b Department of Nanobiotechnology/Biophysics, Faculty of Biological Science, Tarbiat Modares University, Tehran 14115-154, Iran;
c Research and Development Department, Sina Medical Biochemistry Technologies Co., Ltd., Shiraz 7178795844, Iran;
d Department of Mechanical and Industrial Engineering, Qatar University, Doha 2713, Qatar;
e Biomedical Research Center, Qatar University, Doha 2713, Qatar;
f Independent Researcher, W Nazar ST, Boostan Ave, Isfahan 81756-33551, Iran;
g Centre of Research Impact and Outcome, Chitkara University, Rajpura, Punjab 140401, India;
h Department of Biomaterials, Saveetha Dental College and Hospitals, SIMATS, Saveetha University, Chennai 600077, India;
i Department of Science & Technology, Department of Urology, NanoBioMed Group, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People's Hospital, Quzhou 324000, China
Dermal wound healing, an intricate biological process that restores tissue integrity and function following injury, represents a significant challenge in healthcare and regenerative medicine [1]. The ability to accelerate wound closure, reduce scarring, and enhance tissue regeneration has long been the focus of intense research and innovation. In recent years, the intersection of advanced materials science and biomedical research has given rise to a novel and promising approach: the integration of metal-organic frameworks (MOFs) with polymers to create composite materials tailored for the specific demands of dermal wound healing [2-4]. Wound healing involves complex cellular events including inflammation, tissue proliferation, and remodeling [5]. Imbalances in this process can lead to chronic wounds, complications, and prolonged suffering [6]. Traditional therapies such as dressings and ointments have drawbacks like uncontrolled drug release, limited antimicrobial properties, and insufficient mechanical support for tissue regeneration [7]. Integrating MOFs and polymers can help overcome these limits, paving the way for advanced wound-healing solutions [8]. MOFs possess remarkable porosity, high surface area, and chemical functionality, ideal for drug delivery and aiding wound healing processes [5]. Meanwhile, polymers, known for biocompatibility and mechanical strength, making them pivotal in wound dressings and tissue engineering. Combining MOFs' absorption abilities and controlled release with polymers' flexibility and strength offers a unique avenue for advanced wound care solutions [6]. This review emphasizes the potential of MOF-polymer composites in dermal wound healing, highlighting their distinctive properties, preparation methods, and dermal wound healing applications, and discussing future prospects and challenges.
2. Preparation of MOF-polymer compositesMOFs, introduced in 1995 by Yaghi and Li, are crystalline, porous materials composed of metal centers linked by organic molecules, offering high surface area and adjustable pore sizes [7-10]. Due to these qualities, they are favored in drug delivery, biosensing, and biomedical research [11-14]. In recent years, MOFs have gained significant traction in wound healing research. This heightened interest relates to their unique surface modulation capabilities, enabling targeted delivery and controlled release of wound healing agents. Additionally, their comparatively lower toxicity, coupled with inherent angiogenicm, anti-microbial, and anti-inflammation properties, makes them stand out from other nanomaterials used in this field [15]. The structures, components, and main roles of various MOF materials in wound healing applications are presented in Table S1 (Supporting information).
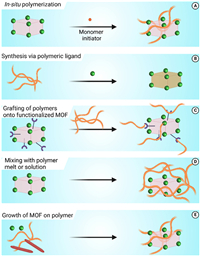
Although MOFs have remarkable physicochemical features, their application in biomedicine faces limitations. Combining porous MOFs with flexible polymers creates composites with enhanced properties, addressing issues like limited degradability and stability in aqueous solutions. Integrating MOFs into polymer networks forms advanced materials with superior properties compared to individual components. These composites combine the strengths of MOFs and polymers, providing versatile applications in different fields [16]. The literature presents diverse strategies for designing MOF-polymer composites, offering different structural layouts. Current research outlines five primary synthetic approaches for creating these composite systems (Fig. 1).
|
Download:
|
| Fig. 1. General methods are frequently employed in the preparation of MOF-polymer composite including (A) In-situ polymerization, (B) synthesis via ligand, (C) grafting of polymers onto functionalized MOF, (D) mixing of melt polymer or solution with MOF, (E) growth of MOF on polymer. | |
This method involves polymerization within MOF pores using monomers that can enter these pores and then continue polymerization using chemicalinitiators or other inventors, or straight reactions with the MOF structure [17,18]. Several vinyl polymers, such as poly(vinyl acetate), poly(acrylonitrile), and poly(methyl methacrylate) have been incorporated into MOFs.
2.2. MOF creation by employing polymeric ligandsIn 2015, Seth Cohen's team pioneered polyMOFs. This innovative approach merged metal ions/clusters with organic polymers, creating composite systems with unique properties. They designed polymeric linkers directly used in MOF synthesis, integrating polymers during crystallization instead of traditional organic ligands. These polyMOFs combine MOFs' strengths (porosity and crystallinity) with polymers' benefits (processability and stability) [19]. This technique allowed the synthesis of diverse polyMOF materials with varying structures, from spherical superstructures to crystalline films [20,21].
2.3. Grafting of polymers onto functionalized ligands of MOFsAnother method involves post-synthetic polymer grafting by attaching polymers to pre-formed MOFs, often focusing on the outer surface to enhance stability or introduce new functionalities. This method involves using pre-functionalized ligands containing reactive groups during MOF creation, although limitations arise due to compatibility issues in high-temperature, high-pressure conditions [22]. Researchers commonly employ post-synthetic polymer grafting to enhance MOF properties. These modifications enable dynamic responses in bio-applications but rely on reactive ligands, limiting specific uses [23]. However, studies on how altering polymer properties affects MOF performance remain limited [24,25].
2.4. Polymer attachment to MOFs via post-synthetic modificationWhen traditional polymerization within MOFs is not viable or direct MOF formation from polymeric ligands is not feasible, integrating polymers into MOFs employs innovative techniques. "Polymer melt processing" involves blending pre-formed polymers with pre-prepared MOF powder, heating the mixture beyond the polymer's melting point, and allowing softened chains to intercalate into the MOF upon cooling [26].
2.5. Template-directed growth of MOFs on polymersScientists explore methods to grow MOF crystals around pre-formed polymer, either tethering polymer to inner pore surfaces or fully encapsulating them within MOF interiors through a "ship-in-a-bottle" effect during crystal growth. This approach overcomes challenges like uneven polymer distribution and pore blockage in composite synthesis, allowing diverse polymer integration. Using metal ions as cross-linkers in natural polymer hydrogels, researchers have constructed MOF/polymer composites. This method allows for different MOFs by immersing metal-containing hydrogels [27,28].
3. Physicochemical and biological features of MOF-polymer compositesMOF-based polymer composites, particularly in the context of biomedical applications and wound dressing, offer a brilliant property that makes them promising materials.
3.1. Chemical stabilityDespite the development of chemically stable MOFs such as zirconium and titanium-based MOFs, several MOFs such as HKUST-1 (stands for Hong Kong University of Science and Technology) can be vulnerable to chemical instability in aquatic environments. Exposure to H+, OH−, and H2O, leading to framework transformation or decomposition [29]. Strategies to enhance stability include increasing coordination points [30,31], using alternative ligands with higher pKa [32], locally modifying labile coordination bonds [33,34], and surface functionalization with hydrophobic molecules or polymeric coatings [35,36]. This method aims to bolster MOF stability for diverse bioapplications.
3.2. Mechanical stabilityPolymers enhance mechanical support and flexibility in wound dressing composites. Maintaining stability during activation procedures) such as UV-based and supercritical CO2 (which is vital for MOFs, especially with large mesoporous networks. Challenges arise from solvent removal, leading to framework distortion and reduced active site accessibility. To bolster MOF stability, molecules or polymers are introduced inside pores, preventing collapse during activation [37].
3.3. Thermal stabilityThermal stability is crucial for MOFs, determined by metal-ligand bonds and node connection strength. Certain di- or tri-valent metal cations and ligands (such as carboxylate-based linkers) exhibit high thermal stability [38]. Understanding temperature-induced phase changes helps identify optimal activation temperatures. Introducing polymers has aided in preventing phase transitions.
3.4. Highly porous structures and extensive surface area for therapeutic deliveryMOFs, with their porous structure and very high surface area, excel in transporting therapeutic agents like nitric oxide (NO), essential in wound healing due to its anti-inflammatory and collagen-boosting effects [39]. Li et al. engineered cobalt-based MOFs, ZIF-67 (stands for zeolitic imidazolate frameworks), to deliver dimethyloxalylglycine, aiding diabetic wound treatment. These MOFs, embedded in a poly(l-lactic acid)/gelatin scaffold, formed a cooperative drug-copper ion release system lasting around two weeks [40]. When combined with hydrogels, MOFs amplify porosity, enhancing water absorption and creating a gas exchange-friendly scaffold.
3.5. Antibacterial activitySome MOFs inherently possess antibacterial properties or can be modified to include antibacterial agents, aiding in infection prevention. Recent studies highlight advantages of MOF-polymer platforms in acting as antibacterial agents, namely: (ⅰ) bactericidal metal centers; MOFs with metal ions such as Ag, Zn, Cu, and Ni demonstrate effective inhibition of various bacteria, (ⅱ) adjustable pore sizes of MOF offer adaptable pore sizes to encapsulate diverse antimicrobial agents, (ⅲ) post-modification-functional polymers can be attached to MOFs, boosting therapeutic potential [41].
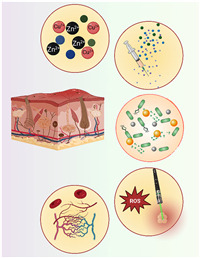
4. Applications in dermal wound healingThis article predominantly delves into the multifaceted applications of MOF materials within the realm of wound healing. Our focus spans different dimensions, exploring how MOF materials contribute significantly to various aspects of the wound-healing process. Specifically, we delve into their role in creating sustained-release systems for metal ions, catalyzing reactions crucial for healing, regulating controlled drug delivery to optimize treatment, and harnessing photoexcited systems to further aid in the wound healing journey (Fig. 2). Each of these applications represents a distinct avenue where MOF materials demonstrate their potential to enhance and advance the overall wound healing mechanisms. Table S2 (Supporting information) presents a comprehensive overview of diverse types of MOFs, detailing their respective properties and applications.
|
Download:
|
| Fig. 2. Applications of the MOF in dermal wound healing including (from top to down) prolonged metal ion and drug delivery, antibacterial properties, photoexcited systems, and angiogenesis. | |
Bacterial infections pose substantial obstacles to wound healing process as they often result in serious complications that are challenging to eliminate. On the other hand, the rise of antibiotic resistance has led to bacterial infections becoming formidable hurdles in clinical treatment [42,43]. Several studies have indicated that MOFs exhibit antibacterial properties by serving as reservoirs for certain metal ions, allowing for controlled release over time.
Copper has long been acknowledged as an exceptionally potent antibacterial agent in wound healing practices [44-46]. Moreover, copper-based MOFs possess remarkable physicochemical properties compared to other MOFs, leading to their increasing popularity in diverse biomedical fields such as anticancer treatments, antibacterial agents, biosensors, biocatalysis, and wound healing. The redox chemistry of copper, with a standard redox potential of Cu2+/Cu+ at 0.153 eV, is particularly intriguing due to its susceptibility to donor atoms and coordination geometry. The inherent ability of Cu2+ to stimulate angiogenesis and exhibit antibacterial properties positions Cu-MOFs as a highly promising solution for enhancing wound healing. Furthermore, the rate of Cu2+ release is a critical factor in numerous biological applications. It is essential to achieve accelerated release of Cu2+ at specific sites, such as tumor or infected wounds, while ensuring sustained release in normal tissues. This dynamic management of Cu-MOFs' stability is crucial for achieving optimal therapeutic effects while minimizing systemic metal toxicity [47]. To address these properties, an impressive study showcased an antibacterial film incorporating copper MOF (HKUST-1) and the natural polysaccharide, chitosan (CS). By manipulating either the metal ion centers or the organic linkers, the physical and chemical attributes of MOFs can be adjusted, offering precise control over copper ion storage and release within the HKUST-1/CS film. Integrating MOFs into versatile CS aids in metal ion chelation, overcoming water stability limitations of HKUST-1, and enhancing powdered material characteristics. Additionally, the fabrication method involving HKUST-1 nanoparticle coating with CS facilitates the regulated release of copper ions. In vivo experiments have showcased the efficacy of the HKUST-1/CS film in preventing wound infections caused by Staphylococcus aureus (S. aureus) and expediting wound closure rates in local infection treatments. These beneficial effects stem from the film's capacity to prompt blood vessel formation (angiogenesis) and facilitate repair across both the epidermis and dermis [48].
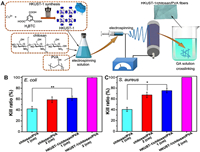
The utilization of electrospinning technology in the preparation of fiber membranes has garnered significant interest in wound dressing applications. These membranes possess a notable surface area-to-volume ratio, excellent air permeability, and the flexibility to adjust pore sizes. Consequently, electrospun fiber membranes have gained substantial popularity in wound dressing applications [7]. To enhance wound treatment capabilities, an upgraded preparation method was adopted to craft HKUST-1/CS/polyvinyl alcohol (PVA) fibers by integrating Cu-MOFs (HKUST-1) into a composite of electrospun CS and PVA (Fig. 3). These fibers displayed advantageous physical characteristics for wound healing, validated through water absorption tests, mechanical evaluations, and rates of water vapor transmission. Biocompatibility assessments demonstrated that HKUST-1/CS/PVA fibers were non-toxic, fostering cell attachment and proliferation. Incorporating HKUST-1 conferred effective antibacterial properties upon the fibers against both S. aureus and E. coli. In a rat wound model, HKUST-1/CS/PVA fibers exhibited substantial wound-healing effects with minimal inflammation (Figs. 2B and C). Overall, the HKUST-1/CS/PVA fiber emerges as a promising dressing to facilitate the regeneration of complete cutaneous tissue [49].
|
Download:
|
| Fig. 3. (A) The manufacturing method for HKUST-1/CS/PVA fibers and the efficacy of CS/PVA fibers and HKUST-1/CS/PVA fibers, measuring their killing efficiency against E. coli (B) and S. aureus (C) at diameters of 1 and 2 cm. Abbreviations: HKUST-1, Hong Kong University of Science and Technology; CS, chitosan; PVA, polyvinyl alcohol, GA, glutaraldehyde. Copied with permission [49]. Copyright 2020, Elsevier. | |
Zinc (Zn2+) serves as a safe antimicrobial agent in dermatology, finding extensive use as an astringent, moisturizer, and antibacterial agent [50]. In a pioneering study, researchers harnessed a MOF coating as a localized antibacterial dressing, eliminating reliance on antibiotics or chemicals. Unlike traditional MOFs, zinc-based zeolitic imidazolate framework (ZIF) nano-dagger arrays (ZIF-L) employed a distinct approach to inhibit bacterial growth. The pointed tips of the nano-array ruptured microbial cells, causing bacterial death through a physical mechanism rather than releasing metal ingredients. This innovative approach resulted in significant reductions in both S. aureus and E. coli, surpassing log reductions of 7. Comparative analysis with commercially available silver gauze revealed that ZIF-L coated gauze displayed superior biocompatibility, reduced hemolytic activity, lower cytotoxicity, and improved wound healing. Animal studies further demonstrated ZIF-L-coated gauze's exceptional bacterial disinfection capacity, effectively eradicating bacteria in infected wounds and expediting healing [51].
To address wound infections and promote wound healing a multifunctional wound dressing was designed. The study involved the preparation of a curcumin-based metal-organic framework (QCSMOF-Van) loaded with vancomycin and coated with quaternary ammonium salt chitosan (QCS). Composite hydrogels were synthesized by combining gelatin methacrylate (GelMA) and sodium methacrylic acid oxidized alginate (OSAMA) with QCSMOF-Van through radical polymerization and Schiff base reaction. The positive charges on the surface of QCSMOF-Van facilitated the capture of bacteria to minimize potential toxic effects on normal human tissues. The combined action of broad-spectrum antibacterial Zn2+ and vancomycin effectively facilitated the rapid killing of captured bacteria. The QCSMOF-Van hydrogels exhibited control over the balance of M1/M2 phenotypes of macrophages, promoting nerve and blood vessel regeneration and thereby accelerating chronic wound healing. Consequently, this novel cascade management approach serves as multifunctional composite hydrogels in the field of chronic wound dressings [52].
Silver-based products are widely used in antimicrobial wound dressings [53]. To address the rising concern over antibiotic resistance caused by prolonged antibiotic use, a study focused on developing two highly effective MOFs. These MOFs capitalized on the combined effects of reactive organic radicals and silver (Ag) cations to combat bacterial infections. By integrating Ag-based bioactive compounds, these MOFs displayed robust antimicrobial properties against both Gram-positive and Gram-negative bacteria. Animal experiments carried out on mice showcased these MOFs' remarkable capacity to expedite the healing of infected wounds while exhibiting minimal cytotoxicity. This research underscored the potential of Ag-MOFs as potent therapies against infections and highlighted their promising applications in clinical settings [9].
4.2. Catalytic systems and antibacterial propertiesRecently, catalytic systems utilizing MOF materials have demonstrated significant promise in combating bacterial infection. MOF-based catalytic systems primarily harness the peroxidase (POD) or oxidase (OXD) activities to generate reactive oxygen species (ROS) from hydrogen peroxide (H2O2) and oxygen (O2). By decomposing H2O2, MOFs produce hydroxyl radicals (•OH), enhancing antibacterial activity in laboratory conditions and significantly expediting wound healing [54,55]. Recent advancements in functional group development, such as guanidine, amines, and carboxyl groups, aim to replicate amino acid residue functions [56].
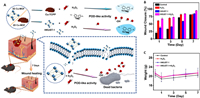
Amidst growing concerns about antibiotic resistance from traditional antibiotics, a recent study concentrated on developing MOFs, complex chemical structures in which copper coordinates with organic ligands. This investigation explored various dimensions of Cu-MOFs for potential applications in antibacterial therapy and wound healing enhancement. Among these, the three-dimensional (3D) MOF known as HKUST-1 exhibited superior peroxidase-like (POD-like) activity in comparison to the two-dimensional (2D) ultrathin metal-organic framework nanosheets (Cu-TCPP). The remarkable POD-like activity of HKUST-1 facilitated the swift generation of ROS by decomposing H2O2 into highly potent hydroxyl radicals (•OH). Leveraging this POD-like activity and •OH generation, Cu-MOFs were employed for in vivo antibacterial efficacy and wound healing. In vitro experiments showcased HKUST-1's effective catalysis of H2O2 into •OH, resulting in the successful eradication of E. coli and methicillin-resistant Staphylococcus aureus (MRSA) (Fig. 4). Moreover, HKUST-1 demonstrated its capability to expedite wound healing without inducing significant biological toxicity in major organs or adverse effects (Figs. 3B and C). This study introduced MOFs of diverse structural dimensions and varying POD-like activity as a promising approach for antimicrobial strategies (Fig. 4) [4].
|
Download:
|
| Fig. 4. (A) Understanding the POD-like activity mechanism in Cu-MOFs of varying dimensional structures for their antibacterial and wound healing properties. (B) The size of wound closure in mice following various treatments. (C) The body weights of mice undergoing different therapies. Abbreviations: MOF, metal-organic-frameworks; Cu-TCPP, ultrathin metal-organic framework nanosheets; TMB, 3,3′, 5,5′-tetramethylbenzidine; POD-like, superior peroxidase-like. Copied with under access license [4]. Copyright 2023, MDPI. | |
In medical science, a significant emphasis is placed on enhancing targeted drug delivery and controlled drug release to minimize harm to healthy tissues. The release speed of metal ions from MOFs can be influenced by the choice of different organic ligands. In the context of drug delivery systems based on MOFs, the selection of organic ligands can impact the release kinetics of therapeutic agents.
Composites based on MOFs can serve as carrier systems for loading antimicrobial ions, gases, or drugs, enabling sustained and controlled release. The design of nanoscale MOFs allows for efficient drug transportation within the circulatory system of organisms, improving the pharmacokinetic properties of drugs. Additionally, different organic ligands can result in MOFs with varying pore sizes, enhancing cargo encapsulation efficiency. For example, in wound healing and skin regeneration applications, MOFs can encapsulate bioactive agents, trap them within their inherent pores, or attach them to their surfaces through electrostatic and hydrophobic interactions. This allows for localized and controlled release of therapeutic agents at the wound site. Recent developments have focused on utilizing MOFs as delivery carriers for bioactive agents in wound healing applications. These advancements highlight the potential of MOFs in achieving precise drug delivery, sustained release, and improved therapeutic outcomes [57]. The field has seen growing interest in the utilization of nanotechnology to facilitate controllable drug delivery and release [58].
Silver nanoparticles (AgNPs) have emerged as highly effective antibacterial nanomaterials, showing promise as alternatives to conventional antibiotics [59]. Yet, the excessive release of silver ions can pose risks to healthy tissues. MOFs present advantageous traits as drug carriers, featuring natural biodegradability and effective drug-loading capabilities. As a result, MOFs can serve as carriers for AgNPs. These carriers not only maintain the antibacterial efficacy of AgNPs but also curb the excessive release of silver ions, consequently averting prolonged direct interaction between silver and healthy tissues [60-62].
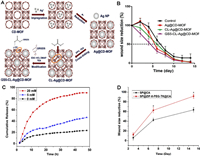
In a recent study, γ-cyclodextrin metal-organic frameworks (CD-MOFs) were utilized as templates for synthesizing ultrafine AgNPs, approximately 2 nm in size. This method served a dual purpose: anchoring AgNPs and reducing metal ions (Ag+), resulting in smaller particle sizes and heightened stability. Additionally, the hydrophilic nature of CD-MOFs enables easy dispersion of AgNPs and the release of Ag+ ions, leading to a bacteriostatic effect. Through further cross-linking and surface modification using the Gly-Arg-Gly-Asp-Ser (GRGDS) peptide, the hemostatic and synergistic effects with antibacterial properties were amplified, fostering wound healing (Fig. 5A). Despite the presence of inflammatory factors near the injured tissue during GS5-CL-Ag@CD-MOF treatment, a significant decrease in expression levels of cytokines, angiogenesis, and maturation of granulation tissue was observed, effectively facilitating wound healing. Researchers incorporated GS5 oligopeptides into crosslinked CD-MOFs and post-surface modification, the resulting hybrid material based on MOFs demonstrated capabilities in enhancing platelet hemostatic function, boosting hemostatic effects, adhering to the wound site, collaborating with the antibacterial effects of AgNPs, and advancing wound healing (Fig. 5B). These findings offer promising prospects for designing effective wound repair devices [63].
|
Download:
|
| Fig. 5. (A) Synthesis of AgNPs utilizing CD-MOF templates via solution impregnation, reduction, crosslinking, and surface modification with GRGDS. (B) Assessing wound size reduction in rats over time post-treatment with control, Ag@CD-MOF, CL-Ag@CD-MOF, and GS5-CL-Ag@CD-MOF. Abbreviations: CD-MOF, γ-cyclodextrin metal-organic frameworks; GS5, an oligopeptide; CL, cross-linked; TEA, triethylamine; DPC, diphenyl carbonate. Adapted from [63]. Copyright 2019, Wiley. (C) Evaluating the release patterns of SP from SP@ZIF-8-PEG-TK nanoparticles under varying H2O2 concentrations. (D) Measuring wound area reduction following treatment with SP@CA and SP@ZIF-8-PEG-TK@CA. Abbreviations: SP, substance P; PEG-TK, polyethylene glycol-thioketal; CA, calcium chloride; ZIF-8, zeolite imidazolate framework-8. Copied with permission [66]. Copyright 2020, Dove Medical Press. | |
Stimulus-responsive biopolymer systems have garnered considerable attention in biomedical applications, especially in creating wound dressings responsive to ROS. Among these systems, substance P (SP), a natural factor involved in initiating injury responses, has shown its capability to regulate inflammation and advance wound healing processes [64,65]. In a recent investigation, SP-loaded ZIF-8 nanoparticles (NPs) were coated with polyethylene glycol thioacetal (PEG-TK), which possesses ROS-responsive properties. This led to the formation of SP@ZIF-8-PEG-TK NPs. These NPs were then integrated into an injectable hydrogel composed of pectin and sodium alginate, cross-linked with calcium chloride (CA), creating the SP@ZIF-8-PEG-TK@CA hydrogel dressing, a novel SP drug delivery system. The SP@ZIF-8-PEG-TK NPs demonstrated high drug loading efficiency, maintained drug activity despite ROS presence, and displayed responsive drug release. In vitro studies highlighted the NPs' promotion of human dermal fibroblast proliferation, upregulation of inflammation-related gene expression in macrophages, and favorable cytocompatibility. Furthermore, in an animal wound model, the SP@ZIF-8-PEG-TK@CA dressing accelerated wound healing by initiating early inflammatory responses and subsequent M2 macrophage polarization (Figs. 5C and D) [66].
4.4. Photoexcited systemsPhotodynamic therapy (PDT) is a medical procedure employing photosensitizers (PSs) and appropriate light sources to create ROS. These ROS possess the ability to oxidize and disrupt nearby biomolecules, resulting in the elimination of bacteria and disease-causing microorganisms [67]. PDT offers several advantages, including a low likelihood of drug resistance and minimal safety concerns [68,69].
Moreover, the escalating occurrence of antibiotic resistance presents a considerable hurdle in clinical therapy, underscoring the necessity for alternative strategies against bacterial infections [70]. Recently, there has been mounting enthusiasm for the utilization of MOFs that operate independently of antibiotics [71,72].
While many MOFs have demonstrated antibacterial capabilities through controlled framework decomposition, typically achieved by adjusting the pH, this approach presents challenges in terms of control and stability under acidic conditions. Moreover, excessive release of intrinsically bactericidal cations (such as Zn2+ and Cu2+) or encapsulated antimicrobial agents can lead to biological toxicity in humans [15,73,74]. Therefore, there is a continued need to explore MOFs for antibacterial purposes, aiming for higher stability, reduced toxicity, and improved bactericidal properties.
To address this concern, scientists have investigated the amalgamation of PS MOFs and PDT as a means to counter multidrug-resistant bacteria (MDRA). The integration of PSs as ligands within MOFs not only alleviates the self-quenching effect of PSs but also introduces novel attributes to MOFs, rendering them highly responsive to visible light and enhancing ROS production. Antibacterial photodynamic therapy (APDT) relies on the ROS generation initiated by PSs when exposed to suitable light. These ROS undergo chemical interactions with diverse biological components like DNA, RNA, lipids, and proteins, causing structural alterations that disrupt bacterial function and eventually lead to bacterial demise [75,76]. Moreover, a recent study investigates the influence of size on the photodynamic activity of porphyrin-based MOF nanosheets. The smaller nanosheets exhibited higher photodynamic activity for generating ROS compared to larger nanosheets and bulk MOF crystals, indicating the size-dependent enhancement of photodynamic antimicrobial therapy and wound healing [77].
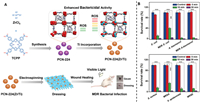
As discussed above, in a recent study, a straightforward synthesis strategy was developed to create porous MOFs called PCN-224. These MOFs possess inherent non-antibacterial properties, enabling their utilization in PDT for treating chronic wounds infected with bacteria, without the necessity for supplementary antibacterial elements. By employing a cation exchange technique, researchers synthesized bimetallic PCN-224(Zr/Ti), where titanium (Ti) partially substituted zirconium (Zr) clusters within PCN-224, while TCPP functioned as the photosensitizer in the frameworks (Fig. 6A). The introduction of titanium notably enhanced the production of ROS and displayed impressive photocatalytic activity, thereby augmenting the antibacterial effectiveness. To craft a wound dressing that exhibits outstanding biocompatibility and minimal cytotoxicity, PCN-224(Zr/Ti) nanoparticles were loaded onto poly(lactic-co-glycolic acid) (PLGA) nanofibers. In vivo experiments showcased the successful treatment of chronic wounds infected by multidrug-resistant bacteria (MDRA) using PDT-based wound dressings. Remarkably, this method obviates the need for additional antimicrobial agents, reducing the risks associated with allergies, toxicity, and the development of microbial drug resistance stemming from excessive antibiotic usage. This research underscores the substantial potential of MOFs in serving as influential non-antimicrobial agents in PDT (Fig. 6B) [78].
|
Download:
|
| Fig. 6. (A) The improved bactericidal effectiveness of PCN-224(Zr/Ti) due to heightened ROS production via titanium integration, and the application of a biocompatible PCN-224(Zr/Ti)-derived dressing as a light-driven antibacterial remedy for managing bacterial infections. (B) Assessing the viability of both Gram-negative and Gram-positive bacteria when exposed to irradiation while treated with 50 µg/mL PCN-224(Zr/Ti). Abbreviations: TCPP, tetrakis(4-carboxyphenyl)porphyrin); MDR, multidrug-resistant; ROS, reactive oxygen species; PCN-224, porous MOFs. Copied with permission [78]. Copyright 2020, Wiley Online Library. | |
Angiogenesis, the formation of new blood vessels, is a crucial stage of the wound-healing process. In chronic wounds, reduced microvascular regeneration causes inflammation and oxygen shortage [79]. This leads to a persistent inflammatory response and hinders the transition of wounds from inflammation to healing phases [80]. Enhancing angiogenesis proves effective in treating metabolic issues and reducing inflammation by improving tissue oxygenation and nutrient supply for better wound healing [79]. Copper is crucial for wound healing, influencing growth factors like platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), and angiopoietin. It aids by boosting collagen deposition and acting as an antibacterial agent [81]. Research suggests that Cu-MOFs offer promising benefits in promoting cell migration, angiogenesis, and collagen deposition. They achieve continuous release of Cu2+ ions, thereby reducing Cu2+ cytotoxicity and aiding in the healing process of diabetic wounds. However, the therapeutic potential of current MOF carriers is restricted due to their limited tissue penetration, as they only interact with the surface of the wound [82]. NO deficiency in wound healing slows collagen deposition collagen and weakens wounds. Studies highlight boosting or supplying NO to reduce swelling, foster blood vessels, and accelerate collagen synthesis [83]. The MOF, with unsaturated metal sites, tightly binds NO. Zhang et al. synthesized NO@HKUST-1 by modifying 4-(methylamino)pyridine and introducing NO under pressure. This approach combined HKUST-1's amine groups with NO for controlled loading, showing synergistic effects of NO and Cu2+ on angiogenesis, collagen enhancement, and inflammation reduction at wound sites [84]. Cobalt ions have the potential to stabilize hypoxia and stimulate angiogenesis by stabilizing hypoxia-inducible factor 1α (HIF-1α) [85]. They are often used in biomaterials to promote vascularization. Co-MOF can serve as both a drug carrier and Co ion release system [86]. Li et al. devised a controlled release system, incorporating ZIF-67-loaded nanoparticles into micropatterned poly(l-lactic acid) (PLLA)/gelatin nanofibrous scaffolds for prolonged angiogenic therapy in diabetic wound beds. In vitro studies suggest that Co ions and dimethylallyl glycine (a pro-angiogenic agent) released from specific nanofiber scaffolds enhance tube formation, migration, and growth of human umbilical vein endothelial cells (HUVECs). This effect is linked to increased VEGF, HIF-1α, and endothelial nitric oxide synthase (eNOS), inducing a hypoxic response. In vivo experiments demonstrate that these scaffolds promote collagen deposition, and angiogenesis, and reduce inflammation in diabetic wounds [87]. Hypomagnesemia has been associated with insulin resistance and heightened platelet reactivity, factors that might elevate the susceptibility to diabetic foot ulcers [88]. Magnesium exhibits promising pro-angiogenic properties [89]. Furthermore, recent studies demonstrated that macrophages exposed to magnesium demonstrated heightened expression of the M2 subtype and increased secretion of anti-inflammatory cytokines. This suggests that Mg(Ⅱ) could potentially function as a novel anti-inflammatory agent [90]. Yin et al. developed a multifunctional magnesium organic framework-graphene oxide-silver microneedle patch (MN-MOF-GO-Ag) based on Mg-MOFs to expedite diabetes healing. These MOFs released Mg(Ⅱ) and gallic acid gradually in acidic environments. Mg(Ⅱ) encouraged angiogenesis and regulated inflammation, while gallic acid countered intracellular ROS and reduced macrophage inflammation. MN-MOF-GO-Ag exhibited high efficacy in wound healing, attributed to its combined effects of promoting angiogenesis, antioxidation, and antibacterial activity [91]. Zinc ions, and zinc oxide play a significant role in skin regeneration, promoting wound healing. Deficiency in zinc is linked to delayed wound recovery, contrasting previous studies confirming zinc's efficacy in healing thermal injuries [92]. Zinc positively impacts wound healing by enhancing platelet activity for [93], promoting angiogenesis [94] and regulating extracellular protein expression[95]. For wound healing and skin regeneration, Zn-MOFs serve as effective antibacterial agents due to zinc's biocompatibility and non-accumulative nature in the body. Chen et al. synthesized Zn-MOFs (Zn-BTC) via a solvothermal method. In experimental test, Zn-BTC exhibited prolonged zinc ion release and superior antibacterial effects. In a wound infection model involving methicillin-resistant bacteria, the Zn-BTC scaffold displayed accelerated wound healing, retaining only 6 % of the wound area by day 14, while the control group retained about 26 % [96].
5. Conclusions, limitations and outlooksThe wound-healing process in acute and chronic wounds is an undeniable challenge. To overcome this challenge, designing innovative materials can be a good solution. Nowadays, the use of MOF/polymer composites is considered a suitable option due to their biocompatibility, suitable biological properties, and good performance in wound healing. These composite materials increase the ability of tissue regeneration and wound healing by continuous release of metal ions along with biological properties of polymers (e.g., non-toxicity, degradability, and in some cases antimicrobial properties). The biological properties of these materials depend on the structure of the MOF (e.g., the type of central metal and ligand attached to the metal) and the polymer used (natural or synthetic). For example, the presence of metal ions such as copper and zinc as the central metal in MOF can cause antimicrobial properties in them. Also, the presence of positive charge (e.g., chitosan and doped polyaniline) or negative charge (e.g., sodium alginate and polycarboxylate) in the polymer structure can cause antimicrobial properties. Therefore, the combination of MOFs with natural and synthetic polymers can lead to biological synergistic effects for wound healing. These composite materials can be used as carriers of antibiotic drugs for the treatment and healing of wounds, angiogenesis, or deposition of free collagen. However, the following limitations can affect their potential applications.
1. Cytotoxicity testing before placing a composite bed on the wound or drug carrier for wound treatment and the mechanism of its action in the body creates limitations. Because the ligands used in the MOF structure as well as the solvents used in the synthesis of most of these materials are toxic.
2. MOF pore size, morphology, and crystallinity depending on the type of central metal and ligand used can affect cell permeability and biocompatibility.
3. Appropriate interactions between MOF and polymer play an important role in improving its performance and creating bio-synergy. Depending on the chemical structure of MOF and the polymer used, these interactions can be weak van der Waals or strong hydrogen interactions.
4. To overcome these challenges and develop MOF/polymer composite materials, the following are suggested.
5. Designing green methods in the synthesis of MOFs, for example using ethanol or water instead of the toxic solvent dimethylformamide.
6. Improving the stability of MOFs by using surfactants or polymers such as polyvinyl pyrrolidone to increase their stability in aqueous environments and their better dispersion in the polymer matrix
7. Using nanoparticles synthesized in a green way such as zinc oxide, copper oxide, and silver nanoparticles to decorate MOF/polymer nanocomposite to improve biological properties such as reducing cytotoxicity, increasing antimicrobial and antioxidant properties
8. Using chemically modified natural polymers such as carboxymethyl cellulose, carboxymethyl starch, and carboxymethyl gums to improve biocompatibility, increase MOF stability, and improve wound healing.
In summary, MOF/polymer composite platforms offer a promising prospect for skin wound healing. Continuous research and development of these materials can improve the treatment process of patients with acute and chronic wounds. By considering current limitations, exploring innovative approaches, and addressing ethical considerations, MOF/polymer composite materials can pave the way for more effective treatments for acute and chronic wounds.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsA. Hasan acknowledges the partial financial support from the Grant (No. NPRP12S-0310-190276) from the Qatar National Research Fund (a member of The Qatar Foundation). The statements herein are the sole responsibilities of authors.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.109564.
| [1] |
H. Wang, R. Zheng, P. He, et al., Adv. Compos. Hybrid Mater. 7 (2024) 10. DOI:10.1007/s42114-023-00814-1 |
| [2] |
M. Mohiti-Asli, B. Pourdeyhimi, E.G. Loboa, Acta Biomater. 10 (2014) 2096-2104. DOI:10.1016/j.actbio.2013.12.024 |
| [3] |
G.D. Mogoşanu, A.M. Grumezescu, Int. J. Pharm. 463 (2014) 127-136. DOI:10.1016/j.ijpharm.2013.12.015 |
| [4] |
C. Lin, X. Guo, F. Mo, D. Sun, Int. J. Mol. Sci. 24 (2023) 3173. DOI:10.3390/ijms24043173 |
| [5] |
Y. Cheng, S.J. Datta, S. Zhou, et al., Chem. Soc. Rev. 51 (2022) 8300-8350. DOI:10.1039/D2CS00031H |
| [6] |
N.D. Rudd, H. Wang, E.M.A. Fuentes-Fernandez, et al., ACS Appl. Mater. Interfaces 8 (2016) 30294-30303. DOI:10.1021/acsami.6b10890 |
| [7] |
J. Chen, G. Zhang, Y. Zhao, et al., Adv. Compos. Hybrid Mater. 5 (2022) 1111-1125. DOI:10.1007/s42114-022-00439-w |
| [8] |
Q. Li, K. Liu, T. Jiang, et al., Mater. Sci. Eng. C 131 (2021) 112519. DOI:10.1016/j.msec.2021.112519 |
| [9] |
J. Lin, J. Qiao, H. Tian, et al., Adv. Compos. Hybrid. Mater. 6 (2023) 177. DOI:10.1007/s42114-023-00762-w |
| [10] |
D. Wang, J.Yang H.Yang, et al., Adv. Compos. Hybrid. Mater. 6 (2023) 138. DOI:10.1007/s42114-023-00708-2 |
| [11] |
L.Q. Fu, X.Y. Chen, M.H. Cai, et al., Front. Bioeng. Biotechnol. 8 (2020) 576348. DOI:10.3389/fbioe.2020.576348 |
| [12] |
D. Giliopoulos, A. Zamboulis, D. Giannakoudakis, D. Bikiaris, K. Triantafyllidis, Molecules 25 (2020) 185. DOI:10.3390/molecules25010185 |
| [13] |
O.M. Yaghi, G. Li, H. Li, Nature 378 (1995) 703-706. DOI:10.1038/378703a0 |
| [14] |
Q.Y. Xu, Z. Tan, X.W. Liao, C. Wang, Chin. Chem. Lett. 33 (2022) 22-32. DOI:10.1016/j.cclet.2021.06.015 |
| [15] |
G. Wyszogrodzka, B. Marszałek, B. Gil, P. Dorożyńnski, Drug Discov. Today 21 (2016) 1009-1018. DOI:10.1016/j.drudis.2016.04.009 |
| [16] |
B.V.K.J. Schmidt, Macromol. Rapid Commun. 41 (2020) 1900333. DOI:10.1002/marc.201900333 |
| [17] |
T. Kitao, Y. Nagasaka, M. Karasawa, et al., J. Am. Chem. Soc. 141 (2019) 19565-19569. DOI:10.1021/jacs.9b10880 |
| [18] |
V.P. Santos, T.A. Wezendonk, J.J.D. Jaén, et al., Nat. Commun. 6 (2015) 6451. DOI:10.1038/ncomms7451 |
| [19] |
Z. Zhang, H.T.H. Nguyen, S.A. Miller, S.M. Cohen, Angew. Chem. Int. Ed. 54 (2015) 6152-6157. DOI:10.1002/anie.201502733 |
| [20] |
K.C. Bentz, K. Gnanasekaran, J.B. Bailey, et al., Chem. Sci. 11 (2020) 10523-10528. DOI:10.1039/D0SC03651J |
| [21] |
K.C. Bentz, S. Ayala, M. Kalaj, S.M. Cohen, Aust. J. Chem. 72 (2019) 848-851. DOI:10.1071/CH19271 |
| [22] |
K.K. Tanabe, S.M. Cohen, Chem. Soc. Rev. 40 (2011) 498-519. DOI:10.1039/C0CS00031K |
| [23] |
Y. Li, J. Liu, K. Zhang, L. Lei, Z. Lei, Ind. Eng. Chem. Res. 57 (2018) 559-567. DOI:10.1021/acs.iecr.7b03398 |
| [24] |
W. Zhang, Y. Hu, J. Ge, H.L. Jiang, S.H. Yu, J. Am. Chem. Soc. 136 (2014) 16978-16981. DOI:10.1021/ja509960n |
| [25] |
H. Ejima, N. Yanai, J.P. Best, et al., Adv. Mater. 25 (2013) 5767-5771. DOI:10.1002/adma.201302442 |
| [26] |
T. Uemura, N. Yanai, S. Watanabe, et al., Nat. Commun. 1 (2010) 83. DOI:10.1038/ncomms1091 |
| [27] |
S.J. Lee, T. Hann, S.H. Park, ACS Appl. Mater. Interfaces 12 (2020) 16319-16326. DOI:10.1021/acsami.9b22843 |
| [28] |
H. Zhu, Q. Zhang, S. Zhu, ACS Appl. Mater. Interfaces 8 (2016) 17395-17401. DOI:10.1021/acsami.6b04505 |
| [29] |
M. Todaro, G. Buscarino, L. Sciortino, et al., J. Phys. Chem. C 120 (2016) 12879-12889. DOI:10.1021/acs.jpcc.6b03237 |
| [30] |
A.J. Rieth, A.M. Wright, M. Dincă, Nat. Rev. Mater. 4 (2019) 708-725. DOI:10.1038/s41578-019-0140-1 |
| [31] |
S. Yuan, L. Feng, K. Wang, et al., Adv. Mater. 30 (2018) 1704303. DOI:10.1002/adma.201704303 |
| [32] |
K. Wang, X.L. Lv, D. Feng, et al., J. Am. Chem. Soc. 138 (2016) 914-919. DOI:10.1021/jacs.5b10881 |
| [33] |
L.N. McHugh, M.J. McPherson, L.J. McCormick, et al., Nat. Chem. 10 (2018) 1096-1102. DOI:10.1038/s41557-018-0104-x |
| [34] |
J. Castells-Gil, F. Novio, N.M. Padial, et al., ACS Appl. Mater. Interfaces 9 (2017) 44641-44648. DOI:10.1021/acsami.7b15564 |
| [35] |
Y. Sun, Q. Sun, H. Huang, et al., J. Mater. Chem. A 5 (2017) 18770-18776. DOI:10.1039/C7TA05800D |
| [36] |
D. Song, J. Bae, H. Ji, et al., J. Am. Chem. Soc. 141 (2019) 7853-7864. DOI:10.1021/jacs.9b02114 |
| [37] |
S. Yang, V.V. Karve, A. Justin, et al., Coord. Chem. Rev. 427 (2021) 213525. DOI:10.1016/j.ccr.2020.213525 |
| [38] |
V. Colombo, S. Galli, H.J. Choi, et al., Chem. Sci. 2 (2011) 1311-1319. DOI:10.1039/c1sc00136a |
| [39] |
X. Zhou, H. Wang, J. Zhang, et al., Acta Biomater. 54 (2017) 128-137. DOI:10.1016/j.actbio.2017.03.011 |
| [40] |
J. Li, F. Lv, J. Li, et al., Nano Res. 13 (2020) 2268-2279. DOI:10.1007/s12274-020-2846-1 |
| [41] |
A.K. Bindra, D. Wang, Y. Zhao, Adv. Mater. 35 (2023) 2300700. DOI:10.1002/adma.202300700 |
| [42] |
M.M. Mihai, M.B. Dima, B. Dima, A.M. Holban, Materials 12 (2019) 2176. DOI:10.3390/ma12132176 |
| [43] |
T.U. Berendonk, C.M. Manaia, C. Merlin, et al., Nat. Rev. Microbiol. 13 (2015) 310-317. DOI:10.1038/nrmicro3439 |
| [44] |
A.P. Kornblatt, V.G. Nicoletti, A. Travaglia, J. Inorg. Biochem. 161 (2016) 1-8. DOI:10.1016/j.jinorgbio.2016.02.012 |
| [45] |
J. Li, D. Zhai, F. Lv, et al., Acta Biomater. 36 (2016) 254-266. DOI:10.1016/j.actbio.2016.03.011 |
| [46] |
S. Chen, J. Lu, T. You, D. Sun, Coord. Chem. Rev. 439 (2021) 213929. DOI:10.1016/j.ccr.2021.213929 |
| [47] |
J.E. Cun, X. Fan, Q. Pan, et al., Adv. Colloid Interface Sci. 305 (2022) 102686. DOI:10.1016/j.cis.2022.102686 |
| [48] |
X. Ren, C. Yang, L. Zhang, et al., Nanoscale 11 (2019) 11830-11838. DOI:10.1039/C9NR03612A |
| [49] |
S. Wang, F. Yan, P. Ren, et al., Int. J. Biol. Macromol. 158 (2020) 9-17. DOI:10.1016/j.ijbiomac.2020.04.116 |
| [50] |
M. Abendrot, L. Chęcińska, J. Kusz, et al., Molecules 25 (2020) 951. DOI:10.3390/molecules25040951 |
| [51] |
Y. Yuan, H. Wu, H. Lu, et al., Chem. Commun. 55 (2019) 699-702. DOI:10.1039/C8CC08568D |
| [52] |
K. Huang, W. Liu, W. Wei, et al., ACS Nano 16 (2022) 19491-19508. DOI:10.1021/acsnano.2c09593 |
| [53] |
D. Leaper, Int. Wound J. 9 (2012) 461. DOI:10.1111/j.1742-481X.2012.01091.x |
| [54] |
D. Yang, Z. Chen, Z. Gao, S.K. Tammina, Y. Yang, Colloids Surf. B: Biointerfaces 195 (2020) 111252. DOI:10.1016/j.colsurfb.2020.111252 |
| [55] |
G. Liu, Z. Bao, J. Wu, Chin. Chem. Lett. 31 (2020) 1817-1821. DOI:10.1016/j.cclet.2020.03.005 |
| [56] |
M. Zhao, H.B. Wang, L.N. Ji, Z.W. Mao, Chem. Soc. Rev. 42 (2013) 8360-8375. DOI:10.1039/c3cs60162e |
| [57] |
C. Jian, X. Li, H. Tian, Curr. Med. Chem. 27 (2020) 5949-5969. DOI:10.2174/0929867326666190618152518 |
| [58] |
J.K. Patra, G. Das, L.F. Fraceto, et al., J. Nanobiotechnol. 16 (2018) 1-33. DOI:10.1186/s12951-017-0328-8 |
| [59] |
T. Bruna, F. Maldonado-Bravo, P. Jara, N. Caro, Int. J. Mol. Sci. 22 (2021) 7202. DOI:10.3390/ijms22137202 |
| [60] |
M. Noga, J. Milan, A. Frydrych, K. Jurowski, Int. J. Mol. Sci. 24 (2023) 5133. DOI:10.3390/ijms24065133 |
| [61] |
R.C. Huxford, J. Della Rocca, W. Lin, Curr. Opin. Chem. Biol. 14 (2010) 262-268. DOI:10.1016/j.cbpa.2009.12.012 |
| [62] |
W. Zhang, G. Ye, D. Liao, et al., Molecules 27 (2022) 7166. DOI:10.3390/molecules27217166 |
| [63] |
S. Shakya, Y. He, X. Ren, et al., Small 15 (2019) 1901065. DOI:10.1002/smll.201901065 |
| [64] |
M. Psarrou, A. Mitraki, M. Vamvakaki, C. Kokotidou, Polymers 15 (2023) 986. DOI:10.3390/polym15040986 |
| [65] |
S. Suvas, J. Immunol. 199 (2017) 1543-1552. DOI:10.4049/jimmunol.1601751 |
| [66] |
Y. Zhu, Z. Yao, Y. Liu, et al., Int. J. Nanomed. (2020) 333-346. |
| [67] |
U. Chilakamarthi, L. Giribabu, Chem. Rec. 17 (2017) 775-802. DOI:10.1002/tcr.201600121 |
| [68] |
X. Zheng, L. Wang, M. Liu, et al., Chem. Mater. 30 (2018) 6867-6876. DOI:10.1021/acs.chemmater.8b03043 |
| [69] |
K. Lu, C. He, W. Lin, J. Am. Chem. Soc. 136 (2014) 16712-16715. DOI:10.1021/ja508679h |
| [70] |
D. Chinemerem Nwobodo, M.C. Ugwu, C. Oliseloke Anie, et al., J. Clin. Lab. Anal. 36 (2022) e24655. DOI:10.1002/jcla.24655 |
| [71] |
L. Yan, A. Gopal, S. Kashif, et al., Chem. Eng. J. 435 (2022) 134975. DOI:10.1016/j.cej.2022.134975 |
| [72] |
S. Cheng, M. Pan, D. Hu, et al., Chin. Chem. Lett. 34 (2023) 108276. DOI:10.1016/j.cclet.2023.108276 |
| [73] |
K.Y. Djoko, Y.O. Cheryl-lynn, M.J. Walker, A.G. McEwan, J. Biol. Chem. 290 (2015) 18954-18961. DOI:10.1074/jbc.R115.647099 |
| [74] |
X. Zhang, F. Peng, D. Wang, J. Funct. Biomater. 13 (2022) 215. DOI:10.3390/jfb13040215 |
| [75] |
Y. Ye, Y. Zhao, Y. Sun, J. Cao, Int. J. Nanomed. 17 (2022) 2367-2395. DOI:10.2147/IJN.S362759 |
| [76] |
Y. Li, X. Liu, L. Tan, et al., Adv. Funct. Mater. 28 (2018) 1800299. DOI:10.1002/adfm.201800299 |
| [77] |
B. Xue, X. Geng, H. Cui, et al., Chin. Chem. Lett. 34 (2023) 108140. DOI:10.1016/j.cclet.2023.108140 |
| [78] |
M. Chen, Z. Long, R. Dong, et al., Small 16 (2020) 1906240. DOI:10.1002/smll.201906240 |
| [79] |
W. Zou, L. Zhang, J. Lu, D. Sun, Chem. Eng. J. 480 (2023) 148220. |
| [80] |
A.P. Veith, K. Henderson, A. Spencer, A.D. Sligar, A.B. Baker, Adv. Drug Deliv. Rev. 146 (2019) 97-125. DOI:10.1016/j.addr.2018.09.010 |
| [81] |
A. Martin, M.R. Komada, D.C. Sane, Med. Res. Rev. 23 (2003) 117-145. DOI:10.1002/med.10024 |
| [82] |
K. Ahmed Saeed AL-Japairai, S. Mahmood, S. Hamed Almurisi, et al., Int. J. Pharm. 587 (2020) 119673. DOI:10.1016/j.ijpharm.2020.119673 |
| [83] |
M. Champeau, V. Póvoa, L. Militão, et al., Acta Biomater. 74 (2018) 312-325. DOI:10.1016/j.actbio.2018.05.025 |
| [84] |
P. Zhang, Y. Li, Y. Tang, et al., ACS Appl. Mater. Interfaces 12 (2020) 18319-18331. DOI:10.1021/acsami.0c01792 |
| [85] |
F. Xing, H. Ma, P. Yu, et al., Mater. Des. 233 (2023) 112252. DOI:10.1016/j.matdes.2023.112252 |
| [86] |
P. Qiu, M. Li, K. Chen, et al., Biomaterials 227 (2020) 119552. DOI:10.1016/j.biomaterials.2019.119552 |
| [87] |
J. Zhang, Y. Cai, K. Liu, Ind. Eng. Chem. Res. 58 (2019) 4199-4207. DOI:10.1021/acs.iecr.8b05656 |
| [88] |
R. Razzaghi, F. Pidar, M. Momen-Heravi, et al., Biol. Trace Elem. Res. 181 (2018) 207-215. DOI:10.1007/s12011-017-1056-5 |
| [89] |
M. Liu, R. Wang, J. Liu, et al., Biomater. Adv. 133 (2022) 112609. DOI:10.1016/j.msec.2021.112609 |
| [90] |
L. Sun, X. Li, M. Xu, et al., Regen. Biomater. 7 (2020) 391-401. DOI:10.1093/rb/rbaa010 |
| [91] |
M. Yin, J. Wu, M. Deng, et al., ACS Nano 15 (2021) 17842-17853. DOI:10.1021/acsnano.1c06036 |
| [92] |
S. Shimizu, R. Tei, M. Okamura, et al., Nutrients 12 (2020) 764. DOI:10.3390/nu12030764 |
| [93] |
A.I.S. Sobczak, K.G.H. Katundu, F.A. Phoenix, et al., Chem. Sci. 12 (2021) 4079-4093. DOI:10.1039/D0SC06605B |
| [94] |
S. Hozain, A. Hernandez, J. Fuller, G. Sharp, J. Cottrell, Exp. Cell Res. 399 (2021) 112436. DOI:10.1016/j.yexcr.2020.112436 |
| [95] |
A. Mónico, S. Zorrilla, G. Rivas, D. Pérez-Sala, Int. J. Mol. Sci. 21 (2020) 2426. DOI:10.3390/ijms21072426 |
| [96] |
Y. Chen, J. Cai, D. Liu, et al., Regen. Biomater. 9 (2022) rbac019. DOI:10.1093/rb/rbac019 |
 2024, Vol. 35
2024, Vol. 35 

