b School of Materials Science and Engineering, Hunan Institute of Technology, Hengyang 421002, China;
c National Engineering Research Center of Low-Carbon Processing and Utilization of Forest Biomass, Nanjing Forestry University, Nanjing 210037, China
Visible-light photoredox catalysis has emerged as a highly versatile tool in synthetic synthesis, which employs sustainable light energy to promote organic bond formations [1–5]. Much progress has been achieved in the field of photocatalytic synthesis over the past decades. However, the practical application of photocatalytic synthesis still has limitations due to long reaction times and accompanying undesired side reactions. In recent years, ultrasound (US) has demonstrated its efficacy as an alternative and milder approach to conventional thermal synthesis by accelerating reaction rates, increasing product yields, and reducing the formation of unwanted by-products [6–9]. The cavitation effect produced by ultrasound energy produces extremely high localized pressures and temperature which lead to the enhancement in solubility, diffusivity, and mass transportation. Sono-photocatalysis, which is characterized by merits of the combination of ultrasonic synthesis and visible light photosynthesis, shows interesting advantages at the kinetic level and energy consumption [10–13]. The synergistic effect of ultrasound irradiation and light irradiation can be observed when sono-photocatalytic oxidation rate constant of organic pollutants degradation is higher than that of the photocatalytic counterpart. To the best of our knowledge, the sono-photocatalytic organic bond formation reaction has never been reported.
Quinoxalin-2(1H)-one and its derivatives are extensively recognized as valuable N-heterocycles due to their abundance in tremendous biologically active molecules [14–15]. Therefore, a series of synthetic protocols for functionalized quinoxalin-2(1H)-ones through C—H functionalization have been well developed [16–19], which includes alkylation [20–27], arylation [28–33], acylation [34–37], amidation [38–39], amination [40–45], aminoalkylation [46–51], sulfenylation [52–53] and trifluoromethylation [54–55]. Among these derivatives, 3-aminoquinoxalin-2(1H)-ones are particularly attractive due to their unique physiochemical and pharmacological properties. Consequently, various efforts have been made to develop the C—H amination of quinoxalin-2(1H)-ones.
The direct application of abundant simple chemical feedstocks for producing value-added chemicals has become a long-standing goal in green and sustainable chemistry. In this regard, amines have been widely used as the amino source in C—N bond formations because of its low cost and commercial availability. In 2016, Cui and colleagues pioneered the copper-catalyzed oxidative amination of quinoxalin-2(1H)-ones with aliphatic amines (Scheme 1a) [40]. Later, Nidhi Jain's group reported the molecular iodine-catalyzed aminations with THBP as the oxidant (Scheme 1b) [41]. In 2018, Wei and co-workers developed the first example of visible light induced amination of quinoxalin-2(1H)-ones with Eosin Y as the photocatalyst (Scheme 1c) [42]. Although the above-mentioned methods are favorable, there are still some limitations as follows: (1) The requirement of external homogeneous catalysts and/or stoichiometric harmful chemical oxidants; (2) long reaction time (> 12 h). Consequently, developing an environmentally friendly and practical method for the construction of 3-aminoquinoxalin-2(1H)-ones under external catalyst-free and mild conditions is highly challenging and desirable. As part of our continuing interest in green synthesis [56–62], we hereby report the sono-photocatalytic amination of quinoxalin-2(1H)-ones with aliphatic amines under external photocatalyst-free conditions. To the best of our knowledge, this is the first example of sono-photocatalytic bond formation.
|
Download:
|
| Scheme 1. Amination of quinoxalin-2(1H)-ones with aliphatic amines. | |
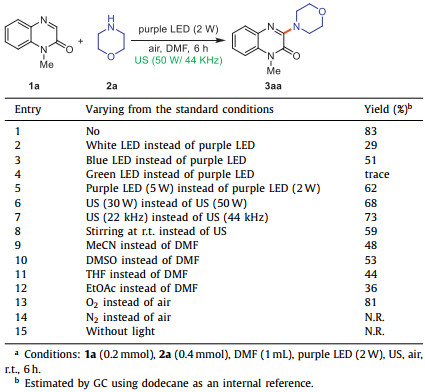
In the initial assessment of sono-photocatalytic amination, the reaction of quinoxalin-2(1H)-ones (1a) and morpholine (2a) was selected as the model reaction. The 3-aminoquinoxalin-2(1H)-one (3aa) was produced in 83% GC yield by carrying out this reaction with ambient air in DMF upon the irradiation of a purple LED (390 nm, 2 W) under 44 KHz ultrasound irradiation in a cleaning bath (50 W) for 6 h without the addition of any photocatalyst and additive (Table 1, entry 1). Changing the LED light with different wavelengths reduced reaction efficiency (entries 2–4). Increasing the power of LED light from 2 W to 5 W has a negative impact on this amination process, leading to the decomposition of 3aa (entry 5). Performing the sono-photocatalytic reaction under 30 W/44 KHz or 50 W/22 KHz delivered 3aa in a lower yield (entries 6 and 7). Only 59% GC yield of 3aa was observed when the reaction was conducted under conventional stirring at room temperature (entry 8). Replacing DMF with other common solvents resulted in a lower yield (entries 9–12). No improvement in this transformation was observed when pure oxygen was used instead of air (entry 13). The reaction did not occur under a nitrogen atmosphere, demonstrating the critical role of molecular oxygen (entry 14). In addition, no progress was observed in the absence of visible light, which indicated the necessity of visible light to promote the reaction (entry 15).
|
|
Table 1 Optimization of reaction conditions.a |
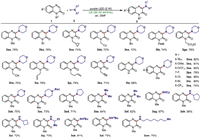
Having established the optimized conditions (Table 1, entry 1), the scope of quinoxalin-2(1H)-ones was firstly investigated (Scheme 2). To our delight, a series of N-substituted quinoxalin-2(1H)-ones 1 reacted well with morpholine 2a to generate the target products 3 with moderate to good yields. N-Substituted quinoxalinones with a variety of aliphatic groups (3aa-3ha) including methyl, ethyl, cyclopropylmethyl, cyclohexylmethyl, benzyl, p-methoxybenzyl (PMB), ethoxycarbonylmethyl and cyanomethyl could well couple with 2a in the sono-photocatalytic system. The unsaturated alkenyl and alkynyl groups were also compatible under the standard conditions to deliver 3ia-3ka in good yields, respectively. Moreover, the unprotected quinoxalin-2(1H)-one was found to be a suitable substrate to give the desired product 3la with 68% yield. The N-methylquinoxalin-2(1H)-one 1, comprising an electron-donating substituents (-tBu, -OMe and OCF3) or an electron-withdrawing substituents (-F, -Cl, -Br and -CF3) at the phenyl part was also compatible with this procedure to afford the corresponding product (3ma-3sa) with yields in the range of 74%–86%. Subsequently, the compatibility of aliphatic amines was explored. Both cyclic (thiomorpholine, N-Boc-piperazine and pyrrolidine) and acyclic (dimethylamine and N-benzylmethylamine) secondary amines showed good reactivities, and the target products 3ab-3af were generated in moderate to good yields. The present protocol also worked well with primary amines. In addition, primary amines with various isomeric structures and chain lengths had little influence in the reaction outcome as the yields of aminated products (3ag-3am) were good. However, no reaction was observed between 1a and aniline.
|
Download:
|
| Scheme 2. Reaction scope. Conditions: 1 (0.2 mmol), 2 (0.4 mmol), DMF (1 mL), purple LED (2 W), US, air, r.t. | |
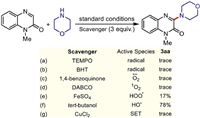
To gain insights into the mechanism, a series of mechanistic investigations were performed. With a radical scavenger (TEMPO or BHT) as the additive, the amination reaction was fully suppressed (Schemes 3a and b), suggesting that this photocatalytic process was a radical pathway. The addition of a superoxide radical anion (O2˙ˉ) scavenger 1,4-benzoquinone (Scheme 3c), a singlet oxygen (1O2) scavenger 1,4-diazabicyclo[2.2.2]octane (Scheme 3d) or peroxide radical (HO2˙) scavenger FeSO4 (Scheme 3e) significantly inhibited this transformation, indicating that O2˙ˉ, 1O2 and HO2˙ played critical roles in this reaction. By comparison, hydroxyl radical (HO˙) scavenger isopropanol had no effect upon the reaction efficiency (Scheme 3f). Only a trace amount of 3aa was observed when a single electron transfer (SET) inhibitor (CuCl2) was added (Scheme 3g), revealing that a SET process might be involved in the coupling process [63]. In addition, the results of ultraviolet visible absorption experiments manifested that the both substrate 1a and product 3aa could absorb visible light and act as photocatalysts in the present transformation (Fig. S2 in Supporting information). The light on/off experimental results revealed that continuous light irradiation was indispensable for the transformation, which ruled out any possibility of radical propagation pathway (Fig. S3 in Supporting information).
|
Download:
|
| Scheme 3. Control experiments. | |
On the basis of the above experimental results and related literature [64–66], a possible mechanism for this self-photocatalyzed radical amination was proposed in Scheme 4. Upon irradiation by purple LED, the absorption of photons in the substrate 1a resulted in the formation of excited-species 1a*, which underwent an energy transfer (EnT) process with ground-state triplet oxygen (3O2) to generate the active excited-state singlet oxygen (1O2) along with regeneration of ground-state 1a. The 1O2 reacted with morpholine (2a) gave a morpholine radical cation (IM1) and a O2˙ˉ, which abstracted a proton from IM1 to produce the HO2˙ and nitrogen-centered morpholine radical (IM2). Next, the radical IM2 region-selectively attacked quinoxalinone 1a to yield the nitrogen-centered radical IM3, which converted into carbon centered morpholine radical (IM4) through the process of a 1,2-H shift. The higher active HOO˙ oxidized IM4 into carbocation intermediate IM5 via a SET process. Finally, the dehydrogenation and re-aromatization of IM5 gave the target aminated product 3aa along with the generation of H2O2 (detected by H2O2 test paper). In addition, 3aa could also serve as the photo-catalyst to achieve the photoredox catalytic cycle.
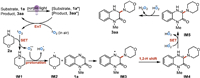
|
Download:
|
| Scheme 4. Proposed reaction mechanism. | |
To sum up, we have developed the first example of sono-photocatalytic C—N bond formation. With both visible light and ultrasound wave as the energy, a series of 3-aminoquinoxalin-2(1H)-ones were efficiently obtained with good functional group tolerance in the absence of any additive or external photocatalyst. Compared with the conventional photocatalysis, sono-photocatalysis not only dramatically improved the reaction rates and yields, but also reduced energy consumption. The mechanistic studies manifested that the dual function of quinoxalin-2(1H)-ones simplified the reaction system. Moreover, this reaction involved an EnT process, a protonation process, a 1,2-H shift and two SET processes. We believe that the present strategy not only provides an eco-friendly synthetic method for 3-aminoquinoxalin-2(1H)-one derivatives but also develops the ultrasound chemistry and visible light photocatalysis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statementWen-Tao Ouyang: Validation. Jun Jiang: Supervision. Yan-Fang Jiang: Validation. Ting Li: Validation. Yuan-Yuan Liu: Validation. Hong-Tao Ji: Validation. Li-Juan Ou: Supervision. Wei-Min He: Writing – original draft.
AcknowledgmentWe are grateful for financial support from the University of South China.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2024.110038.
| [1] |
J.P. Barham, B. König, Angew. Chem. Int. Ed. 59 (2020) 11732-11747. DOI:10.1002/anie.201913767 |
| [2] |
X.Y. Yu, J.R. Chen, W.J. Xiao, Chem. Rev. 121 (2021) 506-561. DOI:10.1021/acs.chemrev.0c00030 |
| [3] |
H. Wang, Y.M. Tian, B. König, Nat. Rev. Chem. 6 (2022) 745-755. DOI:10.1038/s41570-022-00421-6 |
| [4] |
N. Holmberg-Douglas, D.A. Nicewicz, Chem. Rev. 122 (2022) 1925-2016. DOI:10.1021/acs.chemrev.1c00311 |
| [5] |
Y. Lv, H. Ding, J. You, W. Wei, D. Yi, Chin. Chem. Lett. 35 (2024) 109107. DOI:10.1016/j.cclet.2023.109107 |
| [6] |
S. Saranya, S. Radhika, C.M. Afsina Abdulla, G. Anilkumar, J. Heterocycl. Chem. 58 (2021) 1570-1580. DOI:10.1002/jhet.4261 |
| [7] |
I.V. Machado, J.R.N. dos Santos, M.A.P. Januario, A.G. Corrêa, Ultrason. Sonochem. 78 (2021) 105704. DOI:10.1016/j.ultsonch.2021.105704 |
| [8] |
K.S. Wahan, G. Bhargava, A.P. Chawla, Curr. Org. Chem. 27 (2023) 1010-1019. DOI:10.2174/1385272827666230911130127 |
| [9] |
T. Xiong, H. Yuan, F. Yang, J. Jiang, Green Synth. Catal. 3 (2022) 46-52. DOI:10.1016/j.gresc.2021.10.005 |
| [10] |
C.G. Joseph, G.Li Puma, A. Bono, D. Krishnaiah, Ultrason. Sonochem. 16 (2009) 583-589. DOI:10.1016/j.ultsonch.2009.02.002 |
| [11] |
D. Paustian, M. Franke, M. Stelter, P. Braeutigam, Catalysts 12 (2022) 754. DOI:10.3390/catal12070754 |
| [12] |
A.A. Isari, F. Hayati, B. Kakavandi, et al., Chem. Eng. J. 392 (2020) 123685. DOI:10.1016/j.cej.2019.123685 |
| [13] |
A. Mukherjee, S. Das, Y.H. Ahn, J. Water Process Eng. 49 (2022) 103186. DOI:10.1016/j.jwpe.2022.103186 |
| [14] |
M. Montana, V. Montero, O. Khoumeri, P. Vanelle, Molecules 25 (2020) 2784. DOI:10.3390/molecules25122784 |
| [15] |
X. Jiang, K. Wu, R. Bai, P. Zhang, Y. Zhang, Eur. J. Med. Chem. 229 (2022) 114085. DOI:10.1016/j.ejmech.2021.114085 |
| [16] |
P.Rani Kiran, S. Chahal, et al., New J. Chem. 45 (2021) 18722-18763. DOI:10.1039/D1NJ03445F |
| [17] |
K.L. Wang, H.T. Ji, L.J. Ou, W.M. He, Eur. J. Org. Chem. 26 (2023) e202300752. |
| [18] |
Z. Wang, N. Meng, Y. Lv, et al., Chin. Chem. Lett. 34 (2023) 107599. DOI:10.1016/j.cclet.2022.06.022 |
| [19] |
N. Meng, Y. Lv, Q. Liu, et al., Chin. Chem. Lett. 32 (2021) 258-262. DOI:10.1016/j.cclet.2020.11.034 |
| [20] |
J. Sun, H. Yang, B. Zhang, Green Chem. 24 (2022) 858-863. DOI:10.1039/D1GC03992J |
| [21] |
M. Wang, J. Liu, Y. Zhang, P. Sun, Adv. Synth. Catal. 364 (2022) 2660-2665. DOI:10.1002/adsc.202200455 |
| [22] |
H. Zhang, J. Xu, Y. Ouyang, et al., Chin. Chem. Lett. 33 (2022) 2036-2040. DOI:10.1016/j.cclet.2021.09.069 |
| [23] |
L. Ding, K. Niu, Y. Liu, Q. Wang, Chin. Chem. Lett. 33 (2022) 4057-4060. DOI:10.1016/j.cclet.2021.12.053 |
| [24] |
Y. Deng, X. Cheng, H. Tan, et al., Adv. Synth. Catal. 365 (2023) 865-870. DOI:10.1002/adsc.202300035 |
| [25] |
X. Shi, Y. Cao, Y. Liu, et al., Org. Chem. Front. 10 (2023) 1296-1300. DOI:10.1039/D2QO02019J |
| [26] |
J. Zhu, Y. Guo, Y. Zhang, et al., Green Chem. 25 (2023) 986-992. DOI:10.1039/D2GC04521D |
| [27] |
F. Cheng, L. Fan, Q. Lv, X. Chen, B. Yu, Green Chem. 25 (2023) 7971-7977. DOI:10.1039/D3GC02545D |
| [28] |
J. Ren, C. Pi, X. Cui, Y. Wu, Green Chem. 24 (2022) 3017-3022. DOI:10.1039/D1GC04825B |
| [29] |
J.C. Hou, J. Jiang, Y.C. Wen, et al., J. Org. Chem. 89 (2024) 6117-6125. DOI:10.1021/acs.joc.4c00087 |
| [30] |
H.Y. Song, J. Jiang, C. Wu, et al., Green Chem. 25 (2023) 3292-3296. DOI:10.1039/D2GC04843D |
| [31] |
H. Hou, C. Wang, X. Cheng, et al., Catal. Sci. Technol. 13 (2023) 305-309. DOI:10.1039/D2CY01640K |
| [32] |
J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 6632-6638. DOI:10.1039/D1GC01899J |
| [33] |
L. Dai, G. Zhong, Chin. J. Org. Chem. 43 (2023) 2589-2590. DOI:10.6023/cjoc202300042 |
| [34] |
J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182. DOI:10.1002/adsc.202000116 |
| [35] |
L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378. DOI:10.1039/D0GC02844D |
| [36] |
K.C.C. Aganda, B. Hong, A. Lee, Adv. Synth. Catal. 363 (2021) 1443-1448. DOI:10.1002/adsc.202001396 |
| [37] |
Y. He, G. Wang, W. Hu, et al., ACS Sustain. Chem. Eng. 11 (2023) 910-920. DOI:10.1021/acssuschemeng.2c04720 |
| [38] |
J.W. Yuan, J.L. Zhu, H.L. Zhu, et al., Org. Chem. Front. 7 (2020) 273-285. DOI:10.1039/C9QO01322A |
| [39] |
J. Li, J. Hu, Y. Xiao, et al., Tetrahedron Lett. 88 (2022) 153511. DOI:10.1016/j.tetlet.2021.153511 |
| [40] |
Y. Li, M. Gao, L. Wang, X. Cui, Org. Biomol. Chem. 14 (2016) 8428-8432. DOI:10.1039/C6OB01283C |
| [41] |
A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792. DOI:10.1021/acs.joc.7b00464 |
| [42] |
W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130. DOI:10.1021/acs.orglett.8b03079 |
| [43] |
L. Zheng, Y.E. Qian, Y.Z. Hu, et al., Org. Lett. 23 (2021) 1643-1647. DOI:10.1021/acs.orglett.1c00064 |
| [44] |
Q. Yang, Z. Yang, Y. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667. DOI:10.1002/adsc.201801661 |
| [45] |
Y. Tan, B. Liu, Y.P. Han, et al., Chem. Asian J. 15 (2020) 3365-3369. DOI:10.1002/asia.202000916 |
| [46] |
Y.F. Si, X.L. Chen, X.Y. Fu, et al., ACS Sustain. Chem. Eng. 8 (2020) 10740-10746. |
| [47] |
Y. Li, C. Dai, S. Xie, P. Liu, P. Sun, Org. Lett. 23 (2021) 5906-5910. DOI:10.1021/acs.orglett.1c02014 |
| [48] |
W.T. Ouyang, H.T. Ji, J. Jiang, et al., Chem. Commun. 59 (2023) 14029-14032. DOI:10.1039/D3CC04020H |
| [49] |
Y.H. Lu, Z.T. Zhang, H.Y. Wu, et al., Chin. Chem. Lett. 34 (2023) 108036. DOI:10.1016/j.cclet.2022.108036 |
| [50] |
J.F. Cui, W.Q. Zhong, J.M. Huang, J. Org. Chem. 88 (2023) 1147-1154. DOI:10.1021/acs.joc.2c02654 |
| [51] |
Y.H. Lu, S.Y. Mu, J. Jiang, et al., ChemSusChem 16 (2023) e202300523. DOI:10.1002/cssc.202300523 |
| [52] |
J. Zhou, Z. Li, Z. Sun, et al., J. Org. Chem. 85 (2020) 4365-4372. DOI:10.1021/acs.joc.0c00050 |
| [53] |
L. Zhang, J. He, P. Zhang, et al., Green Synth. Catal. 4 (2023) 226-230. DOI:10.1016/j.gresc.2022.04.004 |
| [54] |
Y. Li, X. Liang, K. Niu, et al., Org. Lett. 24 (2022) 5918-5923. DOI:10.1021/acs.orglett.2c02150 |
| [55] |
J. Liu, Z. Huang, C. Wang, et al., Eur. J. Org. Chem. 26 (2023) e202300129. DOI:10.1002/ejoc.202300129 |
| [56] |
H. Li, P. Chen, Z. Wu, et al., Chin. J. Org. Chem. 42 (2022) 3398-3404. DOI:10.6023/cjoc202207009 |
| [57] |
S. Mu, H. Li, Z. Wu, et al., Chin. J. Org. Chem. 42 (2022) 4292-4299. DOI:10.6023/cjoc202211002 |
| [58] |
Q.X. Luo, H.T. Ji, Y.H. Lu, et al., J. Org. Chem. 88 (2023) 16790-16796. DOI:10.1021/acs.joc.3c01271 |
| [59] |
H.Y. Song, J. Jiang, Y.H. Song, et al., Chin. Chem. Lett. 35 (2024) 109246. DOI:10.1016/j.cclet.2023.109246 |
| [60] |
H.Y. Song, F. Xiao, J. Jiang, et al., Chin. Chem. Lett. 34 (2023) 108509. DOI:10.1016/j.cclet.2023.108509 |
| [61] |
K. Wang, J. Huang, W. Liu, et al., Chin. J. Org. Chem. 42 (2022) 2527-2534. DOI:10.6023/cjoc202203055 |
| [62] |
R. Yi, W. He, Chin. J. Org. Chem. 43 (2023) 2985-2987. DOI:10.6023/cjoc202300047 |
| [63] |
T. Liu, F. Xue, Z. Chen, et al., J. Catal. 414 (2022) 76-83. DOI:10.1016/j.jcat.2022.08.029 |
| [64] |
Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245. DOI:10.1039/C9GC03045J |
| [65] |
J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042. DOI:10.1039/D0QO00872A |
| [66] |
L. Xuan, R. Du, P. Lei, et al., Green Chem. 24 (2022) 9475-9481. DOI:10.1039/D2GC02874C |
 2024, Vol. 35
2024, Vol. 35