b Department of Radiology, the Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450003, China
A century ago, Paul Ehrlich, known as the pioneer of chemotherapy, revolutionized the medical field by introducing the concept of "magic bullets" to combat human ailments. This visionary idea of precise drug delivery, with its emphasis on high efficacy, continues to shape modern drug discovery and serves as a driving force for generations of scientists in their quest to develop innovative strategies for drug administration [1,2]. There is no doubt that this idea is very forward-looking and innovative. In recent years, the rapid development of cutting-edge technologies in chemistry, biology, materials, and medicine has led to widespread use of various engineered targeted delivery systems based on nanobiology in disease diagnosis and personalized precision therapy.
For traditional engineered targeted delivery systems, researchers have utilized the unique mechanical, chemical, and biological properties of nanomaterials to encapsulate biological agents or drugs. The main purpose is to enhance drug stability and solubility, facilitate drug penetration through tissue barriers, and prolong drug circulation time in the body to improve safety and efficacy [3-8]. The development and application of these drug delivery systems have partially addressed issues such as rapid blood clearance, systemic toxicity, side effects, and poor stability of drugs when administered in isolation [9-11]. However, traditional nanomaterials are primarily synthesized using physical and chemical methods, which often involve raw materials, solvents, and modifying molecules with potential biosafety and biocompatibility issues. Additionally, the incomplete understanding of the underlying biology of diseases and the yet unexplored heterogeneity among patients limits the ability of nanomedicine delivery systems to precisely respond to physiological and pathological differences. Consequently, these systems fail to achieve the anticipated therapeutic benefits [12].
With the introduction of the Precision Medicine Initiative in 2015 and the increasing popularity of precision medicine, there is a need for custom-designed drug delivery systems in the next generation. These systems should be able to address multiple, distinct key targets, which will lead to greater demands on various disciplines such as genomics, molecular biology, chemical biology, and materials science [13,14]. Therefore, realizing breakthroughs in the synthesis theory and biological applications of nanomaterials has become a key scientific issue for the transformative development of this field, and the emergence of synthetic biology provides a new innovative space for this purpose.
Synthetic biology primarily involves the targeted design, modification, and even complete resynthesis of existing living organisms using engineering technology. The goal is to mimic the traits and life processes of the target organisms, and when needed, to create entirely new functions not found in nature [15,16]. There are two main guiding concepts in synthetic biology research: the top-down strategy and the bottom-up strategy. The top-down strategy enables existing biological systems to acquire completely new functions by constructing synthetic components with predictable and controllable genetic, metabolic, or bio signaling networks based on the current properties and functions of natural organisms [17,18]. On the other hand, the bottom-up strategy involves designing independent modules composed of non-natural components from scratch, independent of existing biological systems and their functions. These modules are then assembled and synthesized to create synthetic artificial biological systems that do not exist in nature [19-21]. In light of the distinct characteristics and requirements of synthetic biology and nanobiology, the integration of nanomaterials and synthetic biology will be a pivotal trend in the advancement of next-generation drug delivery systems. On one hand, synthetic biology technology enables the intelligent fusion of chassis cells, bacteria, and their modified derivatives with nanomaterials, resulting in the development of nano-artificial heterotrimeric systems. These systems organically combine the functionalities of both materials and facilitate significant breakthroughs and optimization of biological functions [22-24]. On the other hand, guided by synthetic biology, the self-assembly of modular nano-assemblies with biocatalytic or responsive functions mimics essential features of living cells, such as compartmentalization of enzymatic reactions and susceptibility to external stimuli. This approach offers novel design concepts for the construction of artificial cells [25-27].
In summary, this paper focuses on the intersection of nanomaterials science and synthetic biology. We review the application and research progress of the new generation of synthetic biology-based nanomedicine delivery systems in precision and personalized treatment of diseases (Table S1 in Supporting information). Furthermore, we analyze the potential of these systems for clinical applications such as vaccine development, early diagnosis, and precision treatment of diseases. We discuss the advantages and limitations of the current synthetic biology-based nano drug delivery systems for clinical translation, and we also envision the future development of nanotechnology for intelligent drug diagnostic and therapeutic systems.
2. Living cell-based drug delivery system 2.1. Whole cell-based drug delivery systemCells have the inherent ability to sense the physiological environment within the body and integrate physiological signals to respond to its dynamics. As a result, cells have emerged as a promising natural vehicle for delivering therapeutic drugs [28-30]. In comparison to synthetic nanoparticles, natural cell-based drug delivery systems exhibit excellent biocompatibility, biodegradability, and low immunogenicity, all while retaining the intrinsic biological properties and functions of the cells [31-33]. Firstly, specific types of cells can leverage their unique biological properties to traverse physiological tissue barriers, enhancing drug enrichment and distribution in challenging-to-reach lesion sites. For instance, mesenchymal stem cells, neural stem cells, and macrophages, which are natural cell carriers, cross the blood-brain barrier (BBB) for effective treatment of neurodegenerative diseases like Alzheimer's and Parkinson's [34-36]. Moreover, the cell membrane structure, as well as the proteins and carbohydrates present on its surface, enable cells to effectively carry out their specific functions in complex physiological environments [37]. For example, erythrocytes have a lifespan of up to 120 days in the circulatory system, while stem cells from various sources possess different degrees of reparative and regenerative-promoting effects on neural, cardiac, and bone tissues, among others [38-40]. Furthermore, immune cells such as macrophages, neutrophils, T cells, and natural killer (NK) cells exhibit inflammatory homing abilities and the ability to specifically recognize and eliminate tumor cells through surface markers [41-43]. At the same time, cell-based drug carriers also have great drug-carrying potential and can be adapted to different drug loading strategies. For instance, the phospholipid bilayer of the cell membrane is well-suited for loading hydrophobic drugs, while the cytoplasmic compartments provide ample space for loading hydrophilic drugs. Specifically, the membrane lipids and proteins, which possess charged or reactive groups (e.g., amino and sulfhydryl groups), can enable drug loading onto the cell surface through physical adsorption (e.g., electrostatic interactions) or chemical bonding [44]. Simultaneously, the separation of intracellular space provides protection for drugs against degradation and clearance by enzymes, acids, and other harsh factors in the physiological environment, resulting in more efficient drug delivery to the target lesion [45,46].
Notably, an increasing number of clinical studies have demonstrated the positive and highly promising effects and progress of chimeric antigen receptor T-cell (CAR-T) therapies in antitumor therapy [47,48]. As a type of immune cell therapy, CAR-T therapy utilizes the CAR gene to genetically engineer T cells. The modified T cells are capable of recognizing and killing tumor cells without relying on major histocompatibility complex class I (MHC-Ⅰ) [49]. Compared to traditional anti-tumor methods, CAR-T cell therapy offers advantages such as high specificity, strong lethality, and long-lasting efficacy. However, while CAR-T cells have demonstrated good results in the treatment of hematological malignancies, there are still challenges to overcome in the treatment of solid tumors. These include immune escape due to tumor antigen heterogeneity, physiological or cytokine barriers, and the tumor microenvironment which contains immunosuppressive molecules and cells that impede the entry and persistence of CAR-T cells [50-52]. To address the limitations of conventional CAR-T therapies, researchers have explored the modification of nanomaterials with various functions, such as targeting, controlling, responding, and tracing, on T cells [53-55]. Nonetheless, single CAR-T therapy still retains some limited efficacy in solid tumors, and greater therapeutic effects can be achieved by combining chemotherapy, photothermal therapy, metabolic modulation, or other immunotherapies. For example, Gu's team combined photothermal therapy with CAR-T therapy by locally injecting PLGA nanoparticles loaded with the photosensitizer indocyanine green (ICG) at the tumor site. This approach promoted the enrichment and activation of CAR-T cells in solid tumors through photothermal therapy, enhancing the therapeutic effect on tumor treatment [54].
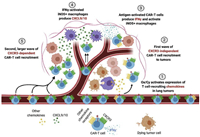
Limitations of current CAR-T therapies include decreased effectiveness for epithelial malignancies with higher mortality rates. As a result, Shivani et al. utilized cyclophosphamide (Cy) and oxaliplatin (Ox) to deplete lymphocytes in mice while simultaneously activating tumor macrophages to express T-cell-recruiting chemokines (Fig. 1). This approach uses CAR-T cells to target the tumor-associated antigen receptor tyrosine kinase-like orphan receptor 1 for treatment. Experimental results showed that the combination of Ox/Cy and anti-programmed cell death-ligand 1 (anti-PD-L1) therapy improved CAR-T cell infiltration, remodeled the tumor microenvironment, and increased tumor sensitivity to anti-PD-L1 [56]. In vivo cell tracking techniques can provide better understanding of the distribution, local infiltration, persistence, and therapeutic efficacy of CAR-T cells. The Aras team developed a dual-mode positron emission tomography/near-infrared fluorescence-based artificial heterogeneous CAR-T imaging platform. This platform employs 89Zr and near-infrared fluorescence-containing silica nanoparticles to label CAR-T cells. Dual-mode positron emission tomography/near-infrared fluorescence imaging enables in vivo biodistribution monitoring of CAR-T cells, providing guidance for evaluating the treatment modalities of CAR-T therapies in solid tumors [57].
|
Download:
|
| Fig. 1. Srivastava et al. demonstrate that adding oxaliplatin to the lymphodepletion regimen given before CAR-T cell infusion activates lung tumor macrophages to produce T cell-recruiting chemokines. This result improved CAR-T cell infiltration, tumor remodeling, and response to checkpoint blockade, providing a strategy to improve CAR-T cell efficacy in the clinic. Copied with permission [56]. Copyright 2020, Elsevier. | |
In summary, natural cells from different kinds of sources are ideal for drug delivery carriers due to their different ingenious and efficient innate biological functions. More excitingly, the addition of genetic engineering technology has provided an indispensable driving force for the in-depth development of cell carrier technology. Cellular carriers are endowed with the ability to autonomously sense and respond to different physiological environments, and realize intelligent spatio-temporal controlled release of drugs according to the microenvironmental dynamics or the degree of disease progression, thus accomplishing highly effective targeted therapy of diseases.
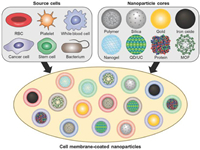
2.2. Cell membrane-based drug delivery systemsCell membrane-based drug delivery systems refer to the nanosizing of extracted cell membranes using nanotechnology to obtain either nanoparticles encapsulated by cell membranes or cell membrane nanoparticles alone. These systems are nature-inspired biomimetic nano-delivery systems that combine synthetic nanoparticles with natural biologically-derived materials, allowing for the integration of the highly tunable physicochemical properties of synthetic nanomaterials and the complex biological functions of host cell membranes [58-61]. During the early stages of cell membrane encapsulation technology development, erythrocyte membranes were utilized to encapsulate synthetic nanoparticles in order to extend their circulation cycle in vivo. Zhang et al. first developed erythrocyte-encapsulated polymeric nanoparticles in 2011, which exhibited a significantly longer cycling half-life (39.6 h) compared to polyethylene glycol (PEG)-modified nanoparticles (15.8 h) (Fig. 2) [62,63]. Furthermore, mature erythrocytes lack nuclei and various organelles, making them more convenient for membrane separation and purification [39]. In addition, cell membrane-encapsulated nanoparticles demonstrated satisfactory stability in 100% fetal bovine serum medium compared to unencapsulated nanoparticles. This stability can be attributed to the natural characteristics of cell membranes, particularly the presence of membrane biomarkers that play a crucial role in maintaining serum stability and resisting nonspecific protein adsorption.
|
Download:
|
| Fig. 2. Cell membrane-coated nanoparticles. A variety of cell types have been used as sources of membranes to coat over nanoparticles. Each cell membrane type can utilize unique properties to provide functionalities to nanoparticulate cores, the material of which can be varied depending on the desired application. Copied with permission [62]. Copyright 2022, Wiley. | |
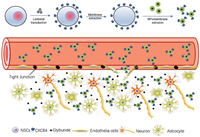
With the rapid development of cell membrane camouflage nanotechnology, an increasing number of cell membrane nanocarriers have been developed. These include platelet membrane nanoparticles that can target inflammatory sites, tumor cell membrane nanoparticles with a homing effect, neutrophil membrane nanoparticles with a nano-sponge effect, and dendritic cell membrane nanoparticles that are capable of stimulating the maturation of T cells. For example, Liu et al. successfully constructed the ASPIRE nanovaccine by transfecting dendritic cells with recombinant adenovirus to co-express specific peptide major histocompatibility complex type 1 (MHC-I), anti-programmed cell death protein 1 (PD1) antibody, and B7 co-stimulatory molecules on the surface of dendritic cells. The ASPIRE nanovaccine significantly improves the delivery of antigens to the lymphoid organs and directly activates both natural and depleted T cells, thereby clearing generated tumors [64]. Zhang et al. used genetic engineering to express the co-stimulatory marker CD80 on the surface of melanoma cells, which were then encapsulated on the surface of nanoparticles. This allowed the nanoparticles to present antigens in an immunostimulatory environment and activated T cells to control tumor growth, enabling the development of multiantigenic, personalized cancer immunotherapies [65]. Gu et al. used genetic engineering to modify megakaryocyte progenitor cells to generate platelets that overexpress programmed cell death protein ligand 1 (PD-L1). These platelets accumulated in the inflamed pancreas of NOD mice, inhibited the activity of pancreatic autoreactive T cells, and protected insulin-secreting β-cells from destruction. At the same time, this approach increased the percentage of regulatory T cells to maintain immune tolerance in the pancreas, effectively maintaining normoglycemia and reversing diabetes in NOD mice [66]. The Zhou Jiang Corps team at Yale University encapsulated membranes of C-X-C motif chemokine receptor 4 (CXCR4) overexpressing neural stem cells on the surface of poly(lactic-ethanolic acid) acid nanoparticles (NPs). This utilization of the interaction between CXCR4 and stromal cell-derived factor-1 (SDF-1) significantly enhanced the efficacy of glibenclamide for stroke treatment, providing a new idea to improve drug delivery to the ischemic brain (Fig. 3) [67]. Chen et al. constructed a nanovaccine by integrating CD47KO/CRT dual bioengineered B16F10 cancer cell membranes and unmethylated cytosine-phosphoguanine (CpG) adjuvant. This nanovaccine promotes endocytosis of antigens and adjuvants in murine bone marrow-derived dendritic cells and induces their maturation and antigen cross-presentation. To avoid immune checkpoint molecule-induced T-cell dysfunction, an immune checkpoint inhibitor, anti-PD-L1 antibody, was introduced to promote tumor immunotherapy by combining with DBE@CCNPs nanovaccine. This combination therapy strategy significantly alleviated tumor growth and provided potential strategies and ideas for future clinical tumor immunotherapy [68].
|
Download:
|
| Fig. 3. Schematic diagram of targeted delivery of NPs to the ischemic brain through surface coating of membrane of CXCR4-overexpressing NSCs. NSCs are engineered through lentiviral transduction to overexpress CXCR4. Cell membrane is extracted and coated onto the surface of PLGA NPs via extrusion. After intravenous administration, the resulting CMNPs selectively accumulate in the ischemic microenvironment through interaction with SDF-1, a ligand of CXCR4 that is preferentially accumulated. CMNPs locally release cargo therapeutics, such as glyburide, and promote stroke repair and recovery. Copied with permission [67]. Copyright 2019, Wiley. | |
In summary, the combination of modifying cells using synthetic biology technology and reducing the size of their cell membranes through nanotechnology can lead to the construction of cell membrane nanocarriers with special functions. This advancement greatly advances the development of this field.
2.3. Exosome-based drug delivery systemExosomes are extracellular vesicles with a diameter of approximately 80–180 nanometers and a classical "tear-drop-like" morphology. They contain complex RNAs and proteins and are secreted by cells. Exosomes play a crucial role in intercellular communication, affecting both physiological and pathological conditions [69-75]. These vesicles contain lipids, proteins, and various types of nucleic acids derived from cells and act as important carriers of cellular communication. Due to their unique attributes, such as low toxicity, low immunogenicity, biodegradability, and the ability to encapsulate endogenous bioactive molecules across physiological barriers, exosomes hold significant therapeutic potential [69,76-83].
However, the targeting ability of natural exosomes is poor and cannot achieve real-time response to physiological signals or controlled release of drugs. Therefore, synthetic biology techniques have been used to modify targeting molecules on the surface of exosomes or load nanoparticles into their lumen to enhance their functions of active targeting, drug delivery, and therapy [84-89]. For example, Wood et al. developed an efficient, tissue-specific, and non-immunogenic delivery technique. The investigators modified dendritic cells to overexpress Lamp2b, an exosomal membrane protein fused to a neuron-specific rabies virus glycoprotein (RVG) peptide. They then isolated and purified exosomes secreted by the cells and loaded exogenous siRNA into them using electroporation. By intravenously injecting these RVG-targeted exosomes, they successfully delivered glyceraldehyde-3-phosphate dehydrogenase (GAPDH) siRNA specifically to neurons in the brain, microglia, effectively reducing the expression of the BACE gene mRNA (60%) and protein (62%) [90]. In addition, Yin et al. used the phage-screening peptide CP05 to capture, target-modify, and load "cargo" into exosomes of different origins for diagnostic and therapeutic tumor research. The phage peptide CP05 can capture exosomes from different sources, including patient-derived exosomes, by binding to the exosome surface protein CD63. Using CP05 as a bridge, they coupled CP05 to target molecules (M12, RVG, SP94) and then modified exosomes to target transport drug molecules to specific organ tissues. In a mouse model of Duchenne muscular dystrophy (DMD), CP05 coupled to phosphoramidodiamidomorpholine oligomers was modified on the surface of exosomes and infused back to treat the mice. This approach significantly increased the jumping efficiency of exon 23 and allowed for an 18-fold increase in the expression of the dystrophin anti-atrophic protein in the quadriceps muscle. Studies have shown that exosome-anchored peptides can directly and efficiently functionalize and capture exosomes, providing tools for exosome engineering, in vivo gene function detection, and targeted therapeutic drug delivery [91]. Furthermore, Zhang et al. designed and synthesized a synthetic multi-antibody-targeted exosome expressing antibodies to CD3, a membrane surface protein specifically expressed by T cells, and epidermal growth factor receptor (EGFR), a membrane protein specifically expressed by triple-negative breast cancer cells. This system is capable of specifically directing T cells to the periphery of triple-negative breast cancer cells, thereby inducing an immune response. The exosome was injected into a transplanted tumor mouse model and demonstrated a significant anti-cancer effect [92]. In summary, whether as a carrier for drug delivery or acting as a display platform for biomolecules, exosomes modified using synthetic biology are highly maneuverable and practical.
3. Drug delivery systems based on bacteria and their derivatives 3.1. Bacteria-based drug delivery systemThe human body serves as a natural habitat for a diverse range of microbial communities, and the intricate interactions between the host and the microorganisms within its body play a critical role in human health and the development of diseases, among other factors. Bacteria constitute an important component of these microorganisms. Various bacterial species, including both commensal and pathogenic bacteria, employ biochemical signals to engage in complex interactions with their hosts and exert influence over host bodily functions by regulating the structure and functionality of the microbiota [93]. Researchers have capitalized on these characteristics by utilizing biomodule modification techniques in synthetic biology, along with chemical component modifications of nanomaterials, to achieve intricate modifications to live bacteria and construct intelligent "nanohybrid bacteria". This has enabled the precise regulation of bacterial motility, metabolism, synthesis of active molecules, controlled release, and other related behaviors [94-97]. Due to the unique immunosuppressive and hypoxic environment present in tumor sites, bacteria can selectively colonize tumor tissues, serving as targeted anticancer drugs or drug carriers. Compared to passive therapies such as chemotherapy and radiotherapy, which have low selectivity and permeability to tumor tissues, "nanohybrid bacteria" can effectively target tumors, produce cytotoxic molecules, sense the local environment through physiological signals, and generate externally detectable signals. Consequently, they can be employed as intelligent "robots" for precise and efficient cancer therapy [98-101].
For instance, Danino et al. designed a non-pathogenic E. coli strain containing a synchronous lysis loop to specifically lyse and release a nano-antibody antagonist of CD47, which is an anti-phagocytic receptor commonly overexpressed in several human cancer types, in the tumor microenvironment. The experimental results demonstrated that the colonization of this strain at the tumor site increased the activation of tumor-infiltrating T cells, stimulated rapid tumor regression, prevented tumor metastasis, and stimulated systemic tumor antigen-specific immune responses. This provides a proof-of-concept for engineered bacterial immunotherapy-induced distant effects. Thus, this system enables the targeted delivery of immunotherapeutic drugs in combination with immunostimulatory bacterial lysis adjuvants to stimulate antitumor immunity and promote tumor regression [102]. Additionally, the team designed a probiotic system for controlling the production and intra-tumor release of nanobodies targeting PD-L1 and cytotoxic T-lymphocyte-associated protein-4 using a stable lysate release mechanism. Experimental results in model mice showed tumor regression with a single injection of the engineered E. coli strain, as well as enhanced systemic tumor immune responses compared to similar clinically relevant antibodies. These findings suggest that the engineered probiotic system effectively combines synthetic biology and immunology for improved delivery of immune checkpoint blockers [103]. In another design, Wang's team genetically engineered bacteria using promoter plasmids responsive to blue light to achieve light-controlled protein expression of transforming growth factor-β1 (TGF-β1) and interferon-γ (IFN-γ). The engineered bacteria were then wrapped with rare-earth upconverting nanomaterials through chitosan-sodium alginate crosslinking gel and orally delivered to the intestine. The rare-earth upconverting nanomaterials converted near-infrared light into locally effective blue light, avoiding damage to blue light-sensitive tissues. This "bacteria-nanomaterials" hybrid system can control the behavior of bacteria in the intestinal tract in vitro and effectively inhibit ulcerative colitis and tumor growth in mice [99].
In summary, these preliminary studies demonstrate the safety and efficacy of bacterial-based drug delivery systems in the treatment of diseases. The studies have also shown good biosafety, a convenient route of administration, high potential for customization, and excellent efficacy. These qualities make bacterial-based drug delivery systems a valuable option for future translational medicine applications.
3.2. Drug delivery system based on bacterial outer membrane vesiclesBacterial outer membrane vesicles (OMVs) are natural vesicles primarily produced by the outer membrane outgrowth of Gram-negative bacteria. OMVs are spherical lipid bilayer vesicles with dimensions ranging from approximately 20–250 nm. They are enriched with various pathogen-associated molecules derived from bacteria, such as lipopolysaccharides, peptidoglycans, proteins, and nucleic acids. These molecules have the ability to efficiently activate the immune system and are commonly used as bacterial vaccines, adjuvants, cancer immunotherapeutic agents, and drug delivery vectors [104-109]. Genetic engineering modification of a Gram-negative bacteria like E. coli allows for the expression of antigenic fusions of influenza virus, human papillomavirus, pneumococcus, Staphylococcus aureus, and Acinetobacter baumannii in OMVs. This modification stimulates the production of specific antibodies against these pathogenic microorganisms in the body, leading to effective preventative effects [110-117].
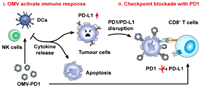
In addition to their role in the field of pathogenic microorganisms, OMVs are also frequently used in tumor immunotherapy. For instance, Ning's team developed OMVs derived from transgenic E. coli that had been modified with αvβ3 integrin peptide-targeting ligand and the photosensitizer ICG. These bacterial OMVs displayed excellent penetration of the stratum corneum and specific targeting of melanoma. Under near-infrared (NIR) stimulation, the photosensitizer ICG dissociates from the vesicles, leading to a strong photothermal effect that rapidly eliminates the primary tumor. Additionally, the NIR stimulation causes the release of tumor necrosis factor related apoptosis-inducing ligand from the OMVs, which activates apoptosis in the tumor, providing the possibility of completely eradicating melanoma with delayed recurrence and metastasis [118]. Furthermore, Peng et al. demonstrated the significant anti-tumor activity of OMVs. Upon intravenous injection, OMVs specifically accumulated in tumor tissues and induced an anti-tumor immune response that ultimately eliminated the tumor. OMVs derived from different bacterial strains displayed excellent inhibition of tumor growth in various tumor models, confirming the overall effectiveness of OMVs in antitumor therapy. Notably, the anti-tumor immune responses induced by OMVs were durable and exhibited immune memory, as mice that were cured did not experience relapse after receiving second and third inoculations of tumor cells [118]. Building on these findings, Nie's team fused and expressed the extracellular region of the PD-1 molecule on the surface of OMVs. This modification allowed the OMVs to not only effectively stimulate the immune system, but also inhibit the depletion of immune cells by tumor cells, leading to a stronger anti-tumor immune effect (Fig. 4) [119].
|
Download:
|
| Fig. 4. OMV-PD1 accumulation at the tumor site increases the infiltration of immune cells, such as dendritic cells (DCs) and NK cells, and activates an immune response in vivo. At the same time, the PD1 ectodomain on the OMV-PD1 surface blocks the PD1/PD-L1 interaction and protects CD8 T cells, which can then attack tumor cells. Copied with permission [119]. Copyright 2020, American Chemical Society. | |
In summary, due to their exceptional performance, bacterial outer membrane vesicles have garnered considerable attention and have been applied in various areas such as drug delivery and vaccine carriers. However, our current understanding of their structures and compositions is still not sufficiently in-depth, and further research and exploration are required before engineered bacteria and their derivatives can be utilized on the clinical frontline.
4. Artificial cell-based drug delivery systemWith the rapid development of synthetic biology technology, based on the "bottom-up" guidance strategy, researchers have isolated the smallest components of living systems and their biochemical components for in vitro synthesis and reconstruction and assembly to mimic biological processes in vivo or to give them unprecedented new functions [120-123]. In contrast to the top-down engineering approach, bottom-up assembled synthetic systems involve only essential components, resulting in reduced susceptibility to unwanted crosstalk, metabolic burdens, and toxic products.
The cell is both the basic structural and functional unit of an organism and the smallest unit that carries out life activities and has the ability to replicate independently. However, in exploring the complex biological processes and the mechanisms and origins of life, the cell's complexity, fragility and susceptibility to inactivation or death in vitro pose many challenges to this process [124-128]. To gain a more comprehensive and profound understanding of these complex biological processes or to bestow unprecedented functions to natural cells, researchers have endeavored to isolate modular components from complex cellular systems. Using bottom-up synthetic biology techniques, these modules are then functionally assembled to construct artificial cells that emulate the basic functions from scratch.
The concept of artificial cells was first proposed in 1957 by Chang, who successfully constructed vesicles using the lipids found in cell membranes [129,130]. An artificial cell, as a closed vesicle, has the ability to encapsulate biologically active substances such as enzymes and gene sequences, allowing for the realization of bionic functions or the development of previously impossible functions. To achieve full autonomy and sustainability, the ideal artificial cell should possess the following qualities [131-142] (ⅰ) compartmentalization, (ⅱ) energy supply, (ⅲ) protein production to perform fundamental functions, (ⅳ) transport mechanisms across compartment boundaries, (ⅴ) minimal metabolism, (ⅵ) ability to replicate genetic information, (ⅶ) a machinery capable of cell division.
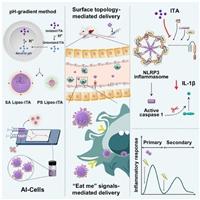
Brinker's team also constructed artificial apoptotic cells (AI-Cells) encapsulated with unmodified Chlamydia. Due to their similarity in morphology and membrane composition to apoptotic cells, AI-Cells were primarily localized to the liver and taken up by hepatic macrophages in a mouse model of acetaminophen (APAP)-induced acute liver failure. Moreover, AI-Cells effectively mitigated APAP-induced liver injury by regulating macrophage function. Notably, AI-Cells trained hepatic macrophages to exhibit an anti-inflammatory memory-like phenotype, significantly protecting mice from liver re-injury. Mechanistically, AI-Cells inhibited the activation of macrophage cysteinyl asparagin-1 and cleavage of pro-interleukin-1β (IL-1β) into its active form via the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome. Therefore, this potential AI-Cells therapy, which fine-tunes hepatic macrophages to orchestrate liver homeostasis, holds promise for the treatment of liver failure (Fig. 5) [143].
|
Download:
|
| Fig. 5. Artificial apoptotic cells loaded with itaconic acid (AI-Cells) are constructed, wherein the compositions of the synthetic plasma membrane and surface topology are rationally engineered. AI-Cells are predominantly localized to the liver and further transport to hepatic macrophages. Intravenous administration of AI-Cells modulates macrophage inflammation to protect the liver from acetaminophen-induced acute liver failure. Copied with permission [143]. Copyright 2023, Elsevier. | |
Using synthetic materials, Gu's team constructed a multicompartmental artificial β-cell with a glucose metabolism system and a membrane fusion mechanism. This bionic artificial cell can accurately distinguish between high and normal glucose levels through a sequential cascade of glucose uptake, enzyme oxidation, and proton exocytosis, thereby mimicking the glucose-responsive insulin secretion of natural pancreatic β-cells. This responsive insulin secretion provides new ideas and strategies for clinically improving diabetic patients [144]. In a different study, Schroeder et al. developed artificial lipid vesicles containing the molecular mechanisms required for transcription and translation. These vesicles obtain nutrients from their biological microenvironment to trigger the synthesis of anti-cancer proteins within tumors. Experimental studies demonstrated that co-incubating the artificial lipid vesicles capable of secreting the exotoxin Pseudomonas aeruginosa A (PE) with 4T1 breast cancer cells resulted in the majority of tumor cells being killed. In mice bearing 4T1 tumors, local injection of PE-secreting artificial lipid vesicles reliably induced apoptosis [145]. Gan et al. constructed smart glucose-responsive polymer vesicles (PEP-PMs) using glucose-responsive polymers with modified peptides that readily bind to the intestinal epithelial ganglioside-monosialic acid receptor. This allowed for efficient intestinal epithelial cell transport and bulk aggregation in the liver, which in turn regulated insulin release in response to glucose levels. The efficiency of in vivo liver-targeted delivery of insulin-releasing polymer vesicles was demonstrated in diabetic rats, and the system was shown to normalize hepatic glucose utilization. This system acts as a therapeutic biomolecular system capable of triggering cargo release through the combination of engineered modules sensitive to a stimulus (hydrogen peroxide), which are generated in response to an external signal (rising glucose concentration) by specialized metabolic modules. Importantly, the polymer vesicle combines core concepts from biosensing and targeted drug delivery, connecting them through a central compartmentalized response and providing broader ideas for the further development of oral insulin therapy [146].
In summary, the emergence of "artificial cells" provides a more accurate and programmable platform for intelligent drug delivery, vaccine design, and biosensing. It also offers greater possibilities for clinical diagnosis and treatment of diseases. However, the technology is not fully developed and mature, and its design principles and assembly laws still need improvement. Furthermore, supporting technologies such as in vivo trajectory detection, monitoring of drug release behavior, and dynamic feedback of diagnostic and therapeutic data need further establishment.
5. Summary and outlookIn recent years, with the vigorous development of synthetic biology research and technology, the new generation of drug delivery systems has also accomplished epoch-making innovations. By understanding the mechanisms and laws of natural life systems, we are able to integrate nanomaterials technology, develop technologies and tools to modify natural biological systems, and construct artificial biological systems through the closed loop of "design-construct-test". This enables the intersection and empowerment of nanomaterials and synthetic biology, and leads to the development of a new generation of drug delivery systems. This intersection and mutual empowerment allow for advancements in both building knowledge and building application in life science research and biotechnology. These advancements involve both gradual evolution and mutational leap. In terms of drug delivery systems, this paper primarily focuses on the research progress of using whole cell-based systems, cell membranes, exosomes, bacteria, and bacterial outer membrane vesicles. It also discusses how synthetic biology technology enhances natural biomaterials in terms of biocompatibility and biological breakthroughs.
Although significant advancements have been made in drug delivery systems engineered by synthetic biology, there are still urgent problems and challenges that need to be addressed in terms of clinical translation. For cell-based drug delivery systems, the safety of nanomaterials used cannot be overlooked. Although the biocompatibility of these nanomaterials has been well verified in laboratory animals such as mice, rats, and rabbits, the feasibility of applying them to clinical patients still presents a major challenge. Additionally, the precise control of chemical modifications, especially in sensitive biomolecules or complex biological systems, remains a direction of future research. Furthermore, the identification and establishment of mass-production methods and standardized routes for obtaining natural materials from different biological origins, and the assembly of materials of natural origin and artificial nanomaterials to achieve simpler handling, higher throughput, and lower-cost preparations, will greatly contribute to the clinical application of such drug delivery systems.
This paper focuses on the latest research progress on artificial cell-based drug delivery systems in relation to "mutational leapfrogging". It summarizes the necessary components and modules for building such systems, as well as the new breakthroughs in drug delivery and disease therapeutics achieved through customized function editing and recombination. However, artificial cell-based drug delivery systems still need to address many challenges. For example, in order to realize continuous monitoring, diagnosis and treatment in vivo without external supervisory intervention, more mature and perfect "artificial cells" should have the following functions: (ⅰ) Ensuring a continuous and renewable energy supply as the self-sustaining power module of the system; (ⅱ) Improving the sensitivity of regulatory mechanisms to effectively respond to conditions and resource allocation; (ⅲ) Correcting functional scaffolding modules, metabolic networks, and replicative processes; (ⅳ) Developing the ability to control and regulate the cellular system's function.
The essence and core of artificial cells lies in design, specifically designing the components and structures at the bottom to obtain purposeful functions at the top. Nevertheless, the intrinsic complexity of natural biomolecules, frequently integral components of synthetic biology, significantly challenges engineering precision and quantitative control. Therefore, it is imperative to introduce and apply computational biology and artificial intelligence-related technologies more widely in the construction of artificial biological systems. This includes creating or screening new biological components, characterizing and improving the properties of biomolecules, guiding the mining and design of catalytic components, regulatory components, and biosensors, and predicting and optimizing the design of screened gene circuits to achieve quantitative and data-driven theories of synthetic biology.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors are grateful to Dr. Zizhuang Li for his assistance in the English language editing.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109576.
| [1] |
K. Strebhardt, A. Ullrich, Nat. Rev. Cancer 8 (2008) 473-480. DOI:10.1038/nrc2394 |
| [2] |
R.S. Schwartz, N. Engl. J. Med. 350 (2004) 1079-1080. DOI:10.1056/NEJMp048021 |
| [3] |
X. You, L. Wang, J. Zhang, et al., Chin. Chem. Lett. 34 (2023) 107720. DOI:10.1016/j.cclet.2022.07.063 |
| [4] |
J. Huang, X. You, P. Xin, et al., Chin. Chem. Lett. 32 (2021) 1737-1742. DOI:10.1016/j.cclet.2020.12.006 |
| [5] |
Z. Fang, X. Zhang, H. Huang, J. Wu, Chin. Chem. Lett. 33 (2022) 1693-1704. DOI:10.1016/j.cclet.2021.11.050 |
| [6] |
Y.B. Meng, J. Wu, Chin. J. Polym. Sci. 40 (2022) 1016-1027. DOI:10.1007/s10118-022-2701-9 |
| [7] |
R. Chen, P. Ouyang, L. Su, et al., Chin. Chem. Lett. 33 (2022) 4610-4616. DOI:10.1016/j.cclet.2022.03.074 |
| [8] |
J. Zhang, J. Yan, Y. Wang, et al., Chin. Chem. Lett. 35 (2024) 108434. DOI:10.1016/j.cclet.2023.108434 |
| [9] |
S. Waheed, Z. Li, F. Zhang, et al., J. Nanobiotechnology 20 (2022) 395. DOI:10.1186/s12951-022-01605-4 |
| [10] |
P. Couvreur, Adv. Drug Del. Rev. 65 (2013) 21-23. DOI:10.1016/j.addr.2012.04.010 |
| [11] |
K. Park, ACS Nano 7 (2013) 7442-7447. DOI:10.1021/nn404501g |
| [12] |
E. Blanco, H. Shen, M. Ferrari, Nat. Biotechnol. 33 (2015) 941-951. DOI:10.1038/nbt.3330 |
| [13] |
F.S. Collins, H. Varmus, N. Engl. J. Med. 372 (2015) 793-795. DOI:10.1056/NEJMp1500523 |
| [14] |
O.S. Fenton, K.N. Olafson, P.S. Pillai, et al., Adv. Mater. 30 (2018) 1705328. DOI:10.1002/adma.201705328 |
| [15] |
S. Hirschi, T.R. Ward, W.P. Meier, et al., Chem. Rev. 122 (2022) 16294-16328. DOI:10.1021/acs.chemrev.2c00339 |
| [16] |
S.A. Benner, A.M. Sismour, Nat. Rev. Genet. 6 (2005) 533-543. |
| [17] |
N. Zhao, Y. Song, X. Xie, et al., Signal Transduct. Target Ther. 8 (2023) 112. DOI:10.1038/s41392-023-01375-x |
| [18] |
A.S. Khalil, J.J. Collins, Nat. Rev. Genet. 11 (2010) 367-379. |
| [19] |
H. Pick, A.C. Alves, H. Vogel, Chem. Rev. 118 (2018) 8598-8654. DOI:10.1021/acs.chemrev.7b00777 |
| [20] |
H. Jia, P. Schwille, Curr. Opin. Biotechnol. 60 (2019) 179-187. DOI:10.1016/j.copbio.2019.05.008 |
| [21] |
B.C. Buddingh, J.C.M. van Hest, Acc. Chem. Res. 50 (2017) 769-777. DOI:10.1021/acs.accounts.6b00512 |
| [22] |
B. An, Y. Wang, Y. Huang, et al., Chem. Rev. 123 (2023) 2349-2419. DOI:10.1021/acs.chemrev.2c00512 |
| [23] |
W. Li, X. Zhang, C. Zhang, et al., Nat. Commun. 12 (2021) 7264. DOI:10.1038/s41467-021-27434-x |
| [24] |
R. Kojima, D. Bojar, G. Rizzi, et al., Nat. Commun. 9 (2018) 1305. DOI:10.1038/s41467-018-03733-8 |
| [25] |
W. Jiang, Z. Wu, Z. Gao, et al., ACS Nano 16 (2022) 15705-15733. DOI:10.1021/acsnano.2c06104 |
| [26] |
F. Lussier, O. Staufer, I. Platzman, J.P. Spatz, Trends Biotechnol. 39 (2021) 445-459. DOI:10.1016/j.tibtech.2020.08.002 |
| [27] |
A. Cubillos-Ruiz, T. Guo, A. Sokolovska, et al., Nat. Rev. Drug Discov. 20 (2021) 941-960. DOI:10.1038/s41573-021-00285-3 |
| [28] |
L. Yang, Y. Yang, Y. Chen, et al., Adv. Drug Del. Rev. 187 (2022) 114394. DOI:10.1016/j.addr.2022.114394 |
| [29] |
Q. Lu, T. Liu, Z. Han, et al., J. Control. Release 361 (2023) 604-620. DOI:10.1016/j.jconrel.2023.08.023 |
| [30] |
S. Tan, T. Wu, D. Zhang, Z. Zhang, Theranostics 5 (2015) 863-881. DOI:10.7150/thno.11852 |
| [31] |
M.Y. Thanuja, C. Anupama, S.H. Ranganath, Adv. Drug Del. Rev. 132 (2018) 57-80. DOI:10.1016/j.addr.2018.06.012 |
| [32] |
H.Y. Chen, J. Deng, Y. Wang, et al., Acta Biomater. 112 (2020) 1-13. DOI:10.1016/j.actbio.2020.05.028 |
| [33] |
Z. Wu, H. Zhang, J. Yan, et al., Theranostics 13 (2023) 20-39. DOI:10.7150/thno.76894 |
| [34] |
H.H. Wu, Y. Zhou, Y. Tabata, J.Q. Gao, J. Control. Release 294 (2019) 102-113. DOI:10.1016/j.jconrel.2018.12.019 |
| [35] |
C. Tran, M.S. Damaser, Adv. Drug Del. Rev. 82- 83 (2015) 1-11. |
| [36] |
B. Crivelli, T. Chlapanidas, S. Perteghella, et al., J. Control. Release 262 (2017) 104-117. DOI:10.1016/j.jconrel.2017.07.023 |
| [37] |
L. Fu, H.N. Kim, J.D. Sterling, et al., Adv. Drug Del. Rev. 184 (2022) 114195. DOI:10.1016/j.addr.2022.114195 |
| [38] |
Q. Xia, Y. Zhang, Z. Li, et al., Acta Pharm. Sin. B 9 (2019) 675-689. DOI:10.1016/j.apsb.2019.01.011 |
| [39] |
P.H.D. Nguyen, M.K. Jayasinghe, A.H. Le, et al., ACS Nano 17 (2023) 5187-5210. DOI:10.1021/acsnano.2c11965 |
| [40] |
J.S. Brenner, S. Mitragotri, V.R. Muzykantov, Annu. Rev. Biomed. Eng. 23 (2021) 225-248. DOI:10.1146/annurev-bioeng-121219-024239 |
| [41] |
A. Choi, K. Javius-Jones, S. Hong, H. Park, Int. J. Nanomedicine 18 (2023) 509-525. DOI:10.2147/IJN.S394389 |
| [42] |
X. Dong, D. Chu, Z. Wang, Theranostics 7 (2017) 751-763. DOI:10.7150/thno.18069 |
| [43] |
K. Jin, Z. Luo, B. Zhang, Z. Pang, Acta Pharm. Sin. B 8 (2018) 23-33. DOI:10.1016/j.apsb.2017.12.002 |
| [44] |
Y.S. Zhu, K. Tang, J. Lv, Trends Pharmacol. Sci. 42 (2021) 857-869. DOI:10.1016/j.tips.2021.07.001 |
| [45] |
S. Sharma, M.K. Masud, Y.V. Kaneti, et al., Small 17 (2021) e2102220. DOI:10.1002/smll.202102220 |
| [46] |
F. Pierigè, S. Serafini, L. Rossi, M. Magnani, Adv. Drug Del. Rev. 60 (2008) 286-295. DOI:10.1016/j.addr.2007.08.029 |
| [47] |
L. Labanieh, R.G. Majzner, C.L. Mackall, Nat. Biomed. Eng. 2 (2018) 377-391. DOI:10.1038/s41551-018-0235-9 |
| [48] |
J. Wei, Y. Guo, Y. Wang, et al., Cell. Mol. Immunol. 18 (2021) 792-804. DOI:10.1038/s41423-020-00555-x |
| [49] |
R.C. Larson, M.V. Maus, Nat. Rev. Cancer 21 (2021) 145-161. DOI:10.1038/s41568-020-00323-z |
| [50] |
R.C. Sterner, R.M. Sterner, Blood Cancer J. 11 (2021) 69. DOI:10.1038/s41408-021-00459-7 |
| [51] |
A. Dimitri, F. Herbst, J.A. Fraietta, Mol. Cancer 21 (2022) 78. DOI:10.1186/s12943-022-01559-z |
| [52] |
Q. Liu, J. Li, H. Zheng, et al., Mol. Cancer 22 (2023) 28. DOI:10.1186/s12943-023-01735-9 |
| [53] |
M.M. Billingsley, N. Singh, P. Ravikumar, et al., Nano Lett. 20 (2020) 1578-1589. DOI:10.1021/acs.nanolett.9b04246 |
| [54] |
Q. Chen, Q. Hu, E. Dukhovlinova, et al., Adv. Mater. 31 (2019) e1900192. DOI:10.1002/adma.201900192 |
| [55] |
W. Ma, D. Zhu, J. Li, et al., Theranostics 10 (2020) 1281-1295. DOI:10.7150/thno.40291 |
| [56] |
S. Srivastava, S.N. Furlan, C.A. Jaeger-Ruckstuhl, et al., Cancer Cell 39 (2021) 193-208. DOI:10.1016/j.ccell.2020.11.005 |
| [57] |
S. Harmsen, E.I. Medine, M. Moroz, et al., Biomaterials 269 (2021) 120630. DOI:10.1016/j.biomaterials.2020.120630 |
| [58] |
D. Zou, Z. Wu, X. Yi, et al., Proc. Natl. Acad. Sci. U. S. A. 120 (2023) e2214757120. DOI:10.1073/pnas.2214757120 |
| [59] |
Q. Jiang, Y. Liu, R. Guo, et al., Biomaterials 192 (2019) 292-308. DOI:10.1016/j.biomaterials.2018.11.021 |
| [60] |
Q. Zhang, D. Dehaini, Y. Zhang, et al., Nat. Nanotechnol. 13 (2018) 1182-1190. DOI:10.1038/s41565-018-0254-4 |
| [61] |
P. Dash, A.M. Piras, M. Dash, J. Control. Release 327 (2020) 546-570. DOI:10.1016/j.jconrel.2020.09.012 |
| [62] |
R.H. Fang, A.V. Kroll, W. Gao, L. Zhang, Adv. Mater. 30 (2018) e1706759. DOI:10.1002/adma.201706759 |
| [63] |
N. Mohandas, P.G. Gallagher, Blood 112 (2008) 3939-3948. |
| [64] |
C. Liu, X. Liu, X. Xiang, et al., Nat. Nanotechnol. 17 (2022) 531-540. DOI:10.1038/s41565-022-01098-0 |
| [65] |
Y. Jiang, N. Krishnan, J. Zhou, et al., Adv. Mater. 32 (2020) e2001808. DOI:10.1002/adma.202001808 |
| [66] |
X. Zhang, Y. Kang, J. Wang, et al., Adv. Mater. 32 (2020) e1907692. DOI:10.1002/adma.201907692 |
| [67] |
J. Ma, S. Zhang, J. Liu, et al., Small 15 (2019) e1902011. DOI:10.1002/smll.201902011 |
| [68] |
S. Liu, J. Wu, Y. Feng, et al., Bioact. Mater. 22 (2023) 211-224. |
| [69] |
M. Xu, T. Feng, B. Liu, et al., Theranostics 11 (2021) 8926-8944. DOI:10.7150/thno.62330 |
| [70] |
O.P.B. Wiklander, M.Á. Brennan, J. Lötvall, et al., Sci. Transl. Med. 11 (2019) eaav8521. DOI:10.1126/scitranslmed.aav8521 |
| [71] |
I. Kimiz-Gebologlu, S.S. Oncel, J. Control. Release 347 (2022) 533-543. DOI:10.1016/j.jconrel.2022.05.027 |
| [72] |
I.K. Herrmann, M.J.A. Wood, G. Fuhrmann, Nat. Nanotechnol. 16 (2021) 748-759. DOI:10.1038/s41565-021-00931-2 |
| [73] |
L. Barile, G. Vassalli, Pharmacol. Ther. 174 (2017) 63-78. DOI:10.1016/j.pharmthera.2017.02.020 |
| [74] |
E.V. Batrakova, M.S. Kim, J. Control. Release 219 (2015) 396-405. DOI:10.1016/j.jconrel.2015.07.030 |
| [75] |
S. Salunkhe, M.Basak Dheeraj, et al., J. Control. Release 326 (2020) 599-614. DOI:10.1016/j.jconrel.2020.07.042 |
| [76] |
Z. Yang, J. Shi, J. Xie, et al., Nat. Biomed. Eng. 4 (2020) 69-83. |
| [77] |
P.H.L. Tran, D. Xiang, T.T.D. Tran, et al., Adv. Mater. 32 (2020) e1904040. DOI:10.1002/adma.201904040 |
| [78] |
Q. Wu, S. Fu, H. Xiao, et al., Adv. Sci. 10 (2023) e2204814. DOI:10.1002/advs.202204814 |
| [79] |
J. Guo, F. Wang, Y. Hu, et al., Cell Rep. Med. 4 (2023) 100881. DOI:10.1016/j.xcrm.2022.100881 |
| [80] |
T. Bu, Z. Li, Y. Hou, et al., Theranostics 11 (2021) 9988-10000. DOI:10.7150/thno.64229 |
| [81] |
R. Tenchov, J.M. Sasso, X. Wang, et al., ACS Nano 16 (2022) 17802-17846. DOI:10.1021/acsnano.2c08774 |
| [82] |
H. Duan, Y. Liu, Z. Gao, W. Huang, Acta Pharm. Sin. B 11 (2021) 55-70. DOI:10.1016/j.apsb.2020.09.016 |
| [83] |
S.M. van Dommelen, P. Vader, S. Lakhal, et al., J. Control. Release 161 (2012) 635-644. DOI:10.1016/j.jconrel.2011.11.021 |
| [84] |
S. Liu, X. Chen, L. Bao, et al., Nat. Biomed. Eng. 4 (2020) 1063-1075. DOI:10.1038/s41551-020-00637-1 |
| [85] |
J. Wang, W. Tang, M. Yang, et al., Biomaterials 273 (2021) 120784. DOI:10.1016/j.biomaterials.2021.120784 |
| [86] |
J. Du, Z. Wan, C. Wang, et al., Theranostics 11 (2021) 8185-8196. DOI:10.7150/thno.59121 |
| [87] |
Y. Guo, Z. Wan, P. Zhao, et al., J. Nanobiotechnology 19 (2021) 402. DOI:10.1186/s12951-021-01145-3 |
| [88] |
D. Deng, X. Li, J.J. Zhang, et al., ACS Nano 17 (2023) 8530-8550. DOI:10.1021/acsnano.3c00839 |
| [89] |
C. Gong, X. Zhang, M. Shi, et al., Adv. Sci. 8 (2021) 2002787. DOI:10.1002/advs.202002787 |
| [90] |
L. Alvarez-Erviti, Y. Seow, H. Yin, et al., Nat. Biotechnol. 29 (2011) 341-345. DOI:10.1038/nbt.1807 |
| [91] |
X. Gao, N. Ran, X. Dong, et al., Sci. Transl. Med. 10 (2018) eaat0195. DOI:10.1126/scitranslmed.aat0195 |
| [92] |
Q. Cheng, X. Shi, M. Han, et al., J. Am. Chem. Soc. 140 (2018) 16413-16417. DOI:10.1021/jacs.8b10047 |
| [93] |
F. Wu, J. Liu, Adv. Drug Del. Rev. 188 (2022) 114443. DOI:10.1016/j.addr.2022.114443 |
| [94] |
N.S. McCarty, R. Ledesma-Amaro, Trends Biotechnol 37 (2019) 181-197. DOI:10.1016/j.tibtech.2018.11.002 |
| [95] |
S.R. Kang, D.H. Nguyen, S.W. Yoo, J.J. Min, Adv. Drug Del. Rev. 181 (2022) 114085. DOI:10.1016/j.addr.2021.114085 |
| [96] |
H. Shen, N. Aggarwal, K.S. Wun, et al., Adv. Drug Del. Rev. 187 (2022) 114364. DOI:10.1016/j.addr.2022.114364 |
| [97] |
F. Farjadian, M. Moghoofei, S. Mirkiani, et al., Biotechnol. Adv. 36 (2018) 968-985. DOI:10.1016/j.biotechadv.2018.02.016 |
| [98] |
N.S. Forbes, Nat. Rev. Cancer 10 (2010) 785-794. DOI:10.1038/nrc2934 |
| [99] |
C. Yang, M. Cui, Y. Zhang, et al., Commun. Biol. 3 (2020) 561. DOI:10.1038/s42003-020-01287-4 |
| [100] |
F. Chen, Z. Zang, Z. Chen, et al., Biomaterials 214 (2019) 119226. DOI:10.1016/j.biomaterials.2019.119226 |
| [101] |
B.W. Park, J. Zhuang, O. Yasa, M. Sitti, ACS Nano 11 (2017) 8910-8923. DOI:10.1021/acsnano.7b03207 |
| [102] |
S. Chowdhury, S. Castro, C. Coker, et al., Nat. Med. 25 (2019) 1057-1063. DOI:10.1038/s41591-019-0498-z |
| [103] |
C.R. Gurbatri, I. Lia, R. Vincent, et al., Sci. Transl. Med. 12 (2020) eaax0876. DOI:10.1126/scitranslmed.aax0876 |
| [104] |
M. Toyofuku, N. Nomura, L. Eberl, Nat. Rev. Microbiol. 17 (2019) 13-24. DOI:10.1038/s41579-018-0112-2 |
| [105] |
M. Li, H. Zhou, C. Yang, et al., J. Control. Release 323 (2020) 253-268. DOI:10.1016/j.jconrel.2020.04.031 |
| [106] |
M.G. Sartorio, E.J. Pardue, M.F. Feldman, M.F. Haurat, Annu. Rev. Microbiol. 75 (2021) 609-630. DOI:10.1146/annurev-micro-052821-031444 |
| [107] |
M. Kaparakis-Liaskos, R.L. Ferrero, Nat. Rev. Immunol. 15 (2015) 375-387. DOI:10.1038/nri3837 |
| [108] |
Q. Feng, X. Ma, K. Cheng, et al., Adv. Mater. 34 (2022) e2206200. DOI:10.1002/adma.202206200 |
| [109] |
O.Y. Kim, H.T. Park, N.T.H. Dinh, et al., Nat. Commun. 8 (2017) 626. DOI:10.1038/s41467-017-00729-8 |
| [110] |
C. Schwechheimer, M.J. Kuehn, Nat. Rev. Microbiol. 13 (2015) 605-619. DOI:10.1038/nrmicro3525 |
| [111] |
V. Gujrati, S. Kim, S.H. Kim, et al., ACS Nano 8 (2014) 1525-1537. DOI:10.1021/nn405724x |
| [112] |
R.C. Laughlin, R.C. Alaniz, Gut Microbes 7 (2016) 450-454. DOI:10.1080/19490976.2016.1222345 |
| [113] |
M.J.H. Gerritzen, D.E. Martens, R.H. Wijffels, et al., Biotechnol. Adv. 35 (2017) 565-574. DOI:10.1016/j.biotechadv.2017.05.003 |
| [114] |
K. Cheng, R. Zhao, Y. Li, et al., Nat. Commun. 12 (2021) 2041. DOI:10.1038/s41467-021-22308-8 |
| [115] |
S. Qing, C. Lyu, L. Zhu, et al., Adv. Mater. 32 (2020) e2002085. DOI:10.1002/adma.202002085 |
| [116] |
Y. Li, K. Zhang, Y. Wu, et al., Small 18 (2022) e2107461. DOI:10.1002/smll.202107461 |
| [117] |
Q. Guo, X. Li, W. Zhou, et al., ACS Nano 15 (2021) 13826-13838. DOI:10.1021/acsnano.1c05613 |
| [118] |
L.H. Peng, M.Z. Wang, Y. Chu, et al., Sci. Adv. 6 (2020) eaba2735. DOI:10.1126/sciadv.aba2735 |
| [119] |
Y. Li, R. Zhao, K. Cheng, et al., ACS Nano 14 (2020) 16698-16711. DOI:10.1021/acsnano.0c03776 |
| [120] |
T. Ozdemir, A.J.H. Fedorec, T. Danino, C.P. Barnes, Cell Syst. 7 (2018) 5-16. DOI:10.1016/j.cels.2018.06.008 |
| [121] |
Y. Elani, Angew. Chem. Int. Ed. 60 (2021) 5602-5611. DOI:10.1002/anie.202006941 |
| [122] |
R.A. Meyer, J.C. Sunshine, J.J. Green, Trends Biotechnol. 33 (2015) 514-524. DOI:10.1016/j.tibtech.2015.07.001 |
| [123] |
S. Toda, L.R. Blauch, S.K.Y. Tang, et al., Science 361 (2018) 156-162. DOI:10.1126/science.aat0271 |
| [124] |
S. Ausländer, D. Ausländer, M. Fussenegger, Angew. Chem. Int. Ed. 56 (2017) 6396-6419. DOI:10.1002/anie.201609229 |
| [125] |
D.N. Nesbeth, A. Zaikin, Y. Saka, et al., Essays Biochem. 60 (2016) 381-391. DOI:10.1042/EBC20160014 |
| [126] |
Y. Lu, G. Allegri, J. Huskens, Mater. Horiz. 9 (2022) 892-907. DOI:10.1039/D1MH01431E |
| [127] |
G. Zubaite, J.W. Hindley, O. Ces, Y. Elani, ACS Nano 16 (2022) 9389-9400. DOI:10.1021/acsnano.2c02195 |
| [128] |
Y.J. Li, J.Y. Wu, J. Liu, et al., J. Nanobiotechnology 19 (2021) 242. DOI:10.1186/s12951-021-00986-2 |
| [129] |
U.T. Bornscheuer, Angew. Chem. Int. Ed. 49 (2010) 5228-5230. DOI:10.1002/anie.201003393 |
| [130] |
Y. Ding, F. Wu, C. Tan, Life 4 (2014) 1092-1116. DOI:10.3390/life4041092 |
| [131] |
P.L. Luisi, P. Stano, Nat. Chem. 3 (2011) 755-756. DOI:10.1038/nchem.1156 |
| [132] |
A.N. Zelikin, B. Städler, Small 16 (2020) e2003442. DOI:10.1002/smll.202003442 |
| [133] |
H. Seo, H. Lee, Nat. Commun. 13 (2022) 5179. DOI:10.1038/s41467-022-32889-7 |
| [134] |
V. Noireaux, Y.T. Maeda, A. Libchaber, Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 3473-3480. DOI:10.1073/pnas.1017075108 |
| [135] |
J.A. Vance, N.K. Devaraj, J. Am. Chem. Soc. 143 (2021) 8223-8231. DOI:10.1021/jacs.1c03436 |
| [136] |
Y. Ai, R. Xie, J. Xiong, Q. Liang, Small 16 (2020) e1903940. DOI:10.1002/smll.201903940 |
| [137] |
X. Wang, H. Du, Z. Wang, et al., Adv. Mater. 33 (2021) e2002635. DOI:10.1002/adma.202002635 |
| [138] |
A. Leathers, M. Walczak, R.A. Brady, et al., J. Am. Chem. Soc. 144 (2022) 17468-17476. DOI:10.1021/jacs.2c06140 |
| [139] |
D. Gaur, N.C. Dubey, B.P. Tripathi, Adv. Colloid Interface Sci. 299 (2022) 102566. DOI:10.1016/j.cis.2021.102566 |
| [140] |
B.C. Buddingh, J. Elzinga, J.C.M. van Hest, Nat. Commun. 11 (2020) 1652. DOI:10.1038/s41467-020-15482-8 |
| [141] |
M. Dwidar, Y. Seike, S. Kobori, et al., J. Am. Chem. Soc. 141 (2019) 11103-11114. DOI:10.1021/jacs.9b03300 |
| [142] |
T. Mashima, M. van Stevendaal, F.R.A. Cornelissens, et al., Angew. Chem. Int. Ed. 61 (2022) e202115041. DOI:10.1002/anie.202115041 |
| [143] |
N. Yin, W. Zhang, X.X. Sun, et al., Cell Rep. Med. 4 (2023) 101132. DOI:10.1016/j.xcrm.2023.101132 |
| [144] |
Z. Chen, J. Wang, W. Sun, et al., Nat. Chem. Biol. 14 (2018) 86-93. DOI:10.1038/nchembio.2511 |
| [145] |
N. Krinsky, M. Kaduri, A. Zinger, et al., Adv. Healthc. Mater. 7 (2018) e1701163. DOI:10.1002/adhm.201701163 |
| [146] |
A. Wang, W. Fan, T. Yang, et al., Adv. Funct. Mater. 30 (2020) 1910168. DOI:10.1002/adfm.201910168 |
 2024, Vol. 35
2024, Vol. 35 

