| [13] |
M. Kim, M. Truss, P.P. Pagare, et al., Bioorg. Med. Chem. Lett. 30 (2020) 127511. |
| [14] |
M. Miliutina, S.A. Ejaz, V.O. Iaroshenko, et al., Org. Biomol. Chem. 4 (2016) 495-502. |
| [15] |
A. Das, G. Das, New J. Chem. 46 (2022) 19002-19008. DOI:10.1039/D2NJ04115D |
| [16] |
R. Kouser, A. Rehman, S.M.A. Abidi, et al., J. Mol. Struct. 1256 (2022) 132533. DOI:10.1016/j.molstruc.2022.132533 |
| [17] |
H. Gómez-Machuca, C. Quiroga-Compano, C. Jullian, et al., ChemistrySelect 7 (2022) e202202581. DOI:10.1002/slct.202202581 |
| [18] |
L. Fu, J.P. Wan, Asian J. Org. Chem. 8 (2019) 767-776. DOI:10.1002/ajoc.201900196 |
| [19] |
P.N. Bagle, M.V. Mane, S.P. Sancheti, et al., Org. Lett. 21 (2019) 335-339. DOI:10.1021/acs.orglett.8b03989 |
| [20] |
Y.F. Lin, C. Fong, W.L. Peng, et al., J. Org. Chem. 82 (2017) 10855-10865. DOI:10.1021/acs.joc.7b01626 |
| [21] |
J. Joussot, A. Schoenfelder, L. Larquetoux, et al., Synthesis 48 (2016) 3364-3372. DOI:10.1055/s-0035-1562513 |
| [22] |
M. Zhang, Z. Liu, L. Chen, et al., Adv. Synth. Catal. 364 (2022) 4440-4446. DOI:10.1002/adsc.202201154 |
| [23] |
W. Zhao, Z. He, X. Yang, et al., J. Org. Chem. 88 (2023) 13634-13644. DOI:10.1021/acs.joc.3c01339 |
| [24] |
K. Wen, Y. Li, Q. Gao, et al., J. Org. Chem. 87 (2022) 9270-9281. DOI:10.1021/acs.joc.2c01005 |
| [25] |
S. Pan, M. Song, L. Zuo, et al., J. Org. Chem. 88 (2023) 5586-5596. DOI:10.1021/acs.joc.3c00093 |
| [26] |
Z.Q. Zhu, J.Y. Hu, Z.B. Xie, et al., Adv. Synth. Catal. 364 (2022) 2169-2173. DOI:10.1002/adsc.202200304 |
| [27] |
Q. Yu, Y. Liu, J.P. Wan, Org. Chem. Front. 7 (2020) 2770-2775. DOI:10.1039/D0QO00855A |
| [28] |
H. Xiang, Q. Zhao, Z. Tang, et al., Org. Lett. 19 (2017) 146-149. DOI:10.1021/acs.orglett.6b03441 |
| [29] |
H. Gao, B. Hu, W. Dong, et al., ACS Omega 2 (2017) 3168-3174. DOI:10.1021/acsomega.7b00383 |
| [30] |
J.P. Wan, Z. Tu, Y. Wang, Chem. Eur. J. 25 (2019) 6907-6910. DOI:10.1002/chem.201901025 |
| [31] |
S. Mkrtchyan, M. Jakubczyk, S. Lanka, et al., Adv. Synth. Catal. 364 (2022) 3512-3521. DOI:10.1002/adsc.202200645 |
| [32] |
L. Fu, Z. Xu, J.P. Wan, et al., Org. Lett. 22 (2020) 9518-9523. DOI:10.1021/acs.orglett.0c03548 |
| [33] |
M.O. Akram, S. Bera, N.T. Patil, Chem. Commun. 52 (2016) 12306-12309. DOI:10.1039/C6CC07119H |
| [34] |
T. Zhang, W. Yao, J.P. Wan, et al., Adv. Synth. Catal. 363 (2021) 4811-4816. DOI:10.1002/adsc.202100617 |
| [35] |
Y.X. Xu, S.Y. Wu, Z.B. Dong, J. Org. Chem. 87 (2022) 15350-15357. DOI:10.1021/acs.joc.2c01924 |
| [36] |
B. Zhang, Z. Fu, H. Yang, et al., Adv. Synth. Catal. 364 (2022) 1602-1606. DOI:10.1002/adsc.202200089 |
| [37] |
G.S. Sorabad, M.R. Maddani, Asian J. Org. Chem. 8 (2019) 1336-1343. DOI:10.1002/ajoc.201900402 |
| [38] |
Y. Guo, Y. Xiang, L. Wei, et al., Org. Lett. 20 (2018) 3971-3974. DOI:10.1021/acs.orglett.8b01536 |
| [39] |
H.Y. Liu, J.R. Zhang, G.B. Huang, et al., Adv. Synth. Catal. 363 (2021) 1656-1661. DOI:10.1002/adsc.202001474 |
| [40] |
Z.W. Wang, Y. Zheng, Y.E. Qian, et al., J. Org. Chem. 87 (2022) 1477-1484. DOI:10.1021/acs.joc.1c02796 |
| [41] |
T. Luo, J.P. Wan, Y. Liu, Org. Chem. Front. 7 (2020) 1107-1112. DOI:10.1039/D0QO00065E |
| [42] |
S. Mkrtchyan, V.B. Purohit, V.O. Iaroshenko, ACS Sustain. Chem. Eng. 11 (2023) 13877-13884. DOI:10.1021/acssuschemeng.3c03467 |
| [43] |
Y. Lin, J.P. Wan, Y. Liu, J. Org. Chem. 88 (2023) 4017-4023. DOI:10.1021/acs.joc.3c00206 |
| [44] |
Y. Lin, J. Jin, C. Wang, et al., J. Org. Chem. 86 (2021) 12378-12385. DOI:10.1021/acs.joc.1c01347 |
| [45] |
Q.L. Zhao, P.J. Xia, L. Zheng, et al., Tetrahedron 76 (2020) 130833. DOI:10.1016/j.tet.2019.130833 |
| [46] |
G. Landelle, A. Panossian, F.R. Leroux, Curr. Top. Med. Chem. 14 (2014) 941-951. DOI:10.2174/1568026614666140202210016 |
| [47] |
N. Thota, P. Makam, K.K. Rajbongshi, et al., ACS Med. Chem. Lett. 10 (2019) 1457-1461. DOI:10.1021/acsmedchemlett.9b00285 |
| [48] |
M. Diaferia, F. Veronesi, G. Morganti, et al., Parasitol. Res. 112 (2013) 163-168. |
| [49] |
J. Gregorc, N. Lensen, G. Chaume, et al., J. Org. Chem. 88 (2023) 13169-13177. DOI:10.1021/acs.joc.3c01373 |
| [50] |
C. Xu, S. Wang, Q. Shen, ACS Sustain. Chem. Eng. 10 (2022) 6889-6899. DOI:10.1021/acssuschemeng.2c01006 |
| [51] |
X. Wang, Z. Wang, Z. Li, et al., Chin. Chem. Lett. 34 (2023) 108045. DOI:10.1016/j.cclet.2022.108045 |
| [52] |
X.H. Yang, D. Chang, R. Zhao, et al., Asian J. Org. Chem. 10 (2021) 61-73. DOI:10.1002/ajoc.202000575 |
| [53] |
G.L. Xie, S.H. Jia, X. Shen, et al., J. Fluorine Chem. 235 (2020) 109524. DOI:10.1016/j.jfluchem.2020.109524 |
| [54] |
H. Xiang, C. Yang, Org. Lett. 16 (2014) 5686-5689. DOI:10.1021/ol502751k |
| [55] |
T.A. Kizhakkan, K.M.S. Ajay, D. Basvaraja, et al., Org. Chem. Front. 9 (2022) 5306-5357. DOI:10.1039/D2QO00278G |
| [56] |
D. Chen, J. Jiang, J.P. Wan, Chin. J. Chem. 40 (2022) 2582-2594. DOI:10.1002/cjoc.202200347 |
| [57] |
Y. Li, D. Shen, H. Zhang, et al., Chin. Chem. Lett. 34 (2023) 108048. DOI:10.1016/j.cclet.2022.108048 |
| [58] |
Z. Xu, J. Wan, Y. Liu, Chin. J. Org. Chem. 43 (2023) 2425-2446. DOI:10.6023/cjoc202212035 |
| [59] |
M.S. Kang, J.Y.X. Khoo, Z. Jia, et al., Green Synth. Catal. 3 (2022) 309-316. DOI:10.1016/j.gresc.2022.09.002 |
| [60] |
Y. Lv, H. Cui, N. Meng, et al., Chin. Chem. Lett. 33 (2022) 97-114. DOI:10.1016/j.cclet.2021.06.068 |
| [61] |
Y.H. Lu, S.Y. Mu, H.X. Li, et al., Green Chem. 25 (2023) 5539-5542. DOI:10.1039/D2GC04906F |
| [62] |
Y.H. Lu, Z.T. Zhang, H.Y. Wu, et al., Chin. Chem. Lett. 34 (2023) 108036. DOI:10.1016/j.cclet.2022.108036 |
| [63] |
J. Jiang, K.L. Wang, X. Li, et al., Chin. Chem. Lett. 34 (2023) 108699. DOI:10.1016/j.cclet.2023.108699 |
| [64] |
H.T. Ji, K.L. Wang, W.T. Ouyang, et al., Green Chem. 25 (2023) 7983-7987. DOI:10.1039/D3GC02575F |
| [65] |
Y. Han, L. Zhou, C. Wang, et al., Chin. Chem. Lett. 35 (2024) 108977. DOI:10.1016/j.cclet.2023.108977 |
| [66] |
H. Guo, Y. Liu, J.P. Wan, Green Synth. Catal. (2024). DOI:10.1016/j.gresc.2023.10.004 |
| [67] |
Y. Guo, Y. Liu, J.-P. Wan, Chin. Chem. Lett. 33 (2022) 855-858. DOI:10.1016/j.cclet.2021.08.003 |
| [68] |
D. Cao, C. Wang, J.-P. Wan, et al., Chem. Commun. 59 (2023) 6383-6386. DOI:10.1039/D3CC01427D |
| [69] |
S. Tian, Y. Liu, C. Wan, et al., J. Org. Chem. 88 (2023) 2433-2442. DOI:10.1021/acs.joc.2c02858 |
| [70] |
W. Song, Y. Liu, N. Yan, et al., Org. Lett. 25 (2023) 2139-2144. DOI:10.1021/acs.orglett.3c00636 |
| [71] |
J. Ye, Y. Liu, J. Luo, et al., Org. Lett. 25 (2023) 8451-8456. DOI:10.1021/acs.orglett.3c03353 |
| [72] |
X. Zhao, A. Wei, B. Yang, et al., J. Org. Chem. 82 (2017) 9175-9181. DOI:10.1021/acs.joc.7b01226 |
| [73] |
L. Zheng, X. Qiu, Z. Xiao, et al., J. Org. Chem. 88 (2023) 7518-7524. DOI:10.1021/acs.joc.3c00342 |
 2024, Vol. 35
2024, Vol. 35 

