Microbial contamination in water distribution systems is an emerging issue that poses a threat to human health [1]. While traditional water treatment processes, including coagulation sedimentation and the use of biofilters, effectively reduce microbial abundance, treated drinking water is not completely sterile and may still host diverse microbial communities [2,3]. The complete sterilization of drinking water is infeasible. Disinfectants are frequently added at the terminal to control microbial growth [4,5]. However, the formation of harmful disinfection by-products has become an increasingly serious problem with current disinfection technologies such as free chlorine, chloramines, and ozone disinfection [6–9]. Moreover, recent research on the microbial ecology of distribution systems has shown that some pathogenic microorganisms have developed resistance to chlorination [10,11]. In particular, sediment accumulation in water storage tanks may offer a protective environment for diverse bacterial growth and microbial activity. And bacteria have demonstrated resistance to free chlorine and potential regrowth after exposure to a high level of chlorine. Therefore, the development of safer biocidal materials that can effectively control microbial contamination without disinfection by-products is crucial.
Quaternary phosphonium salt (QPS) is a promising cationic biocide that exhibits high efficiency, low toxicity, and broad-spectrum antimicrobial activity [12,13]. QPS is superior to traditional quaternary ammonium salt (QAS) due to the larger atomic radius of phosphorus than nitrogen which results in lower electronegativity [14]. Compared to more common quaternary ammonium salts, quaternary phosphonium salts have similar sterilization mechanism and better antibacterial performance. Consequently, QPS molecules are weakly associated cations that can be easily adsorbed onto negatively charged bacterial membranes. The sterilization mechanisms of QPS frequently involve destructive interactions with the cell wall or membrane of microorganisms. QAS with long lipophilic substituent facilitates punching into the phospholipid bilayer, disordering the cell membrane and resulting in cell death [15,16].
Chitosan (CS) is an environment friendly substrate material with abundant sources and reserves. And it also has antibacterial properties [17,18]. CS has been found to exhibit significant antimicrobial effects on bacteria, fungi, and viruses [19,20]. However, the antibacterial activity of CS is strongly influenced by pH, mainly due to the amino group of chitosan can be protonated at pH < 6.3 [21]. Positively charged CS easily interacts with negatively the charged cell membrane of microorganisms [22,23]. The antibacterial activity of CS rapidly decreases with increasing pH. When pH is over 7.0, raw CS loses its antibacterial activity, the phenomenon is primarily attributed to amino groups being no longer significantly charged [24,25].
Therefore, the cationic modification of CS with QPS is necessary to achieve a permanent positive charge over a wide range of pH, making CS suitable for applications in water. In the past, CS modifications primarily relied on metal-based approaches, such as the use of Ag, Cu, and ZnO, utilizing the loading capacity of CS. By contrast, our study synergistically combines CS and QPS, introducing bactericidal sites and enhancing the antibacterial efficacy through cationic modification.
In this study, we reported a high sterilization activity, denoted as PCC, with QPS grafting. This research provides new insight into achieving long-term and sterilization activity of CS-based composites with lesser by products. Drawing inspiration from the features mentioned above, high sterilization activity tributyl (dodecyl)phosphonium bromide was employed to prepare the biocide. By grafting hydroxyl and amino groups with QPS onto CS, functional groups with strong positive electric and antibacterial activities are introduced.
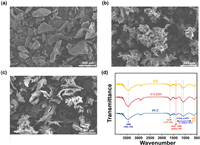
The antibacterial PCC was successfully synthesized via self-assembly with QPS, where Epichlorohydrin (EPI) served as a flexible cross-linker and QPS acted as the quaternization reagent. The morphology of PCC was characterized through scanning electron microscopy (SEM). As shown in Fig. 1, the cross-linked CS-EPI presented a rougher and more clustered appearance compared with CS. After self-assembly with QPS, PCC presented a spike-like structure and its surface appeared smoother than that of CS-EPI (Fig. 1c). This rough surface facilitated easier puncturing of the bacterial cell membrane by PCC, making PCC an effective antibacterial agent.
|
Download:
|
| Fig. 1. SEM image of (a) CS, (b) CS-EPI, (c) PCC, (d) FT-IR spectra of CS, CS-EPI, and PCC. | |
The adsorption peak observed at 3400 cm−1 in the CS spectrum was attributed to O—H and N—H stretching vibrations (Fig. 1d). In the case of CS-EPI, the overlapping peaks in the spectrum indicated the presence of EPI groups. The spectrum of CS-EPI at 3400 cm–1 became wider due to the increase in O—H groups [26]. In addition, the stronger adsorption peaks at 1154 cm−1 and 1075 cm−1 indicated that a cross-linked reaction occurred between C-3 and C-6 with the EPI cross-linker reagent [27,28]. Following self-assembly with QPS, a new adsorption peak was observed near 605 cm−1, which could be attributed to P-C. In addition, the stronger adsorption peak at 1481 cm−1 indicated that more -P+R3 groups were involved in CS-EPI [29].
The changes in surface chemistry after QPS modification were confirmed through zeta potential and water contact angle measurements. The zeta potential of PCC remained positively charged over a wide pH ranging from 3 to 10, signifying that the grafting of QPS introduced more cationic groups (Fig. S2 in Supporting information). As shown in Fig. S3 (Supporting information), an increase in water contact angle due to the introduction of hydrophobic long-chain alkane confirmed the success of the modification. In conclusion, PCC was successfully synthesized through facile self-assembly with QPS.
After confirming that CS-EPI was modified with QPS, we utilized its potential as an antibacterial agent. Two model bacterial strains, gram-negative E. coli and gram-positive S. aureus, were used to evaluate antibacterial performance through colony counting assay. The number of colony-forming units with plate count method was the fastest and direct way for bacterial decontamination. The synthesized PCC described above demonstrated excellent sterilization activity, with the highest activity observed at a dosage of 0.1 g/L, indicating that QPS is an effective bactericide (Fig. S4 in Supporting information).
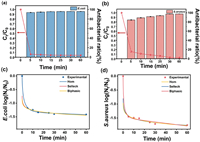
To analyze the bactericidal rate, reaction time and antibacterial performance were studied. As illustrated in Figs. 2a and b, the amounts of both bacteria rapidly decreased with an increase in reaction time. Nearly 95% of E. coli and S. aureus were killed in contact with PCC within 5 min. The kinetic fitting results are presented in the Figs. 2c and d, where three fitting models were calculated. The detailed fitting results are provided in Tables S1 and S2 (Supporting information). According to the correlation coefficient (R2) value, the biphasic model was the most suitable for describing the bactericidal rate of E. coli compared with the other two models. This finding suggests that the bactericidal process is characterized by a first-order linear deactivating rate coupled with a trailing phenomenon. Meanwhile, for S. aureus, Selleck may be the most suitable model for indicating the coupling between hysteresis and the trailing phenomenon.
|
Download:
|
| Fig. 2. Survival kinetics and antibacterial ratio on (a) E. coli and (b) S. aureus. Fit curve of survival kinetics on (c) E. coli and (d) S. aureus. | |
The antibacterial activity of PCC was further demonstrated via live/dead staining assay. In Fig. 3, most of the bacteria treated with CS as the control group were stained green with SYTO-9 acting as a cell-membrane permeant dye. This result indicated that CS exhibited negligible antibacterial activity. By contrast, after treatment with PCC, more than 95% of the E. coli were stained red with propidium iodide, indicating that PCC seriously damaged the cell membrane.

|
Download:
|
| Fig. 3. Fluorescent images of E. coli after treatment with (a) CS and (b) PCC. Fluorescent images of S. aureus after treated with (c) CS and (d) PCC. (e) The percentage diagram of red fluorescence of E. coli. (f) The percentage diagram of red fluorescence intensity of S. aureus. | |
After reacting with PCC, the coverage of red fluorescence increased in Figs. 3b and e compared with the treatment that involved raw CS agents, indicating that bacterial cell membrane was damaged, which can be attributed to the penetration of the bacterial membrane by the hydrophobic alkyl chains of the quaternary phosphonium groups [30,31]. The image data were processed using ImageJ in Figs. 3c and f, around 70% of the bacteria were still alive after reacting with CS, while the survival rate in the PCC group was around 5%. These results also indicated that PCC exhibits high antibacterial activity toward gram-negative bacteria, and this finding is consistent with the antibacterial ratio obtained via colony counting assay.
Different factors, such as pH, humic acid (HA), and temperature, were also investigated to evaluate the sterilization performance of PCC. When pH ranges from 6.5 to 8.5, PCC can completely sterilize against S. aureus and achieve over 95% sterilization performance against E. coli (Fig. S5a in Supporting information) [24]. Additionally, Fig. S5b (Supporting information) illustrates the impact of solution temperature. With an increase in temperature, sterilization performance against E. coli and S. aureus is improved. This result can be attributed to higher temperature producing more active bacterial viability, leading to greater bacterial contact with PCC. The functional groups present in HA make adsorbing onto CS-based antibacterial agents easy through hydrogen bonding, and hydrophobic and electrostatic interactions [32,33]. This phenomenon can lead to competition for binding sites with bacteria on PCC. The effect of HA concentration on the antibacterial activity of PCC was investigated. As the concentration of HA increased from 0 mg/L to 3 mg/L, which is comparable to the concentration in tap water, antibacterial efficiency decreased slightly in Fig. S5c (Supporting information). HA exerted only minimal negative effect on antibacterial performance. Even if the concentration of HA reaches 3.0 mg/L, PCC can still inactivate over 90% E. coli and S. aureus.
The long-term durability of antibacterial agents is an important index for assessing their potential application to actual treatment. Long-term stability was evaluated by soaking the developed agent in water (Fig. S6a in Supporting information). After 30 days of soaking, the antibacterial ratio only decreased to 92.6% and 97.2% for E. coli and S. aureus, respectively. An inference can be made that the antibacterial mechanism of PCC relies on the contact antibacterial mechanism, which differs from the silver releasing mechanism in traditional silver antibacterial agents [34]. The results in Fig. S6b (Supporting information) show that PCC maintained its bactericidal ability over five cycles, indicating good stability and reusability for the sterilization of E. coli and S. aureus.
We also used PCC in river water and tap water antibacterial experiments. Several colonies were found in the raw water coated on a plate as the control group (Figs. S7a and b in Supporting information). After PCC treatment, however, no colony growth was found in both solutions (Figs. S7c and d in Supporting information), demonstrating the strong sterilization activity of PCC in real water. The microbial concentration in the actual water body is lower, that is why the antibacterial result is often better. But it still needs to strengthen continuous flow experiments to better simulate actual environmental remediation than batch experiments.
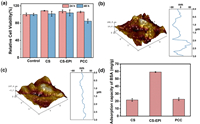
In addition to antibacterial ratio and stability, an equally significant aspect of PCC properties employed in drinking water treatment is cytotoxicity. Achieving a balance between strong antibacterial activity and low toxicity to normal cells is crucial. Several antibacterial agents, such as benzalkonium chloride, exhibit antibacterial reactivity but also pose acute cytotoxicity to humans. The in vitro cytotoxicity of PCC to L929 cells was evaluated via MTT analysis, as shown in Fig. 4a. In contrast with the control group, CS, CS-EPI, and PCC presented no significant cytotoxicity to L929 cells after 24 h of coculture. After 48 h of coculture, the cell viability of PCC was 84%, suggesting that PCC is nontoxic to human cells at a normal dosage. The slight decrease in cell viability may be attributed to cationic polymers interacting easily with negatively charged proteins and plasma membrane, leading to damage to the cell membrane [35]. Overall, PCC demonstrates good biocompatibility in water treatment systems.
|
Download:
|
| Fig. 4. (a) In vitro cytotoxicity on L929 cells for 24 h and 48 h. (b) Adsorption capacity of BSA. Atomic force microscopy diagrams of (c) CS and (d) PCC. | |
This study further investigated the anti-adhesion properties of PCC, and the results are presented in Figs. 4b and c. Smooth antibacterial agents are resistant to scaling compared with a rough surface. After grafting with QPS, the Rq of PCC decreased from 37.4 nm to 21.7 nm, indicating that PCC has a smoother surface (Fig. S5d in Supporting information). It means PCC compared with raw CS and CS-EPI has excellent surface properties for resisting bacterial adhesion on its surface. To simulate actual bacterial adhesion, this study further compared the anti-adhesion properties of PCC by adsorbing bovine serum albumin (BSA) onto the surface of the antibacterial agent [36]. Fig. 4d shows that after cross-linking with EPI, BSA adsorption capacity reached 59.1 mg/g, while the adsorption capacity of PCC was reduced by 22.7 mg/g, proving that surfaced grafting with QPS is an effective method for constructing an anti-adhesion surface. This result further confirmed the excellent anti-adhesion properties of PCC.
The preceding results suggest that PCC can disrupt the structure of bacterial cell membranes and alter their permeability. This finding is evidenced by the consistent trend of K+ and protein leaching concentration with antibacterial kinetics, as shown in Figs. S9a and b (Supporting information). The trends of leaching concentration and time were consistent with that of antibacterial kinetics. Notably, the concentrations of K+ and protein were higher in S. aureus than in E. coli, probably due to the lack of an outer membrane structure in S. aureus, making it more susceptible to the entry of antibacterial groups than E. coli [37,38]. The preceding results illustrated that PCC can destroy the structure and change the permeability of bacterial cell membrane. When taken together, these findings shed light onto the antibacterial mechanism of PCC and highlight its potential as an effective disinfectant agent.
In addition to intracellular substance leakage concentration, the morphology changes of E. coli and S. aureus after PCC treatments were observed via SEM to characterize the bactericidal mechanism. As shown in Fig. 5, untreated E. coli and S. aureus exhibited regular elliptical and spherical shapes with smooth and intact cell walls, representing two types of typical rod-shaped and round-shaped bacteria.

|
Download:
|
| Fig. 5. (a) SEM images of E. coli. (b, c) SEM images of E. coli after PCC treatment. (d) SEM images of S. aureus. (e, f) SEM images of S. aureus after PCC treatment. | |
After exposure to PCC, many cells became wrinkled and shrunken, and some even appeared to be ruptured and broken. The images clearly show that PCC caused severe damage to bacterial cell walls and membranes and the bacteria were distorted or even ruptured [39,40]. In particular, the morphology of most E. coli cells was completely broken and turned into debris because the cell wall was disrupted by PCC. For S. aureus, the degree of damage was slightly less than that for E. coli, and this result can be attributed to the presence of abundant peptidoglycan and teichoic acids in the cell wall of S. aureus compared with that in E. coli [41]. As the representative Gram-negative bacteria, E. coli has a thinner cell wall than the representative Gram-positive bacteria, i.e., S. aureus, which has a thicker cell wall. Its thicker cell wall provides S. aureus with greater resistance to PCC. Speculating that Gram-positive bacteria may be more resistant to sterilization than Gram-negative bacteria is reasonable. The preceding results provided evidence for the damage of the cell membranes of Gram-positive and Gram-negative bacteria, leading to their inactivation. And the proposed antibacterial mechanism is mainly from electrostatic interaction. The positively charged PCC could readily bind to the negatively charged cell walls, and then penetrate cell membrane, and possibly compromising membrane integrity via lipid molecule extraction effectively.
In this study, PCC, a CS-based antibacterial agent, was prepared via self-assembly with QPS. By grafting with QPS, PCC became positively charged over a wide range of pH, overcoming the limitation of antibacterial ability under acidic conditions. PCC achieved an antibacterial ratio of 95% against E. coli and 100% against S. aureus at a low dosage of 0.1 g/L. In a simulated drinking water distribution system, PCC was able to maintain high sterilization activity in different HA concentrations, pH values, and temperatures. After five cycles, PCC presented only a slight decrease in antibacterial performance, suggesting its potential application in real water environments. PCC, which exhibits better anti-adhesion performance than CS and CS-EPI, can reduce the broken bacterial debris that occupies active sites. In vitro cytotoxicity on L929 cells demonstrated that PCC is nontoxic to human cells at a normal dosage, indicating its safe use in water treatment systems. The antibacterial mechanism of PCC is primarily attributed to QPS embedded into the cell membrane, disrupting balance and leading to intracellular leaching and bacterial breakdown. Overall, PCC is a versatile antibacterial agent with considerable potential for use in water treatment system disinfection and environmental remediation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51778230, 22376065), Program of Shanghai Outstanding Technology Leaders (No. 20XD1433900), the Science and Technology Commission of Shanghai Municipality (No. 22ZR1418600), Shanghai Municipal Science and Technology (No. 20DZ2250400).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109406.
| [1] |
Y. Zhou, J. He, J. Lu, et al., Chin. Chem. Lett. 31 (2020) 2623-2626. DOI:10.1016/j.cclet.2020.02.008 |
| [2] |
X. Ma, A. Vikram, L. Casson, et al., Environ. Sci. Technol. 51 (2017) 7648-7657. DOI:10.1021/acs.est.7b00768 |
| [3] |
Q. Lu, Y. Zhou, Q. Sui, et al., Front. Environ. Sci. Eng. 17 (2023) 100. DOI:10.1007/s11783-023-1700-6 |
| [4] |
D. Berry, C. Xi, L. Raskin, Curr. Opin. Biotechnol. 17 (2006) 297-302. DOI:10.1016/j.copbio.2006.05.007 |
| [5] |
M.G. Hilal, B. Han, Q. Yu, et al., Environ. Pollut. 322 (2023) 121185. DOI:10.1016/j.envpol.2023.121185 |
| [6] |
Y. He, M. Gao, Y. Zhou, et al., Chemosphere 311 (2023) 136925. DOI:10.1016/j.chemosphere.2022.136925 |
| [7] |
Z. Liu, Z. Shen, S. Xiang, et al., Front. Environ. Sci. Eng. 17 (2022) 31. |
| [8] |
T. Wang, J. Lu, J. Lei, et al., Sep. Purif. Technol. 307 (2023) 122755. DOI:10.1016/j.seppur.2022.122755 |
| [9] |
Y. Zhou, L. Zhou, Y. Zhou, et al., Appl. Catal. B: Environ. 279 (2020) 119365. DOI:10.1016/j.apcatb.2020.119365 |
| [10] |
A. Kassem, G.M. Ayoub, L. Malaeb, Sci. Total Environ. 668 (2019) 566-576. DOI:10.1016/j.scitotenv.2019.02.446 |
| [11] |
T. Li, Z. Wang, J. Guo, et al., Sci. Total Environ. 860 (2023) 160461. DOI:10.1016/j.scitotenv.2022.160461 |
| [12] |
X. Cai, S. Tan, M. Lin, et al., Langmuir 27 (2011) 7828-7835. DOI:10.1021/la201499s |
| [13] |
T. Kuang, L. Deng, S. Shen, et al., Chin. Chem. Lett. 34 (2023) 108584. DOI:10.1016/j.cclet.2023.108584 |
| [14] |
Y. Zhu, P. Li, C. Liu, et al., Chin. Chem. Lett. 34 (2023) 107543. DOI:10.1016/j.cclet.2022.05.057 |
| [15] |
V.V. Ermolaev, D.M. Arkhipova, V.A. Miluykov, et al., Int. J. Mol. Sci. 23 (2022) 86. |
| [16] |
Y. Zhao, L.N. He, Y.Y. Zhuang, et al., Chin. Chem. Lett. 19 (2008) 286-290. DOI:10.1016/j.cclet.2007.12.033 |
| [17] |
R. Huang, J. Xu, L. Xie, et al., Front. Environ. Sci. Eng. 16 (2022) 117. DOI:10.1007/s11783-022-1549-0 |
| [18] |
Y. Yang, Z. Fu, Q. Zhang, Front. Environ. Sci. Eng. 18 (2023) 15. |
| [19] |
C. Duan, J. Wang, Q. Liu, et al., Sep. Purif. Technol. 282 (2022) 120013. DOI:10.1016/j.seppur.2021.120013 |
| [20] |
S. Cheng, M. Pan, D. Hu, et al., Chin. Chem. Lett. 34 (2023) 108276. DOI:10.1016/j.cclet.2023.108276 |
| [21] |
S.H. Lim, S.M. Hudson, Carbohydr. Res. 339 (2004) 313-319. DOI:10.1016/j.carres.2003.10.024 |
| [22] |
I.M. Helander, E.L. Nurmiaho, R. Ahvenainen, et al., Int. J. Food Microbiol. 71 (2001) 235-244. DOI:10.1016/S0168-1605(01)00609-2 |
| [23] |
J. Hao, C. Zhang, C. Feng, et al., Chin. Chem. Lett. 34 (2023) 107650. DOI:10.1016/j.cclet.2022.06.073 |
| [24] |
Y. Zhou, J. Lu, Q. Liu, et al., J. Hazard. Mater. 384 (2020) 121267. DOI:10.1016/j.jhazmat.2019.121267 |
| [25] |
Y. Zhou, X. Gu, R. Zhang, et al., Ind. Eng. Chem. Res. 54 (2015) 426-433. DOI:10.1021/ie503414k |
| [26] |
Y. Li, Y. Zhou, Y. Zhou, et al., Water Sci. Technol. 78 (2018) 2553-2563. DOI:10.2166/wst.2019.009 |
| [27] |
D. Jiang, D. Huang, C. Lai, et al., Sci. Total Environ. 644 (2018) 1181-1189. DOI:10.1016/j.scitotenv.2018.06.367 |
| [28] |
J.S. Yamani, A.W. Lounsbury, J.B. Zimmerman, Water Res. 88 (2016) 889-896. DOI:10.1016/j.watres.2015.11.017 |
| [29] |
W. Tan, J. Zhang, F. Luan, et al., Int. J. Biol. Macromol. 102 (2017) 704-711. DOI:10.1016/j.ijbiomac.2017.04.073 |
| [30] |
X. Hu, G. Xu, H. Zhang, et al., ACS Appl. Mater. Interfaces 12 (2020) 12165-12175. DOI:10.1021/acsami.0c00597 |
| [31] |
L. Sun, G. Xu, Y. Tu, et al., Water Res. 222 (2022) 118917. DOI:10.1016/j.watres.2022.118917 |
| [32] |
X. Tian, H. Yu, J. Yang, et al., Front. Environ. Sci. Eng. 16 (2021) 89. |
| [33] |
Q. Ji, C. Zhang, D. Li, Front. Environ. Sci. Eng. 14 (2020) 108. DOI:10.1007/s11783-020-1287-0 |
| [34] |
S. Ma, S. Zhan, Y. Jia, et al., ACS Appl. Mater. Interfaces 7 (2015) 21875-21883. DOI:10.1021/acsami.5b06264 |
| [35] |
X. Qi, Y. Huang, S. You, et al., Adv. Sci. 9 (2022) 2106015. DOI:10.1002/advs.202106015 |
| [36] |
S. Fulaz, S. Vitale, L. Quinn, et al., Trends Microbiol. 27 (2019) 915-926. DOI:10.1016/j.tim.2019.07.004 |
| [37] |
X. Zhou, X. Ren, Y. Chen, et al., Front. Environ. Sci. Eng. 17 (2022) 29. |
| [38] |
M. Li, G. Song, R. Liu, et al., Front. Environ. Sci. Eng. 16 (2021) 70. |
| [39] |
Q. Liu, J. Wang, C. Duan, et al., J. Hazard. Mater. 426 (2022) 128074. DOI:10.1016/j.jhazmat.2021.128074 |
| [40] |
X. Wang, H. Chen, J. Wang, et al., Water Switz. 13 (2021) 3004. |
| [41] |
Z. Liu, M. Huang, A. Li, et al., Water Res. 119 (2017) 57-66. DOI:10.1016/j.watres.2017.04.043 |
 2024, Vol. 35
2024, Vol. 35 

