Advanced oxidation processes (AOPs) have been universally recognized strategy for the treatment of organic contaminants [1]. The traditional Fenton can non-selectively remove the organics by hydroxyl radical (HO•), which is generated from the ferrous ion (Fe2+) activated hydrogen peroxide (H2O2) process at acidic conditions (Eqs. 1–3) [2]. Nevertheless, there are a few inherent limitations for the Fenton reaction, including the narrow application range of pH [3,4], the slow ferric ion (Fe3+)/Fe2+ circulation rate [5,6], and the instability of H2O2 [7]. As a solid oxidant, calcium peroxide (CaO2) is easier to store and transport, which can slowly release H2O2 (Eq. 4) [8]. The decomposition rate of CaO2 could be controlled by adjusting pH, which could avoid the disproportionation of H2O2 [9]. Therefore, CaO2 is considered as an effective substitute for H2O2 [10].
On the other side, as another common transition metal, cupric ion (Cu2+) is ubiquitous in multiple waterbodies [11,12]. Cu2+ has a larger solubility (Ksp Cu(OH)2 = 4.8 × 10−20) than that of Fe3+ (Ksp Fe(OH) = 2.8 × 10−39) [13], and the circulation of Cu2+/cuprous ion (Cu+) is easier than that of Fe3+/Fe2+ due to the lower standard potential (E0 (Fe3+/Fe2+) = 0.77 V vs. SHE, E0 (Cu2+/Cu+) = 0.17 V vs. SHE) [14], which means the Cu-based Fenton process is superior with a greater pH tolerance and better catalytic activity (Eqs. 5 and 6) than the Fe-based Fenton method [11]. Therefore, if the Cu2+ existed in water matrix can be in-situ used to activate Fenton/Fenton-like reactions, which could put forward to the practicality of the homogeneous Cu-based Fenton-like system. However, similar to the conventional Fenton reaction, the slow reduction of Cu2+ to Cu+ (k = 1 L mol−1 s−1) remains the rate-limiting step [15,16]. Thus, exploring a skill to accelerate the Cu2+/Cu+ circulation is imperative for promoting this Fenton-like reaction.
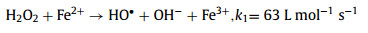
|
(1) |

|
(2) |
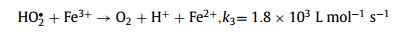
|
(3) |
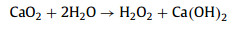
|
(4) |
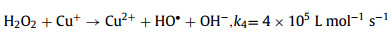
|
(5) |
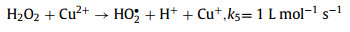
|
(6) |
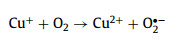
|
(7) |

|
(8) |
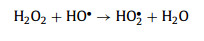
|
(9) |
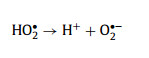
|
(10) |
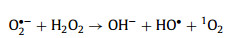
|
(11) |
The complexation of Cu2+ with organic ligands could alter the activity of Cu2+/Cu+ couple, and stabilize Cu ions in terms of electronic property and steric effect [12]. Moreover, some Cu2+ complexes could efficiently trigger H2O2 or persulfates to produce radicals, for degrading diverse organic pollutants [17]. However, some organic ligands, such as ethylenediaminetetraacetic acid [18,19], pyridine [17], and ethylenediamine [20], they are themselves contaminants, which not only increase the amount of organic matter and bring secondary pollution, but also quench free radicals by their self-degradation effect [21]. Hence, searching an environmentally friendly organic ligand is the key for improving homogeneous Cu-based Fenton-like system.
Plant-derived polyphenols belong to natural organic matter (NOM) and also low-molecular-weight organic acids, which are commonly found in the natural environments, including water [11,22]. The hydroxyl groups of polyphenols have the property of strong electron donor [23], which could mediate the Cu2+/Cu+ cycle and then reduce Cu2+ to Cu+ [11,24], triggering a series of chain reactions (Eqs. 5-11) to generate HO•, superoxide (O2•−), and singlet oxygen (1O2) [25,26]. Tartaric acid (TA) is a typical polyphenol, comes from grape and tamarind [10,27], which could be strongly complexed with Fe ions and regulate their redox cycling [28,29]. Nevertheless, there has been an obvious knowledge gap in the function of TA complexed with Cu ions for Fenton/Fenton-like processes.
Based on above, the in-situ simultaneous utilizations of Cu2+ and NOM in water might realize the most efficient implementation for Fenton/Fenton-like reactions [30]. Thus, in this study, the effectiveness of Cu2+-TA complex for catalyzing CaO2-based Fenton-like system was verified for metronidazole (MTZ) elimination. The systematical study centered around the followings: (1) Determine the influences of key factors (the dosages of Cu2+, TA, and CaO2, and pH) of the ternary processes; (2) investigate the catalytic function of Cu2+-TA complex; (3) explore the evolution and contribution of reactive oxygen species (ROS) during this Cu-based Fenton-like reaction.
The specific chemicals of the work were recorded in Text S1 (Supporting information). The batch experiment was conducted in a 150 mL glass vessel under electromagnetic stirring at room-temperature. The desired concentration of MTZ was configured with phosphate buffer, which fixed the initial pH of the reaction solution. TA and cupric chloride (CuCl2) were successively added into 100 mL of MTZ solution, the experiment was triggered once added the desired concentration of CaO2. All the tests were conducted at least in twice. MTZ concentration was detected through UV spectroscopy at 320 nm [13,31]. Tert-butyl alcohol (TBA), l-Histidine (l-His), and p-benzoquinone (p-BQ) were selected as the ROS scavenger, and the generation of ROS was detected by electron spin resonance technique (ESR). The concentration of H2O2 was determined by potassium titanium oxalate colorimetry [13]. The content of Cu ions was measured by neocuproine spectrophotometry [32]. Electro-chemical workstation was applied to conduct the cyclic voltammetry measurement [13]. MTZ decomposed intermediates were determined by high performance liquid chromatography-mass spectrometry (HPLC-MS). The detailed analysis and operation were listed in Text S2 (Supporting information).
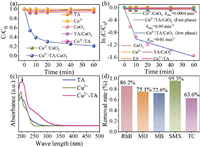
The enhancement effect of TA on Cu2+/CaO2 was evaluated by comparing the degradation efficiencies of MTZ in diverse processes. Fig. 1a shows the removal of MTZ was negligible in Cu2+/CaO2 after 60 min. Nevertheless, the elimination of MTZ augmented to 78.7% in the Cu2+/TA/CaO2 system. The apparent rate constant (kobs) of MTZ degradation in Cu2+/TA/CaO2 process was 225 times higher than that in Cu2+/CaO2 in the first 10 min, and 25 times higher in the following 50 min reaction (Fig. 1b). The decrease of kobs during the following 50 min might be ascribed to the quickly falling H2O2. Furthermore, the insignificant degradation of MTZ was observed in TA/CaO2 and Cu2+/TA systems, which indicated that TA could not activate CaO2 directly, and CaO2 was the motivity of MTZ removal [33]. UV–vis spectrum was employed to verify the formation of Cu2+-TA complex. As depicted in Fig. 1c, compared with the spectra of Cu2+ and sole TA, an emerging absorption peak was appeared around 213 nm in the mixed sample of Cu2+ and TA, which testified the production of Cu2+-TA metal organic ligand. TA might serve as the chelating agent to mediate the redox potential of Cu ions and promote the generation of ROS [18], therefore enhancing the performance of the Cu2+/CaO2 system.
|
Download:
|
| Fig. 1. (a) Elimination for MTZ in different systems and (b) corresponding pseudo-first-order kinetic rate constant. (c) UV–vis absorption spectra of Cu2+, TA, and Cu2+-TA. (d) Degradation of different organic contaminants in Cu2+/TA/CaO2 system. Experimental condition: [Cu2+]0 = 0.1 mmol/L, [TA]0 = 2 mmol/L, [CaO2]0 = 2 mmol/L, pH0 5, [MTZ] = [MB] = [MO] = [RhB] = [SMX] = [TC] = 5 mg/L. | |
Besides, Fig. 1d shows the Cu2+/TA/CaO2 process also had the remarkable capability for other contaminants removal, including rhodamine b (RhB), sulfamethoxazole (SMX), methyl orange (MO), tetracycline (TC), and methylene blue (MB). Furthermore, Fig. S1 (Supporting information) shows that 34.6% of chemical oxygen demand (COD) removal ratio was reached for domestic sewage by the synergistic process after 180 min reaction. Besides, the total organic carbon (TOC) removal achieved 20.2% after 180 min (Fig. S2 in Supporting information). Thus, the ternary system possessed an extensive applicability and actual potential for removing organic pollutants in wastewater.
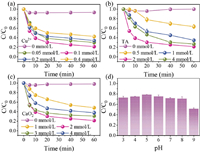
The influences of specific reaction parameters on the elimination of MTZ in the Cu2+/TA/CaO2 system were systematically investigated, including Cu2+ dosage (Fig. 2a), TA dosage (Fig. 2b), and CaO2 concentration (Fig. 2c). The results manifested that Cu2+, TA, and CaO2 were all indispensable factors, the absence of any one of them would seriously affect the degradation of MTZ. Furthermore, the higher dosages of Cu2+ and CaO2 would accelerate the generation of HO• to trigger the self-quenching reaction (Eq. 9) to restrain the elimination of MTZ. TA as an organic compound would compete HO• with MTZ, hence the excess TA also hindered the removal of MTZ. Therefore, after the comprehensive investigation, the optimal experimental dosages were as follows: 0.1 mmol/L Cu2+, 2 mmol/L TA, and 2 mmol/L CaO2. Moreover, the ternary process indicated a wide pH tolerance, as described in Fig. 2d, MTZ could be eliminated efficiently as the pH values increased from 3 to 8. Above results proved the benign catalytic ability of Cu2+-TA complex.
|
Download:
|
| Fig. 2. Influences of (a) Cu2+, (b) TA, (c) CaO2, and (d) pH on MTZ degradation in Cu2+/TA/CaO2 system. Experimental condition: [Cu2+]0 = 0.05–0.4 mmol/L, [TA]0 = 0.5–4 mmol/L, [CaO2]0 = 1–4 mmol/L, pH0 3–9, [MTZ] = 5 mg/L. | |
Moreover, the resistant ability for common anions (chloridion (Cl−), sulfate ion (SO42−), nitrate ion (NO3−), and bicarbonate ion (HCO3−)) on the Cu2+/TA/CaO2 system was investigated (Fig. S3 in Supporting information). The complexing system represented a great resistant ability for different anions expect HCO3−. Carbonate radical anion (CO3•−) with lower redox potential (1.59 V) would be generated by HCO3− quenching HO• (Eqs. 12–14), which would inhibit the degradation of MTZ [34,35].
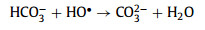
|
(12) |
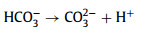
|
(13) |
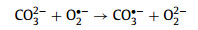
|
(14) |
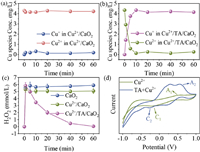
To investigate the role of TA in the enhanced Cu2+/CaO2 process, the change of Cu2+/Cu+ cycle and H2O2 concentration during the reaction were compared in Cu2+/CaO2 and Cu2+/TA/CaO2. As displayed in Fig. 3a. 0.19 mg/L of Cu+ was produced in Cu2+/CaO2 system, the weak reduction of Cu2+ to Cu+ might be the reason for hindering MTZ degradation. However, the Cu2+ was almost completely converted into Cu+ in the present of TA (Fig. 3b), which testified that the Cu2+-TA complex could effectually accelerate the reduction of Cu2+. In addition, the consumption of H2O2 in different systems was also investigated (Fig. 3c). Firstly, 5.88 mmol/L H2O2 could be released by CaO2 alone, and only 0.2 mmol/L of H2O2 was decomposed in Cu2+/CaO2. In Cu2+/TA/CaO2 system, H2O2 could be consumed rapidly, and the concentration of H2O2 was declined from 5.82 mmol/L to 0.05 mmol/L after 60 min. These results proved that the Cu2+-TA complex could availably promote the disintegration of H2O2.
|
Download:
|
| Fig. 3. Variation of Cu2+ and Cu+ concentration in Cu2+/CaO2 (a) and Cu2+/TA/CaO2 systems (b). (c) Change of H2O2 concentration in different systems. (d) CV of Cu2+-TA. Experimental condition: [Cu2+]0 = 0.1 mmol/L, [TA]0 = 2 mmol/L, [CaO2]0 = 2 mmol/L, pH0 5, [MTZ] = 5 mg/L. | |
To demonstrate the redox ability of Cu2+-TA complex, the cyclic voltammetry (CV) was conducted in the Cu2+ system with or without TA. As shown in Fig. 3d, the peaks of A1 and A2 were owed to the oxidation of Cu+ to Cu2+, while C1 and C2 were the reducing peaks of Cu2+ to Cu+ [13]. The higher peak potential in the mixed Cu2+ and TA solution (A2, C2) than that in the without TA (A1, C1) indicated that the Cu2+-TA complex had the greater redox capacity. Hence, TA chelated with Cu2+ in the Cu2+/TA/CaO2 could reduce the Cu2+/Cu+ redox potential to improve the Cu2+/Cu+ cycle, enhancing the H2O2 disintegration and the ROS production [13].
The primary ROS in the Cu2+/TA/CaO2 were clarified firstly by quenching experiment. TBA was applied to quench HO• (kHO = 6 × 108 L mol−1 s−1) [36,37]. As described in Fig. 4a, MTZ removal efficiency significantly declined to 24.2% under 2 mmol/L TBA, indicating that HO• played the dominant role for MTZ elimination. Moreover, p-BQ and l-His were selected to suppress O2•− and 1O2 (kO, p-BQ = (0.9–1) × 109 L mol−1 s−1, k1, l-His = 3.2 × 107 L mol−1 s−1), separately [38–40]. As shown in Figs. 4b and c, the slight quenching effect of p-BQ and l-His illustrated that O2•− and 1O2 both played the minor role in this system. Ulteriorly, ESR was employed to confirm the existence of ROS. 5,5-dimethyl-1-pyrrolidine-N-oxide (DMPO) was used to trap HO• and O2•−, 2,2,6,6-tetramethylpiperidine (TEMP) was employed to capture 1O2 [38,41]. As presented in Figs. 4d– f, the typical signals of DMPO-HO•, DMPO-O2•−, and TEMP-1O2 adducts were detected in both processes with and without TA. Of note, the signal intensity of Cu2+/TA/CaO2 was obviously stronger than that of Cu2+/CaO2, which affirmed the role of TA on the enhancement of ROS generation in this Cu-based Fenton-like reaction.

|
Download:
|
| Fig. 4. Influences of (a) TBA, (b) p-BQ, and (c) l-His on MTZ degradation in Cu2+/TA/CaO2 system. EPR spectra of (d) DMPO-HO• adduct, (e) DMPO-O2•− adduct, and (f) TEMP-1O2 adduct in both systems. Experimental condition: [Cu2+]0 = 0.1 mmol/L, [TA]0 = 2 mmol/L, [CaO2]0 = 2 mmol/L, [TBA]0 = 0.5–2 mmol/L, [p-BQ]0 = [l-His]0 = 0.1–0.5 mmol/L, pH0 5, [MTZ] = 5 mg/L. | |
The generated HO• exhibited triple roles in this system: (1) contributing to the degradation of MTZ; (2) reacting with the excess H2O2 to generate the secondary O2•− and 1O2 (Eqs. 9–11); (3) attacking the electrophilic TA into the small molecule. Furthermore, Cu+-TA also reacted with O2 to promote the production of O2•− (Eq. (15)), and the generated Cu2+-TA reacted with the excess H2O2 to sustain the Cu2+/Cu+ cycle (Eq. 16) [42]. However, O2•− and 1O2 could not effectively remove MTZ due to the low oxidation capacity (kO2•−, MTZ = 0 L mol−1 s−1, k1O2, MTZ = 1.5 × 108 L mol−1 s−1), so HO• was the dominant ROS for MTZ elimination (kHO•, MTZ = 6 × 109 L mol−1 s−1) in the ternary system, followed by 1O2 and O2•− [43,44].
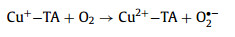
|
(15) |

|
(16) |
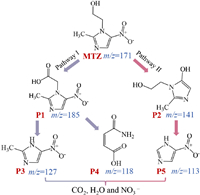
According to the result of HPLC-MS, the possible degradation pathways of MTZ during Cu2+/TA/CaO2 were proposed. As revealed in Fig. 5, firstly, (2-methyl-5-nitroimidazol-1-yl) acetic acid (P1, m/z = 185) might be produced by the N-ethanol group oxidation of MTZ [45,46]. Next, HO• attacked the N-acetic acid group of P1 and generated 2-methyl-5-nitro-1H-imidazole (P3, m/z = 127) [45,47], or P1 underwent hydroxylation and ring opening reaction to form 3-carbonyl propylene glycol 2-oleic acid (P4, m/z = 118) [45,48]. In another pathway, 3-(2-hydroxy-ethyl)-2-methyl-3H-imidazole-4-ol (P2, m/z = 141) was formed by the direct hydroxylation of nitro group [45,49]. The crack of N-acetic acid group and nitration reaction were occurred on P2 to form byproduct P5 (m/z = 113) [50]. In addition, the biotoxicity of MTZ and its elimination byproducts was evaluated by toxicity assessment software, the results demonstrated that the oral rat LD50 (Fig. S4a in Supporting information) and the developmental toxicity (Fig. S4c in Supporting information) of part intermediates were higher than that of MTZ. However, the bioaccumulation factor (Fig. S4b in Supporting information) and the mutagenicity (Fig. S4d in Supporting information) of the intermediates were all decreased after the treatment of the synergic process. Therefore, the Cu2+/TA/CaO2 process could decrease the biotoxicity of MTZ to some extent, and appropriate prolongation of reaction might be required to minimize toxicity in practical treatment.
|
Download:
|
| Fig. 5. Speculated decomposition pathways of MTZ. | |
To sum up, the conceivable mechanism of TA enhanced Cu2+/CaO2 system can be summarized as follows. In the Cu2+/CaO2 system, the sluggish Cu2+/Cu+ cycle hindered the activation of H2O2 (Eqs. 5 and 6), then hampered the elimination of MTZ (Fig. 1a). Upon the TA addition, Cu2+ was rapidly complexed with the two hydroxyl groups of TA to form a soluble Cu2+-TA complex (Eq. 17, Fig. 1c), which could decrease the redox potential of Cu2+/Cu+ (Fig. 3d). The Cu2+-TA could be reduced by H2O2 to form the reductive Cu+-TA, which could improve the reduction of Cu2+ (Eq. 16, Fig. 3b) and the activation of H2O2 (Eq. 18, Fig. 3c) [42]. Next, the production of various ROS was enhanced by the complexation, such as HO•, O2•−, and 1O2 (Figs. 4d–f), and the removal of MTZ was also promoted. Overall, Cu+-TA was the critical species to manipulate this ternary system, and TA severed as the electron donor to promote the electron transfer in metal-ligand and enhance the generation of ROS.

|
(17) |
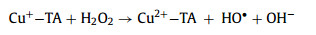
|
(18) |
In summary, the complexation of Cu2+ and TA could efficaciously catalyze CaO2 oxidation of MTZ in the pH extent of 3–8. The addition of TA could accelerate the regeneration of Cu+, which was driven from the formed ligand of Cu2+ and TA. Furthermore, the Cu2+-TA complex could motivate the H2O2 disintegration and the ROS production. HO• was proved to be the main ROS for MTZ degradation, followed by 1O2 and O2•−. MTZ decomposition pathways mainly included hydroxylation, ring opening, attack on specific group, and nitration reactions. This work proved that TA could be a potential chelating agent to promote Fenton-like reaction for degrading different organic contaminants, which provided a referable method for improving the performance of Cu-based Fenton-like system.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the supports from the National Natural Science Foundation of China (No. 51908485), the Central Guidance on Local Science and Technology Development Fund of Hebei Province (No. 226Z3603G), Hebei Province Foundation for Returnees (No. C20210502).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109185.
| [1] |
H. Weng, Y. Yang, C. Zhang, et al., Chem. Eng. J. 453 (2023) 139812. DOI:10.1016/j.cej.2022.139812 |
| [2] |
S. Pan, Z. Zhai, K. Yang, et al., Sep. Purif. Technol. 289 (2022) 120806. DOI:10.1016/j.seppur.2022.120806 |
| [3] |
C. Wang, B. Wei, H. Zhu, et al., Chin. Chem. Lett. 33 (2022) 3073-3077. DOI:10.1016/j.cclet.2021.09.051 |
| [4] |
W. Zheng, Y. Liu, F. Liu, et al., Water Res. 223 (2022) 118994. DOI:10.1016/j.watres.2022.118994 |
| [5] |
W. Hu, M. Yang, Q. Yan, et al., Chin. Chem. Lett. 34 (2023) 108109. DOI:10.1016/j.cclet.2022.108109 |
| [6] |
Q. Zhao, C. Wang, P. Wang, Chin. Chem. Lett. 33 (2022) 4828-4833. DOI:10.1016/j.cclet.2022.01.033 |
| [7] |
S. Tang, E. Zhu, Z. Zhai, et al., Chemosphere 319 (2023) 138025. DOI:10.1016/j.chemosphere.2023.138025 |
| [8] |
D. Yuan, C. Zhang, S. Tang, et al., Chin. Chem. Lett. 32 (2021) 3387-3392. DOI:10.1016/j.cclet.2021.04.050 |
| [9] |
Y. Zhou, X. Fang, T. Wang, Y. Hu, J. Lu, Chem. Eng. J. 313 (2017) 638-645. DOI:10.1016/j.cej.2016.09.111 |
| [10] |
S. Tang, Z. Wang, D. Yuan, et al., J. Clean. Prod. 268 (2020) 122253. DOI:10.1016/j.jclepro.2020.122253 |
| [11] |
Y. Wang, Y. Wu, Y. Yu, et al., Water Res. 186 (2020) 116326. DOI:10.1016/j.watres.2020.116326 |
| [12] |
J. Chen, X. Zhou, P. Sun, Y. Zhang, C. Huang, Environ. Sci. Technol. 53 (2019) 11774-11782. DOI:10.1021/acs.est.9b03873 |
| [13] |
Q. Ye, H. Xu, Q. Wang, et al., J. Hazard. Mater. 407 (2021) 124351. DOI:10.1016/j.jhazmat.2020.124351 |
| [14] |
G. Pan, Z. Sun, Chemosphere 283 (2021) 131257. DOI:10.1016/j.chemosphere.2021.131257 |
| [15] |
N. Li, T. Liu, S. Xiao, et al., J. Hazard. Mater. 445 (2023) 130536. DOI:10.1016/j.jhazmat.2022.130536 |
| [16] |
L. Jin, S. You, N. Ren, B. Ding, Y. Liu, Environ. Sci. Technol. 56 (2022) 11750-11759. DOI:10.1021/acs.est.2c03904 |
| [17] |
J. Li, A.N. Pham, R. Dai, Z. Wang, T.D. Waite, J. Hazard. Mater. 392 (2020) 122261. DOI:10.1016/j.jhazmat.2020.122261 |
| [18] |
A. De Luca, R.F. Dantas, S. Esplugas, Water Res. 61 (2014) 232-242. DOI:10.1016/j.watres.2014.05.033 |
| [19] |
A. Gabet, C. Guy, A. Fazli, et al., Sep. Purif. Technol. 317 (2023) 123877. DOI:10.1016/j.seppur.2023.123877 |
| [20] |
A. Rastogi, S.R. Al-Abed, D.D. Dionysiou, Water Res. 43 (2009) 684-694. DOI:10.1016/j.watres.2008.10.045 |
| [21] |
Y. Xiang, K. Yang, Z. Zhai, et al., Chem. Eng. J. 438 (2022) 135656. DOI:10.1016/j.cej.2022.135656 |
| [22] |
H. Zhou, H. Zhang, Y. He, et al., Appl. Catal. B. 286 (2021) 119900. DOI:10.1016/j.apcatb.2021.119900 |
| [23] |
Q. Ouyang, F. Kou, N. Zhang, et al., Chem. Eng. J. 366 (2019) 514-522. DOI:10.1016/j.cej.2019.02.078 |
| [24] |
Y. Bai, D. Wu, W. Wang, et al., Environ. Res. 192 (2021) 110242. DOI:10.1016/j.envres.2020.110242 |
| [25] |
X. Li, Y. Jia, J. Zhang, et al., Chin. Chem. Lett. 33 (2022) 2105-2110. DOI:10.1016/j.cclet.2021.08.054 |
| [26] |
D. Yuan, C. Zhang, S. Tang, et al., Water Res. 163 (2019) 114861. DOI:10.1016/j.watres.2019.114861 |
| [27] |
M. Rashidipour, Z. Derikvand, A. Shokrollahi, Z. Mohammadpour, A. Azadbakht, Arab. J. Chem. 10 (2017) S3167-S3175. DOI:10.1016/j.arabjc.2013.12.010 |
| [28] |
P. Villegas-Guzman, S. Giannakis, R.A. Torres-Palma, C. Pulgarin, Appl. Catal. B. 205 (2017) 219-227. DOI:10.1016/j.apcatb.2016.12.021 |
| [29] |
Z. Ouyang, C. Yang, J. He, et al., Chem. Eng. J. 402 (2020) 126122. DOI:10.1016/j.cej.2020.126122 |
| [30] |
T. Pan, Y. Wang, X. Yang, X. Huang, R. Qiu, Chem. Eng. J. 384 (2020) 123248. DOI:10.1016/j.cej.2019.123248 |
| [31] |
Y. Xiang, H. Liu, E. Zhu, et al., Sep. Purif. Technol. 295 (2022) 121293. DOI:10.1016/j.seppur.2022.121293 |
| [32] |
X. Zhou, H. Luo, B. Sheng, et al., J. Hazard. Mater. 411 (2021) 125050. DOI:10.1016/j.jhazmat.2021.125050 |
| [33] |
M. Zhao, Y. Xiang, X. Jiao, et al., Sep. Purif. Technol. 276 (2021) 119289. DOI:10.1016/j.seppur.2021.119289 |
| [34] |
S. Pan, T. Zhao, H. Liu, et al., Chem. Eng. J. 452 (2023) 139245. DOI:10.1016/j.cej.2022.139245 |
| [35] |
Y. Huang, L. Bu, Y. Wu, et al., Chem. Eng. J. 442 (2022) 135995. DOI:10.1016/j.cej.2022.135995 |
| [36] |
F. Ye, W. Sun, K. Pang, et al., Chin. Chem. Lett. 34 (2023) 107755. DOI:10.1016/j.cclet.2022.107755 |
| [37] |
M. Wang, M. Fu, J. Li, et al., Chin. Chem. Lett. 35 (2024) 108442. DOI:10.1016/j.cclet.2023.108442 |
| [38] |
Y. Li, H. Dong, J. Xiao, et al., Chem. Eng. J. 455 (2023) 140773. DOI:10.1016/j.cej.2022.140773 |
| [39] |
L. Yang, W. Chen, C. Sheng, et al., Appl. Surf. Sci. 549 (2021) 149300. DOI:10.1016/j.apsusc.2021.149300 |
| [40] |
Z. Yang, Z. Wang, G. Liang, X. Zhang, X. Xie, Chem. Eng. J. 426 (2021) 131777. DOI:10.1016/j.cej.2021.131777 |
| [41] |
P. Yang, A. Shen, Z. Zhu, et al., Chem. Eng. J. 464 (2023) 142724. DOI:10.1016/j.cej.2023.142724 |
| [42] |
J. Guo, X. Chen, Y. Shi, Y. Lan, C. Qin, PLoS One 10 (2015) e0134298. DOI:10.1371/journal.pone.0134298 |
| [43] |
L. Gao, Y. Guo, J. Zhan, G. Yu, Y. Wang, Water Res. 221 (2022) 118730. DOI:10.1016/j.watres.2022.118730 |
| [44] |
Y. Guo, J. Long, J. Huang, G. Yu, Y. Wang, Water Res. 215 (2022) 118275. DOI:10.1016/j.watres.2022.118275 |
| [45] |
H. Ren, H. Liu, T. Cui, et al., Chem. Eng. J. 431 (2022) 133803. DOI:10.1016/j.cej.2021.133803 |
| [46] |
S. Tang, H. Liu, E. Zhu, et al., Sep. Purif. Technol. 301 (2022) 122056. DOI:10.1016/j.seppur.2022.122056 |
| [47] |
J. Cao, J. Li, W. Chu, W. Cen, Chem. Eng. J. 400 (2020) 125813. DOI:10.1016/j.cej.2020.125813 |
| [48] |
X. Guo, B. Hu, K. Wang, et al., Chem. Eng. J. 435 (2022) 132910. DOI:10.1016/j.cej.2021.132910 |
| [49] |
J. Zeng, J. Liu, W. Su, et al., J. Water Process. Eng. 53 (2023) 103733. DOI:10.1016/j.jwpe.2023.103733 |
| [50] |
X. Tian, T. Luo, Y. Nie, et al., Environ. Sci. Technol. 56 (2022) 5542-5551. DOI:10.1021/acs.est.2c00132 |
 2024, Vol. 35
2024, Vol. 35 

