b Shen Zhen Research Institute of Xiamen University, Shenzhen 518057, China;
c Division of Urology and Department of Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, United States;
d Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen 361102, China
Biomacromolecules are large molecules composed of various types of building blocks, such as nucleic acids, proteins, and polysaccharides. They play vital roles in biological systems, including as genetic information, enzymes, structural components and signaling molecules. In recent years, biomacromolecules have been increasingly investigated for their potential as therapeutic agents and drug delivery vehicles due to their high specificity, low toxicity, and biocompatibility [1]. Biomacromolecule-based drugs offer several advantages over small molecule drugs, including targeted delivery, increased half-life, and improved pharmacokinetics [2]. To give an example, functional nucleic acids (FNAs) such as aptamers, DNAzymes, and DNA-based nanomachines (DNMs) serve as the most commonly used tools in cancer therapeutics, combining molecular diagnostics and targeted cancer therapies and facilitating the transition from conventional medicine to personalized and precision medicine [3-5].
What is more, in the field of vaccine development, DNA, RNA and polysaccharide conjugate vaccines have dominated the field for their safety, ease of construction, and rapid production. Various messenger RNA (mRNA) vaccines against corona virus disease 2019 (COVID-19) have been developed, which is a milestone in the development of nucleic acid vaccines [6]. In addition, several polysaccharide conjugate vaccines have been approved for the prevention of Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae serotype b (Hib), e.g., Pedvax HIB (Merck, Kenilworth, NJ, USA), Menveo1® (Novartis), and Hiberix (Glaxi SmithKline, Rixensart, Belgium) [7]. Furthermore, monoclonal antibodies can be engineered to recognize and bind to specific proteins on the surface of cancer cells, enabling targeted drug delivery to the tumor site. Trastuzumab has been used in the clinic to treat human epidermal growth factor receptor 2 (HER2)-positive breast cancer by targeting HER2 receptors on the surface of cancer cells [8]. Similarly, Bevacizumab, which targets vascular endothelial growth factor (VEGF), has been applied in the clinical treatment of various diseases, including colorectal, lung, and kidney cancer [9-11] and corneal neovascularization [12]. This targeted approach reduces off-target effects and increases the therapeutic index of the drug. Furthermore, small molecule drugs are typically metabolized quickly and excreted from the body, resulting in a short duration of action. In contrast, biomacromolecules can be engineered to resist degradation and clearance by the immune system, leading to longer-lasting therapeutic effects. Such as albumin, which is a naturally occurring protein in the body that has many important functions, including the maintenance of oncotic pressure and the transport of various molecules. One approach to enhancing the therapeutic efficacy of albumin is through the use of albumin-based drug delivery systems [13,14]. These systems typically involve the conjugation of a therapeutic agent to albumin, which can protect the agent from degradation and clearance as well as target it to specific tissues or cells. Albumin conjugates have been shown to have improved pharmacokinetic properties compared to free drugs, resulting in longer half-lives and increased therapeutic efficacy. At the same time, tissue engineering takes extensive advantage of the biomacromolecules' high biocompatibility. For instance, the U.S. Food and Drug Administration (FDA) has approved 24 distinct varieties of hydrogel wound dressings made of polysaccharides [15]. This is due to the fact that polysaccharide-based hydrogels are immune-regulating, anti-inflammatory, and have better tissue adhesion, swelling, and water absorption than conventional wound dressings. Moreover, numerous recent studies have demonstrated how polysaccharides significantly limit tumor growth while having few adverse effects and working in concert with chemotherapy drugs [16-19]. The increasing interest in biomacromolecules as therapeutic agents and drug delivery vehicles highlights their potential to transform the field of medicine.
However, the large molecular weight, complex structure, susceptibility to degradation, and low cell entry efficiency of biomacromolecules have limited their effectiveness [20]. A possible strategy to alleviate the inherent limitations of biomacromolecule medications is nanotechnology, which can modify the size, shape, and surface features of biomacromolecule drugs to enhance their pharmacokinetics, biodistribution, and efficacy. Typically, we define particles created by nanotechnology as nanoparticles whose longest and shortest axes are not significantly different from one another and whose external dimensions are all in the nanoscale (lengths in the range of 1–100 nm). In the pharmaceutical sector and the field of nanodrugs, the definition of nanoparticles is usually extended to solid objects with a particle size of less than 1–5 µm, i.e., in the submicron and near-submicron regions. The definition of biomacromolecule nanomedicine considered in this review extends to the submicron and near-submicron regions (i.e., particle sizes less than 1–5 µm) [21]. Cellular uptake and intracellular transport of biomacromolecules will be improved when they are prepared as nanoparticles [22]. By engineering the surface chemistry and composition of nanoparticles, it is possible to enhance their endocytosis and transport across the cell membrane, thereby increasing the bioavailability and therapeutic efficacy of biomacromolecule drugs. Moreover, nanotechnology offers a versatile platform for the co-delivery of multiple biomacromolecule drugs, small molecule drugs, and imaging agents, which can synergistically enhance the therapeutic outcomes while minimizing the adverse effects. By encapsulating or conjugating biomacromolecule drugs within nanoparticles, it is possible to achieve controlled release, sustained action, and reduced toxicity, which are critical factors for the clinical translation of biomacromolecule drugs [23]. For instance, liposome-based nanoparticle carriers can let nucleic acid vaccines enter the cytoplasm and prevent their enzymatic breakdown for efficient anti-tumor immune activation in disease diagnostics and treatment [24]. By encapsulating insulin in nanoparticles, improvements from injectable to oral dosage forms can be achieved in the treatment of chronic disorders like diabetes [25]. In one study, by incorporating insulin nanoparticles made of poly(lactide-co-glycolide) (PLGA) and Eurdragit® RS into HPMCP55-coated capsules, the nanoparticle formulation could be protected from acidic environments, facilitating capsule dissolution and release of the contents in a small intestinal environment with a pH greater than 6.0 [26]. Additionally, the difficulties of conventional small and large molecule medications for the treatment of brain illnesses can be overcome by combining the benefits of biomacromolecules and nanotechnology. For instance, the free amino groups on the surface of chitosan nanoparticles enable chemical alterations for precise targeting of brain tumors and improve drug penetration through the blood-brain barrier by influencing tight junctions [27]. While the conventional methods of nanosizing biomacromolecules, such as precipitation, emulsion, and spray-drying, still have several bottlenecks that need to be addressed [28]. One of the major challenges is maintaining the stability and integrity of the biomacromolecules during the nanosizing process. The harsh processing conditions involved in the nanosizing process can cause denaturation, aggregation, or chemical modification of the biomacromolecules, leading to a loss of biological activity and potential toxicity.
Recently, supercritical fluid (SCF) technology has emerged as a potential alternative to overcome these limitations in the field of biomacromolecule-based nanomedicine [29]. SCF technology utilizes supercritical fluids, which are fluids at a temperature and pressure above their critical point, to dissolve and expand the starting materials, enabling the production of nano-sized particles without solvent residue or active ingredient loss. The SCF technology involves mixing a drug solution with a supercritical fluid, such as supercritical fluid carbon dioxide (SCCO2), and ejecting the mixture via a nozzle with a tiny orifice to form solid nanoparticles with the rapid vaporization of the supercritical fluid [30]. Researchers are interested in SCF technology for nanoparticle preparation compared to conventional methods because of its simplicity, low cost, and use of environmentally friendly solvents that may produce nanoparticles with high purity without leaving any organic solvent residues [31,32]. For example, SCCO2 antisolvent precipitation is applied to nanosize the typical hydrophilic drugs doxorubicin hydrochloride (DOX) and indocyanine green (ICG) [33-35]. The results revealed no significant differences between the structure and action of nanoparticles and the original drug, illustrating an enhanced property than the molecular state, which facilitates the drugs used in clinic successfully [36-38].
SCF technology also offers significant advantages in the preparation of biomacromolecule-based drugs. It maintains the stability and activity of biomacromolecules, which are otherwise prone to degradation and denaturation during traditional preparation processes. SCF technology employs carbon dioxide (CO2) as the solvent, which is nontoxic, non-flammable, and easily removed, thus reducing the possibility of toxic organic solvent residue in the final products. Additionally, SCF technology provides precise control over the particle size, morphology, and crystal form of biomacromolecules [39]. Moreover, SCF technology offers the potential for industrial-scale production of biomacromolecule-based drugs due to its easy scalability and reproducibility. SCF technology can be applied to a wide range of biomacromolecules, including proteins, nucleic acids, and polysaccharides [21]. For instance, SCF technology can be used to produce protein-based nanomedicines with high purity, excellent biological activity, and high stability. Nucleic acids can also be prepared using SCF technology, such as plasmid DNA and siRNA, which can be used for gene therapy applications. Polysaccharides, such as chitosan, can be modified using SCF technology to improve their solubility, bioactivity, and biocompatibility for drug delivery purposes.
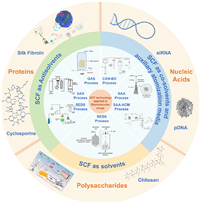
In this review, we highlight the unique features of SCF technology for biomacromolecule-based nanomedicine and its potential biomedical applications (Scheme 1). Firstly, the mechanism and characteristics of SCF technology for the preparations of biomacromolecular drugs were systemically introduced. Specifically, the protein-based, nucleic acid-based, and polysaccharide-based nanomedicine preparation via SCF technology were summarized, along with their biomedical applications such as drug delivery, gene therapy, and tissue engineering. Furthermore, we compared SCF technology with traditional nanosizing techniques, and discussed the challenges and opportunities for the industrial production of biomacromolecular drugs. The goal of this review is to provide a comprehensive understanding of the advantages and limitations of SCF technology in biomacromolecule-based nanomedicine and to stimulate further research in this field.
|
Download:
|
| Scheme 1. Schematic illustration of three types of SCF technologies applied for preparing biomacromolecular nanomedicine, including the use of SCF as solvents, antisolvents, co-solvents and auxiliary atomization media. Operating under mild conditions, SCF technologies reduce the probability of causing damage to biomolecules, thus offering a promising method for preparing biomacromolecular nanodrugs. The potential benefits include enhanced drug efficacy, bioavailability, permeability, and absorption, as well as decreased costs and environmental impact. Copied with permission [40]. Copyright 2007, Elsevier B.V. Copied with permission [41]. Copyright 2011, Elsevier B.V. Copied with permission [42]. Copyright 2006, American Chemical Society. Copied with permission [21]. Copyright 2018, Elsevier B.V. Copied with permission [43]. Copyright 2022, Oxford University Press. Copied with permission [127]. Copyright 2008, Elsevier B.V. Copied with permission [44]. Copyright 2018, American Chemical Society. Copied with permission [45]. Copyright 2022, Elsevier B.V. | |
2. Mechanisms and characteristics of SCF technology for the preparation of biomacromolecular drugs
SCF technology has demonstrated great potential in the field of biopharmaceuticals, primarily due to its environmentally friendly, sustainable, safe, and nontoxic properties. Compared to traditional methods like mechanical crushing and precipitation, employing this technology for biomacromolecular drug preparation offers numerous advantages [46].
Firstly, SCF technology can maintain the structural integrity and biological activity of biomacromolecules, which is critical for their therapeutic effectiveness [47]. Unlike conventional methods that involve harsh processing conditions, such as high temperatures, organic solvents, and shear forces, SCF technology operates under mild conditions (e.g., low temperature and pressure), thereby minimizing damage to biomacromolecules. SCF technology employs various fluids, including H2O, CO2, and NH3, among which CO2 is an important component of SCF and exhibits many attributes suitable for the preparation of biomacromolecular drugs: (1) The critical temperature is 304.2 K, which saves energy and is ideal for the granulation of pharmaceuticals that are sensitive to heat, such as proteins, peptides, and nucleic acids. (2) The critical pressure is 7.37 MPa, which does not necessitate the use of highly specialized equipment. (3) Safe, nontoxic, stable, affordable and easy to obtain. Moreover, SCF technology can minimize the contact of biomacromolecules with organic solvents, which can cause denaturation, aggregation, and toxicity. Therefore, SCF technology offers a gentle and effective way to prepare biomacromolecule-based drugs with high quality and purity.
Secondly, SCF technology can regulate the size, morphology, and distribution of biomacromolecule-based nanoparticles, which are crucial for their pharmacokinetics and pharmacodynamics. The size of nanoparticles plays a significant role in their biological fate, such as cellular uptake, biodistribution, and clearance [48]. SCF technology allows for precise control over the particle size by adjusting the processing parameters, such as pressure, temperature, and flow rate [29]. Additionally, SCF technology can produce uniform and monodisperse nanoparticles with a narrow size distribution, which is beneficial for reducing batch-to-batch variability and enhancing reproducibility [21].
Thirdly, SCF technology facilitates the incorporation of biomacromolecules into various drug delivery systems, such as liposomes, micelles, and nanoparticles, which can improve their therapeutic efficacy and safety [39]. For example, SCF technology can encapsulate biomacromolecules into polymeric nanoparticles or liposomes, which can protect them from enzymatic degradation, increase their solubility, and enhance their cellular uptake. Moreover, SCF technology can functionalize the surfaces of nanoparticles with targeting ligands or stimulus-responsive moieties, which can improve their specificity and selectivity for diseased cells or tissues [46].
Given the diverse roles of SCF in the preparation of biomacromolecular nanoparticles and the different characteristics of each process, the technology can be classified into the following three categories: SCF as solvents, as antisolvents, as co-solvents and auxiliary atomization media [49,50].
2.1. SCF as solventsThe rapid expansion of supercritical solution (RESS) uses a supercritical fluid to dissolve a solute to form a supercritical solution, followed by rapid decompression and expansion through a nozzle [51]. When the pressure suddenly drops, the fluid loses its solubility, leading to solute supersaturation, nucleation and precipitation into a solid form, thereby producing nanoparticles. The RESS process is appropriate for the manufacture of ultrafine particles of all compounds that can be dissolved in SCFs. The characteristics of RESS make it simple to control the size and particle size distribution of the product, and the resulting particles are completely dry, solvent-free, and do not need further processing. For instance, a study illustrated that uniform vanillin particles could be precipitated by the RESS process with a 100-fold reduction in particle size and no significant change in morphology or crystallinity compared to commercial vanillin [52]. Additionally, drug carriers can be prepared using the RESS method. For example, pH-responsive liposomes were encapsulated with a dual coating of chitosan and β-lactoglobulin using a venturi-based rapid expansion of supercritical solution for intestinal drug delivery systems. These systems were equally effective in encapsulating hydrophilic and lipophilic bioactive substances when compared to the commercially available pH-responsive polymer Eudragit® S100 (Eu-SL) coating [53].
In general, RESS technology has a simple process and high yield, leaves no solvent residue and works quickly, but it requires a large amount of SCF and the particles may aggregate and clog the nozzles, making it difficult for industrial production. What is more, the application of RESS to biomacromolecules is limited by the fact that biomacromolecules are insoluble in SCF, which cannot meet the primary conditions for the RESS process.
2.2. SCF as antisolventsSince SCCO2 has poor solubility for the majority of pharmaceuticals in the RESS process, another approach using SCCO2 as an antisolvent was devised. The basic principle is that when SCCO2 is dissolved in an organic solvent solution containing a solute, the solution expands in volume, gets less dense, and the solute's solubility in the solvent decreases, eventually precipitating to form solid particles [54]. The gaseous antisolvent (GAS) process was first proposed in 1992, employing SCF as an antisolvent [55]. The mixing unit in the GAS process also functions as a precipitation unit, where the particles are gathered. GAS is an intermittent process in which SCCO2 needs to be continuously introduced after particle precipitation has occurred to avoid a loss of pressure that would cause the solution to regain solubility. In addition, the GAS process is prone to organic residues. The supercritical antisolvent (SAS) [54] method and solution enhanced dispersion by supercritical fluids (SEDS) [56] are two antisolvent procedures with semi-continuous operation that have been designed to address drawbacks in the GAS process. The SAS process only requires the selection of a suitable solvent, which greatly compensates for the shortcomings of the RESS method and is well developed and widely used. For example, SAS-treated gefitinib has a higher solubility and faster dissolution rate compared with the original drug, which is conducive to improving its bioavailability, reducing dose requirements, and lowering the incidence of adverse reactions [57]. Biodegradable nanoparticles were produced by the SAS method for controlled delivery of paclitaxel. The particle size was controlled by enhancing the mixing between solvent and SCCO2 through ultrasonic treatment, and the resulting composite particles had homogeneous sizes with high encapsulation efficiency and a controlled release of paclitaxel for more than 30 days [58]. The SEDS technology is a variant of the SAS method, which involves nozzle spraying followed by the coaxial nozzle mixing of a solute/drug solution with SCCO2. For improved premixing conditions, the nozzle typically contains two channels as well as a mixing length at the end of the nozzle. The SCCO2 is typically introduced along the outer channel, while the solute/drug solution is usually introduced along the inner channel, which decreases drying time and the chance of particle agglomeration by improving mass transfer efficiency and solvent elimination [59-62]. Therefore, the SEDS technique has been widely used for particle formation. For instance, water-soluble and nanoscale (100–200 nm) PVP/astaxanthin inclusion complexes were successfully made utilizing the SEDS technology [62]. It was discovered that the particle size in the coprecipitates was influenced by the operating pressure, temperature, and PVP/astaxanthin ratio. By maximizing these parameters, high astaxanthin content and fine, water-soluble particles can be produced. Moreover, the biological functions of drugs can be improved after micronization by this technology. In one study, the SEDS technology was applied to micronize curcumin, which not only improved its water solubility, but also its bacteriostatic activity against Pseudomonas aeruginosa [63]. Additionally, using the right organic solvents or adding additives to increase the inter-solubility of water and SCCO2 can micronize various protein and peptide hydrophilic medications for both SAS technology and SEDS technique. The application will be covered in more detail in the following section.
2.3. SCF as co-solvents and auxiliary atomization mediaAlthough semi-continuous antisolvent processes are promising for the preparation of biomacromolecular particles, shortcomings such as insufficient solvent removal and nozzle blockage still need to be overcome. Therefore, continuous processes were developed in which SCF was used as co-solvents and auxiliary atomization media. The CO2-assisted nebulization with bubble dryer (CAN-BD) and the supercritical fluid-assisted atomization method (SAA) are examples of these models.
The CAN-BD method uses SCCO2 mixed with drug solution in a miniature tee to form a microemulsion, which is ejected through a capillary throttling device to create atomized droplets that are heated and dried into solid particles [64-66]. This process uses the expansion effect of SCCO2 to produce ultrafine particles from aqueous solutions or organic solvents. In addition, the drying temperature can be lower than that of conventional spray drying due to the fine and uniform droplets, which makes it more suitable for the micronization of heat-sensitive materials. CAN-BD is a supercritical micronization technique with special interest that changes the scope of application of SCF techniques, making it possible to prepare particles from systems in which water is the single solvent, avoiding organic solvent residues. For instance, Huang et al. employed this technique to micronize mannitol and myo-inositol aqueous solutions to produce tiny particles with sizes between 0.9 and 2 µm [67]. The results showed that the particle size decreased with decreasing solute concentration and increasing SCCO2 to aqueous solution flow rate ratio. Additionally, CAN-BD was utilized to create dried active powders of biopharmaceuticals, including the antibiotic rifampin and the antiviral zanamivir, while preserving their bioactivities [40]. However, in the CAN-BD process, the mixing effect of SCCO2 with the solution is the key to its successful operation. It is challenging to form an emulsion that reaches thermodynamic equilibrium in a short time with collisional flow mixing and a small throughput because it utilizes a miniature three-way mixer with a small dead volume. In addition, the growth of its industrialization is hampered by the use of capillary atomization devices, which are vulnerable to high pressure drop and blockage, leading to less steady spraying and poor process repeatability.
Nevertheless, these shortcomings of the CAN-BD process could be improved by using a thermostatic saturator instead of a miniature tee, and the proposed SAA process could use a thin-walled nozzle instead of a capillary nozzle [68]. The design of the saturator increases the contact area between the two phases of SCCO2 and the solution, enabling SCCO2 to dissolve more fully in the liquid solution and reach a state close to thermodynamic equilibrium. The SAA technology utilizes the secondary atomization of dissolved CO2. When the liquid stream is ejected from the nozzle and the jet is broken by the expansion of undissolved CO2, the CO2 dissolved in the droplet is rapidly released due to pressure relief, breaking the primary droplet into secondary droplets. This results in very small droplet diameters and a very narrow droplet size distribution, making the drying process easier and yielding particles with a narrow particle size distribution. For instance, the SAA method has been used to handle a number of pharmaceuticals, including triclabenzadol, dexamethasone, and carbamazepine. Additionally, it was feasible to customize particle sizes with varied medicinal administration routes and targets by changing the operational conditions. For instance, Reverchon et al. produced rifampicin and tetracycline particles with a regulated particle size for nebulized medication delivery using the SAA approach [69]. On the basis of the SAA technique, another study refined the original technique by introducing a hydraulic cavitation mixer (HCM) to boost the convective mass transfer between the two phases through the hydraulic cavitation, leading to a faster and better mixing of SCCO2 and solution [68]. This design helps with the handling of thermosensitive materials and lowers the risk of process failure caused by the pre-precipitation of solutes in the mixer. Levofloxacin hydrochloride was successfully micronized by the SAA-HCM approach [70]. As a result, the prepared particle morphology is better spherical and the particle size distribution is more uniform. This method has also been utilized to create lysozyme particles while maintaining lysozyme bioactivity with a controlled particle size distribution in the aerosol delivery range (0.2–5 µm). In short, the main benefit of this approach is the use of water as a single solvent, which eliminates the organic solvent residues. Also, the amount of SCF required is relatively low, the pressure utilized is lower than the pressure needed for RESS, and it can operate continuously with good yields. The mild operating conditions and the ability to control the particle size distribution make it suitable for the preparation of water-soluble drug particles, including proteins and peptides and have industrial potential.
3. SCF technology for biomacromolecular nanomedicine preparation and biomedical applicationThe SCF technology is an efficient approach to preparing biomacromolecular drugs that are difficult to formulate using traditional techniques. The SCF technique can avoid the use of harmful organic solvents and provide an eco-friendly and scalable process. The application of SCF strategy to biomacromolecular drugs has been extensively investigated in recent years. SCF technology has been successfully applied to the preparation of various biomacromolecule-based drugs, including proteins, nucleic acids, and polysaccharides. It has been reported that SCF-based nanosizing strategies can improve the stability, solubility, and bioavailability of these biomacromolecules, as well as their pharmacokinetics and therapeutic efficacy. The scalability, high purity, and mild processing conditions make SCF approach a highly attractive approach for the production of biomacromolecular nanomedicine. In addition, SCF-based nanomedicine has been shown to have potential in various biomedical applications, such as cancer therapy, gene delivery, and tissue engineering.
3.1. Protein nanomedicine produced by SCF technologyProteins and peptides have seen substantial development as therapeutic agents over the past two decades [71-74]. Drugs based on these biomacromolecules, which exhibit a specific mode of action and high potency, are indispensable for maintaining healthy bodily functions and treating numerous diseases. They also have excellent application prospects for diseases with high prevalence, such as malignant tumors, hepatitis, hypertension, diabetes, and acquired immunodeficiency syndrome (AIDS), which pose serious risks to human health. Unlike small-molecule drugs such as antibiotics, proteins have specific amino acid sequences, terminal groups, peptide chain composition and disulfide bond positions, while the maintenance of the higher structure that determines their biological or pharmaceutical activity depends only on weak non-covalent bonding interactions. Therefore, proteins and peptides are susceptible to inactivation by various chemical degradation and physical effects [75].
However, protein-based therapeutics encounter constraints in administration routes and are typically administered via injection, such as intravenous, subcutaneous and intramuscular injections. Such modes of administration make patient compliance poor, and as many protein drugs require repeated administration, drug absorption and duration of action may fluctuate when the injection site is changed [76]. Researchers and pharmaceutical corporations have been looking for non-invasive drug delivery methods due to the insufficiency of injectable drug delivery. Among them, pulmonary administration is the most promising alternative, with studies demonstrating the significantly enhanced bioavailability and stability of protein drugs administered via the lungs [77]. The following elements contribute to the lungs' great ability to absorb protein medicines: (1) A huge absorption area. The 300–400 million alveoli found in adult lungs not only serve as the location of daily gas exchange, but also provide an area of almost 100 m2 for drug absorption [78]. (2) Drug molecules only need to travel 0.2–1.0 µm to enter the circulatory system due to the alveolar region's comparatively thin epithelial cell layer [79]. (3) A rich vascular network and blood supply. Adult lungs weigh less than 1 kg, but all the blood output from the heart passes through the lungs, with a blood flow of up to 5700 mL/min, five times that of the gastrointestinal area [80]. (4) Minimizing the effects of hepatic first-pass elimination [81]. (5) No local adverse reactions or immune responses [82]. Therefore, the lungs provide significant benefits for both local and systemic delivery of protein medications. Several drugs, such as Pulmozyme®, Exubera® and Afrezza® have already hit the market. The key to the pulmonary delivery of protein-based drugs lies in the drug micronization technique, however, conventional micronization techniques have a number of problems. For example, although spray drying technology has the characteristics of more mature development and simple equipment operation, the required temperature is high, and when preparing protein particles, the operating temperature is higher than 100 ℃ [66], which is not ideal for the maintenance of protein structure and activity, such as insulin, growth hormone and other protein aqueous solutions that will be significantly degraded after spray drying [30]. Organic solvents are necessary for emulsion and antisolvent treatments, which have an impact on the protein structure and tend to leave residues, leading to time-consuming and expensive post-treatment procedures.
SCF technology has evolved as a new class of green technology with numerous successful protein micronization applications as a result of the difficulties associated with conventional protein micronization technologies and the requirement for constant innovation. The greatest advantage of RESS is the relative simplicity of the process, but the primary condition is that the substance to be treated has a certain solubility in SCF. In one investigation, cyclomycin particles were prepared for administration as pulmonary aerosols using the RESS technique [83]. Cyclomycin is a cyclic peptide with a composition of an aliphatic peptide chain and a high proportion of amino N atoms methylated, making it highly hydrophobic and able to achieve a solubility of close to 1% (w/w) in SCCO2, which satisfies the requirements for RESS micronization. Since most pharmaceutical proteins are spherical proteins, which are highly hydrophilic and insoluble in SCCO2, this process is mostly used in the polymer coating or chemical linkage of proteins, which requires the preparation of proteins by micronization as a prerequisite. For example, melatonin-loaded liposomes (MLL) were successfully prepared using RESS technology and established an in vitro simulated digestion model to evaluate the release performance of MLL. The MLL prepared as a protein carrier was able to release melatonin in a controlled and slow manner, with better release behavior compared with commercially available melatonin tablets. Moreover, the carrier prepared by the RESS method was able to tackle the issue of organic solvent residues, which to some extent facilitated the breaking of the barrier to commercial conversion of liposomes and eliminated some potential dangers in practical applications [84].
In addition, from the perspectives of greenness, safety and feasibility, subcritical or supercritical water is significantly more advantageous than SCCO2 when used as a solvent to dissolve proteins. Nevertheless, its high critical temperature and critical pressure (647.1 K and 22.1 MPa, respectively) prevent its application to protein drugs with high value, instability, and susceptibility to deactivation. Overall, although RESS is simple, convenient and more thoroughly studied, its application in the micronization of protein-based drugs is greatly constrained.
SCF strategies based on antisolvent and nebulization enhancement have received more research attention in the biopharmaceutical processing sector than methods based on solvent. For example, the GAS technique was used to produce insulin-filled microspheres in the range of 0.5–2 µm as a delivery system for inhalation therapy by encapsulating polylactic acid (PLA) and poly(ethylene glycol) (PEG)/PLA [85]. The composite particles have an insulin loading rate of more than 80%, are capable of slow release, could extend the duration of the release by up to 2 months, and retain the same glucose suppressive activity as the raw material in diabetic mice.
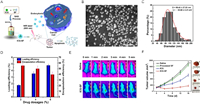
Proteins can either be loaded into nanocarriers [86] or used as drug carriers [87] to improve the water solubility and side effects of hydrophobic drugs. For instance, bovine serum albumin poly(methyl-methacrylate) nanoparticles could be loaded with the hydrophobic anticancer drug camptothecin using the SEDS process [88]. The results showed that the composite nanoparticles obtained had good biocompatibility and efficient cellular uptake, and the preparation process was simple and scalable. The nanoparticles had an enhanced ability to kill cancer cells both in vitro and in vivo in comparison to the free drug. Besides, a stable, highly biocompatible, dual-stimulus-responsive ICG-SF nanoparticle by wrapping ICG with silk fibroin (SF) protein was produced via an antisolvent process (Fig. 1) [44].
|
Download:
|
| Fig. 1. Supercritical fluid-assisted fabrication of ICG encapsulated SF nanoparticles for dual-triggered cancer therapy. (A) Schematic illustration showing the outline of preparation of ICG-SF nanoparticles by the SAS process. (B) SEM images of ICG-SF nanoparticles. (C) Statistical analysis illustrating the average particle diameter of ICG-SF nanoparticles (n = 200) based on the SEM images and surface charge of the ICG-SF nanoparticles determined by zeta potential measurements. (D) The loading and encapsulation efficiency of ICG in SF nanoparticles. (E) Infrared thermographic maps in the MCF7 breast tumor-bearing nude mice irradiated by the 808 nm laser. (F) (i) Graphical representation of tumor volume with respect to time in days after treatment of ICG-SF nanoparticles and corresponding pictures of excised tumors of mice treated with samples for 16 days, (ii) saline, (iii) SF nanoparticles, (iv) ICG, and (v) ICG-SF nanoparticles. Copied with permission [44]. Copyright 2018, American Chemical Society. | |
The method was able to better maintain the biological activity of SF, which is a drug carrier, completely shield ICG from plasma proteins and regulate the clearance rate of ICG under physiological circumstances. The response of SF to the acidic microenvironment of tumors led to a conformational shift that facilitated the release of ICG and improved the efficacy of photothermal therapy for malignancies. These techniques may make it possible to deliver biomacromolecules while exerting tight control over their biological activity and interaction with various targets, such as the vascular endothelium, cancer cells, or the nucleus, which enhances targeting, cell internalization, circulation, and off-target effects [74,89].
Additionally, SCF technology could successfully construct biomacromolecule-modified scaffolds for tissue engineering through the supercritical fluid-polymer interaction. For instance, research has been done to prepare SF nanoparticles using the SEDS process, which were subsequently added to poly-L-lactide (PLLA), and the SF/PLLA composite scaffolds were prepared by the phase-inversion technique in a SCCO2 environment [90]. SF nanoparticles increased the surface strength and hydrophilicity of the PLLA scaffolds, leading to a high affinity for albumin attachment. In vitro cytotoxicity assays of SF/PLLA composite scaffolds on L929 mouse fibroblasts showed good biocompatibility, a better ability to induce osteoblast-specific marker levels, and more bone matrix production, which can manipulate cellular behavior during tissue regeneration and effectively promote osteogenic development and tissue differentiation.
Although the SAS technology is widely used for the micronization of proteins and peptides, its biggest drawbacks are the risk of organic solvent residues and protein structure destruction, and it complicates the phase behavior with a relatively small throughput. Therefore, in order to overcome the limitations of SAS technology, new SCF micronization techniques must be created that can systematically study the production of protein-based water-soluble drug particles or drug-loaded composite particles using pure water as a single solvent.
The CAN-BD method is a significant development in the field of SCF micronization technology since it uses continuous operation to create ultrafine particles from aqueous solutions. The particle morphology and size distribution are well controlled, and there is reduced protein aggregation and biological activity loss. Hepatitis B surface antigen (HBsAg) protein vaccines and live attenuated measles vaccines in the form of dry powder formulations were prepared using the CAN-BD process at near ambient temperature conditions. The activity of HBsAg was adequately preserved when the formulations contained sufficient amounts of alginate. The powder can be stored at −20 ℃ or 66 ℃ for 43 days without loss of activity [40,91]. It is anticipated that the fine powder formulation of the vaccine obtained using this technique will address the issue of its loss of potency during transport. Also, more thorough studies were carried out on three proteins, two of which have therapeutic value: trypsinogen (a model enzyme), anti-CD4 antibodies (for rheumatoid arthritis), α1-antitrypsin (for cystic fibrosis and emphysema). The stability and activity of their dry powders are well maintained to meet inhalation requirements and reach the deep lungs [92].
The improved SAA and SAA-HCM processes based on CAN-BD technology have enhanced the mass transfer process between SCCO2 and solution, resulting in increased throughput and good mixing of SCCO2 and solution in a fairly short time, reducing the possibility of process failure due to pre-precipitation of solutes in the mixer, and also facilitating the operation of protein-like thermosensitive substances to avoid changes in structure and activity.
The feasibility of preparing biomacromolecular particles such as proteins by the SAA-HCM process at different levels from model proteins (lysozyme) [41], pharmaceutical proteins (insulin) [93], and polymer/pharmaceutical protein composite systems (insulin/chitosan composite particles) was verified [94]. The results showed that the chemical structure and advanced structure of the obtained protein particles did not change significantly compared with the original drug, and the particles could be stored for a long time under low temperature and low humidity environments. In contrast to conventional spray drying technology, the thermal stability of the products prepared by the SAA-HCM process was essentially the same as that of the original drug, but the activity of the spray-dried samples decreased, and the control of particle size distribution was not as good as that of SAA-HCM technology. The SAA technique has high application possibilities since it can compensate for the drawbacks of conventional micronization procedures and is suited for the manufacture of heat-sensitive and water-soluble compounds, including proteins and peptides.
Overall, as a new class of green technologies, SCF technology is becoming more and more significant in the field of pharmaceutical particle manufacturing. It is a viable alternative to the present processes for producing stable pharmaceutical protein powders because it enables the quick synthesis of large amounts of protein powder with controlled particle morphology.
3.2. Nucleic acids nanomedicine produced by SCF technologyNucleic acids have great potential for the treatment of lung-related diseases, including cystic fibrosis, lung cancer, chronic obstructive pulmonary disease, asthma, and respiratory infections [95,96]. Plasmid DNA (pDNA) and small interfering RNA (siRNA) are two of the most widely studied nucleic acids for therapeutic development. The most direct and effective method of delivering therapeutic nucleic acids to the lungs is local delivery via inhalation [97]. Compared to intravenous administration, inhalation delivery is non-invasive and more patient-acceptable. In order to achieve intrapulmonary delivery of nucleic acids, an effective inhaled formulation must be able to circumvent the clearance and defense mechanisms of the respiratory system, achieve high lung deposition, and prevent early degradation. In inhaled formulation development, nucleic acids can be formulated in liquid or dry powder. The inability to maintain the structural integrity of nucleic acids in solution, which presents a significant challenge for their transport and storage, has encouraged researchers to select dry powder formulations [98].
SCF technology offers another possibility for producing dry nucleic acid formulations suitable for inhalation. Compared to traditional freeze-drying technology, SCF technology produces dry nucleic acid powders with greater stability, controlled particle size, shorter reaction times, reduced transportation and storage costs, and without the loss of nucleic acid activity associated with freezing and heating [99-102]. In 2000, the SEDS technique was applied to produce pDNA dry powder from aqueous solutions using mannitol as an excipient for the first time and studied the effect of parameters on plasmid stability [103]. The results showed that when the aqueous solution and SCCO2 converged at the nozzle, an acidic solution was inevitably generated, which affected the stability of the plasmid. The addition of sodium acetate was able to counteract this effect and restore the DNA supercoil structure. This endeavor demonstrated that it is feasible to prepare dry nucleic acid powder using SCF technology, and applications for DNA administration by inhalation or even transdermal distribution are possible. The researchers then conducted additional research, including the development of an imaging system to monitor the stability and expression of nucleic acid dry powder prepared by the SCF technique in vivo as well as cancer therapy. Besides, inhalable chitosan-plasmid DNA complex powders were prepared using the SAS method [104]. In the study, chitosan was used as a non-viral delivery vehicle, and mannitol was used as a swelling agent. An aqueous solution of a luciferase expression plasmid driven by a cytomegalovirus promoter (pCMV-Luc) was dispersed in the SCCO2/ethanol mixture to produce the dry powder. Chitosan not only inhibited the degradation of pCMV-Luc and maintained the stability of p-DNA during production and storage, but also increased the yield of the powder. In animal experiments, the gene expression mediated by powder through lung administration was verified, and the pDNA dry powder was able to increase the expression of luciferase activity with a longer duration of action than the corresponding solution form. Meanwhile, a mouse model of lung metastasis has been used by the researchers to successfully demonstrate the therapeutic efficacy of pDNA powder formulations containing the interferon-β gene [105]. Furthermore, they developed a new way of gene drug delivery, demonstrating that the gene powder prepared using SCF technology is expected to treat lung-related diseases. But the study was limited by a lack of gas kinetic data for the powder. In addition, the group has applied the SCF technique to the preparation of siRNA powder, which can effectively and specifically exert gene silencing effects on metastatic tumor cells in mouse lungs and has potential for clinical application [106]. Ion gelation and SAS techniques were used to effectively prepare nanocomposite particles containing siRNA and paclitaxel for overcoming drug resistance in cancer therapy [107]. The composite particles showed a better tumor suppression effect compared to nanoparticles encapsulated with paclitaxel alone. In another study, they prepared PLLA porous particles and loaded them with DOX and siRNA-CS nanoparticles [108]. The composites exhibited excellent aerodynamic properties and were suitable for inhalation co-delivery, which can overcome drug resistance in lung cancer. The two studies mentioned above have developed gene and drug co-delivery systems based on SCF technology.
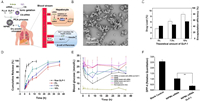
What is more, the SCF technology that produces siRNA nanoparticles has been expanded to treat the chronic condition of diabetes in addition to cancer treatment [109]. As illustrated in Fig. 2, SCCO2-assisted precipitation with a compressed anti-solvent technique decorated the siRNA-incorporated CS nanoparticles over glucagon-like peptide (GLP)−1-dispersed PLLA porous microparticles (PMs) as an inhalation delivery system for diabetes therapy. This study verified that SCF technology provides a convenient way to fabricate nanoparticle-microparticle composites for simultaneous delivery of a gene and a therapeutic peptide, which has the potential to be widely used in the pharmaceutics industry.
|
Download:
|
| Fig. 2. Supercritical fluid-assisted decoration of nanoparticles on porous microcontainers for co-delivery of therapeutics and inhalation therapy of diabetes. (A) Schematic diagram of siRNA nanoparticles-inlaid PMs (NIPMs) for diabetes treatment. (B) SEM images of NIPMs. (C) GLP-1 loading and encapsulation efficiencies in NIPMs at various theoretical loading amounts. (D) Cumulative GLP-1 release characteristics of NIPMs in phosphate buffered saline (PBS) (pH 7.4). (E) Hypoglycemic efficacy of various combinations of NIPMs after administrating through pulmonary route in Type 2 diabetic mice for 36 h. (F) The effect of various combinations of NIPMs on the expression of DPP-4-mRNA. **P < 0.01. Copied with permission [109]. Copyright 2018, American Chemical Society. | |
The application of SCF technology to the development of gene drugs has large application potential. Nucleic acid stability is guaranteed by the mild operating conditions, drying at room temperature, and high recovery (typically > 90%) with minimal losses, making them suitable for the production of pricey gene drugs like pDNA and siRNA. However, SCF technology for gene drug production is still in the development stage, and there are significant intergroup differences in experimental results, reducing comparability. Besides, research on siRNA is limited, and the majority of studies are based on gene powders for in vivo imaging to verify transfection effects, with few studies for cancer therapy. Therefore, it is necessary to investigate the SCF process variables and the aerodynamic properties of the powder to confirm their suitability for inhalation and to raise the clinical value of SCF-produced gene powders.
3.3. Polysaccharides nanomedicine produced by SCF technologyPolysaccharides are macromolecular polymers widely found in nature, and the main forms of their existence include cellulose and its derivatives, alginate, agarose, chitosan, etc. Polysaccharide polymers have the advantages of high structural stability, low toxicity, biocompatibility, and biodegradability [110], and can be used for a variety of functionalized applications in vivo, including body components [111-113], energy storage [114,115], drug delivery [116,117], and regulation of protein structure and function [118].
Among many polysaccharides, chitosan is the most commonly used for the preparation of polysaccharide-related drugs by SCF technology because of its many useful properties, such as biocompatibility [119], biodegradability, low toxicity [120], cellular affinity [121], antimicrobial [122], antioxidant activity [123], and low production cost. The RESS method is an appropriate, novel, and promising methodology for the manufacturing of polysaccharide-based medications due to its small particle products, narrow particle size distribution, solvent-free products, and customizable particle size [124]. The RESS method was also used to micronize chitosan particles and evaluate the effects of process parameters such as temperature, pressure, and the effective nozzle diameter on chitosan particle size using the response surface methodology (RSM) to control the size and particle size distribution of chitosan particles. The results showed that the size distribution of RESS-treated particles was narrower and significantly smaller compared to the original chitosan particles, which had an irregular morphology. With the increase in pressure and temperature and the decrease in nozzle diameter, the chitosan diameter decreased as well [125]. The SAA-HCM technique was utilized to create chitosan nanoparticles, extending the technique's applicability to the production of nanoparticles from nanosuspensions and offering more examples for the formation of finely structured particles [126]. However, research on the effects of process parameters on the size and morphology of polysaccharide particles is more prevalent than research on the application of single-component polysaccharide particles. In fact, due to polysaccharides' positive charge, they are able to interact electrostatically with negatively charged mucosal surfaces to promote transmucosal absorption of drugs and are therefore widely used as drug carriers for anticancer drugs and vaccines [127-129]. Utilizing the SAS drying approach, a sodium alginate-chitosan composite aerogel was established for controlled-release drug delivery. Levomycin was used as a model antibiotic, and the products with porous architectures, large surface areas, high water absorption, and structural stability were able to postpone the release kinetics. It has the ability to not only adsorb medications but also be used to treat wound surfaces and speed up healing [130]. Another study reported DOX-loaded chitosan nanoparticles in one step by the SAA-HCM process [131]. As a drug carrier, chitosan maintains the structural integrity and thermal stability of the drug. In vitro tumor cytotoxicity experiments showed that the drug activity was well maintained. This work validated the use of SAA-HCM technology in drug delivery systems and the efficacy of chitosan as a drug carrier, serving as a benchmark for the development of new synergistic drug delivery systems that are both effective and environmentally friendly.
In addition, polysaccharides can stabilize biomacromolecules such as proteins, peptides, and nucleic acids to create oral and inhaled formulations. Using alginate as an excipient, lysozyme protein powder was constructed by the SAS process and investigated the impact of the protein-to-polysaccharide ratio on the stability of lysozyme [132]. The outcomes demonstrated that the inclusion of alginate was effective in preserving the long-term stability of lysozyme. When the protein: alginate ratio was 1:1, lysozyme exhibited agglomeration, and the polysaccharide content in the formulation was insufficient to inhibit protein interactions. However, when the protein: alginate ratio was 1:4, no structural alterations occurred in lysozyme. An alternate method for enhancing the stability of protein powders made by SCF technology is to optimize the protein-to-polysaccharide ratio. The ideal protein-to-polysaccharide ratio prevents the polysaccharide matrix from crystallizing as well as structural changes and aggregation of the encapsulated protein.
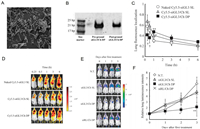
Moreover, polysaccharides may be more effective than viral vectors in gene delivery because they induce lower immune responses and reduce the degree of inflammation. An inhaled siRNA powder containing chitosan and mannitol was prepared by the SAS technique for effective pulmonary gene silencing (Fig. 3) [106]. When chitosan is not included, the acidic microenvironment generated by dissolving SCCO2 into an aqueous solution leads to poor stability of siRNA. When chitosan was added, it was protonated in acidic circumstances to interact electrostatically with the negatively charged siRNA, thereby maintaining its structural integrity and stability [133]. Chitosan was also used as a cationic carrier and it was found that chitosan exerted the best stabilization when it had a molecular weight of 2–5 kDa and an N/P ratio of 5 [134]. The content of nucleic acid and chitosan affects the dispersibility of nucleic acid particles [106]. When powders were prepared using 2% siRNA and 10% chitosan (total mass), the products were rectangular in shape and poorly dispersed. When the powders were prepared using 0.2% pDNA and 0.94% chitosan (total mass), the dispersion was good [104].
|
Download:
|
| Fig. 3. Gene silencing in a mouse lung metastasis model by an inhalable dry siRNA powder prepared using the SAS technique. (A) SEM of the siRNA/Chitosan powders. (B) Integrity of siRNA in the siRNA/Chitosan powders. (C) Time course of lung fluorescence localization derived from Cy5.5 following pulmonary delivery into mice. (D) Optical image of the fluorescence derived from Cy5.5 in mice following pulmonary delivery. (E) Optical image of lung luminescence corresponding to firefly luciferase activity in mice bearing colon26/Luc cells. (F) Time course of lung luminescence intensity. Copied with permission [106]. Copyright 2013, The Pharmaceutical Society of Japan. | |
In conclusion, polysaccharides like chitosan primarily serve as stabilizers and excipients in the field of SCF technology [42], and the buffer function and complexes created will significantly aid in the stable long-term preservation of protein powder and gene powder.
4. Challenges in SCF technology prepared biomacromolecular nanomedicineDespite the many advantages of SCF technology for biomacromolecular nanomedicine preparation and biomedical application, there are also several challenges that need to be addressed. One major challenge is the optimization of process parameters, including pressure, temperature, and co-solvent content, to achieve optimal nanoparticle size and morphology with high drug loading efficiency. The process parameters can significantly affect the properties of the resulting biomacromolecule-based nanoparticles, including drug encapsulation efficiency, drug release kinetics, and particle stability. Therefore, it is necessary to conduct extensive studies to determine the optimal processing conditions for different types of biomacromolecules and drugs.
The lack of a comprehensive understanding of the mechanisms involved in SCF processing also limits the development of biomacromolecular nanomedicine. Although many studies have reported the successful preparation of biomacromolecular nanoparticles using SCF technology, the mechanisms of particle formation and the effects of different processing parameters on particle size, morphology, and stability are still not fully understood. Further research is needed to elucidate the underlying mechanisms and optimize the processing conditions to achieve consistent and reproducible results.
Another challenge is the limited scalability of SCF technology for industrial production. Currently, most SCF-based methods are limited to laboratory-scale production due to the high cost and complexity of the equipment required. To enable large-scale production of biomacromolecular nanoparticles using SCF technology, new processing methods and equipment that are cost-effective and scalable need to be developed.
In addition, the application of SCF technology for the preparation of biomacromolecular nanoparticles is still in its early stages, and more research is needed to fully evaluate the safety and efficacy of these particles in vivo. Although SCF technology has been shown to be effective in maintaining the stability and bioactivity of biomacromolecules, further studies are needed to evaluate their biocompatibility, pharmacokinetics, and long-term safety in animal models and human clinical trials.
Finally, the regulatory approval process for SCF-produced biomacromolecular nanoparticles may be a challenge due to the lack of established guidelines and standards for their manufacture and quality control. It is important to establish clear guidelines and standards to ensure the safety and efficacy of these particles and facilitate their regulatory approval for clinical use.
Overall, while SCF technology holds great promise for the preparation of biomacromolecular nanoparticles for biomedical applications, further research is needed to overcome the challenges and optimize the technology for large-scale production and clinical use.
5. Conclusions and future perspectivesThe synthesis of biomacromolecular pharmaceuticals using SCF technology is reviewed in this study, along with a brief summary of some noteworthy scientific advancements. We summarized several examples of SCF-processed biomacromolecules improving their performance efficacy and outcomes in recent years (Table S1 in Supporting information).
By understanding the nature of SCF and the mechanism of biomacromolecular drug particle formation in SCF, many methods applicable to biomacromolecular nanosizing can be generated. In comparison to currently approved techniques, SCF technology for the production of biomacromolecular drugs has the following benefits: the ability to form fine, smooth particles with a controlled nanometer or micron size range and a narrow size distribution. Furthermore, SCF technology allows for the treatment of delicate biomacromolecules without deterioration in a single step at room temperature, without the need for chemical modification. The absence of organic solvent residues and the ability to design composite particles using approved polymers contribute to the environmentally friendly and energy-efficient nature of SCF technology. Moreover, the SCF method is a desirable alternative for the manufacture of biomacromolecular pharmaceuticals because of its uniform batch size, high yield, long-term stability at room temperature, simplicity of transportation, and preservation. Additionally, water content has a substantial impact on the biomacromolecules' thermal stability and their ability to retain their activity [135]. Sensitive biomacromolecules like proteins and nucleic acids are significantly stabilized by the removal of water to create dry powders since many of the degradation mechanisms affecting biomacromolecules are predominantly mediated by water. Compared to conventional dry powder preparation techniques, the utilization of SCF technology allows for rapid solvent/water removal at much lower temperatures with minimal stress, which not only maintains the stability of the biomacromolecules but also permits relative control over the properties of the final product (e.g., particle size, porosity, morphology, water content), where the residual moisture of biomacromolecule drugs produced using SCF technology is in the range of 5%–10% [136], which meets the requirements for long-term transportation and storage. Lastly, the scalability of SCF technology for cGMP production offers an exciting opportunity for the industrial production of biomacromolecular drugs.
After years of development, SCF technology is currently transitioning from small-scale manufacturing to large-scale production. Not only is this a chance, but it is also a challenge. Large-scale industrial production will provide a strong driving force and a broad market prospect for the in-depth development and application of SCF technology. At the same time, more challenging topics are on the agenda, such as whether laboratory experience can be transferred to large-scale production before it can be scaled up. Aseptic pharmaceutical processes, greater yields and lower energy consumption, recovery of CO2 and solvents, compliance with operating parameters, consistency of product quality, and other issues are the key obstacles in this situation. These issues need to be studied in depth and addressed one by one through production practice.
In conclusion, the SCF process can offer a powerful platform for the creation of biomacromolecular particles. These particles can be used to create new biomacromolecule-based delivery systems as well as directly prepare biomacromolecules as therapeutic drugs while maintaining their stability and activity at the nanoscale. The entire process also adheres to sustainable and green standards. The SCF procedure is anticipated to develop into a cutting-edge platform for drug delivery.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Major State Basic Research Development Program of China (Nos. 2023YFB3810000 and 2018YFA0107301), the National Natural Science Foundation of China (NSFC) (Nos. U22A20333, 81925019, U1705281, and 82202330), the Fundamental Research Funds for the Central Universities (Nos. 20720190088 and 20720200019), the Science Foundation of Fujian Province (No. 2020Y4003), and the Program for New Century Excellent Talents in University, China (No. NCET-13-0502), Shenzhen Science and Technology Program (No. JCYJ20220530143213029), China Postdoctoral Science Foundation (No. 2023T160383).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109169.
| [1] |
Y. Tian, M.V. Tirrell, J.L. LaBelle, Adv. Healthc. Mater. 11 (2022) e2102600. DOI:10.1002/adhm.202102600 |
| [2] |
M.S. Kinch, Drug Discov. Today 20 (2015) 393-398. DOI:10.1016/j.drudis.2014.09.003 |
| [3] |
T. Peng, Z. Deng, J. He, et al., Coord. Chem. Rev. 403 (2020) 213080. DOI:10.1016/j.ccr.2019.213080 |
| [4] |
Z. Guo, B. Jin, Y. Fang, et al., Chin. Chem. Lett. 35 (2024) 108528. DOI:10.1016/j.cclet.2023.108528 |
| [5] |
D. Xiao, T. Chen, T. Zhang, et al., Chin. Chem. Lett. 35 (2024) 108602. DOI:10.1016/j.cclet.2023.108602 |
| [6] |
K.J.D.A. Excoffon, FEBS Lett. 594 (2020) 1828-1837. DOI:10.1002/1873-3468.13794 |
| [7] |
J. Zhao, G. Hu, Y. Huang, et al., Chin. Chem. Lett. 32 (2021) 1331-1340. DOI:10.1016/j.cclet.2020.10.013 |
| [8] |
S. Nowsheen, K. Aziz, J.Y. Park, et al., J. Clin. Oncol. 35 (2017) e12522. DOI:10.1200/JCO.2017.35.15_suppl.e12522 |
| [9] |
M. Maemondo, T. Fukuhara, H. Saito, et al., J. Clin. Oncol. 38 (2020) 9506. DOI:10.1200/JCO.2020.38.15_suppl.9506 |
| [10] |
J. Mooi, F. Chionh, P. Savas, et al., Clin. Cancer Res. 27 (2021) 2159-2167. DOI:10.1158/1078-0432.CCR-20-2714 |
| [11] |
B.I. Rini, R.J. Motzer, T. Powles, et al., Eur. Urol. 79 (2021) 659-662. DOI:10.1016/j.eururo.2020.06.021 |
| [12] |
H. Zhu, J. Yu, J. Ye, et al., Chin. Chem. Lett. 34 (2023) 107648. DOI:10.1016/j.cclet.2022.06.071 |
| [13] |
P.Y. Yang, H. Zou, C. Lee, et al., J. Med. Chem. 61 (2018) 3218-3223. DOI:10.1021/acs.jmedchem.7b00768 |
| [14] |
M. Muttenthaler, G.F. King, D.J. Adams, et al., Nat. Rev. Drug Discov. 20 (2021) 309-325. DOI:10.1038/s41573-020-00135-8 |
| [15] |
A. Gupta, M. Kowalczuk, W. Heaselgrave, et al., Eur. Polym. J. 111 (2019) 134-151. DOI:10.1016/j.eurpolymj.2018.12.019 |
| [16] |
L. Liu, S. Nie, M. Xie, Critical Rev. Food Sci. Nutrit. 56 (2015) S85-S94. |
| [17] |
Y. Zhang, Q. Li, J. Wang, et al., Cancer Lett. 377 (2016) 117-125. DOI:10.1016/j.canlet.2016.04.037 |
| [18] |
C.S. Chiang, Y.J. Lin, R. Lee, et al., Nat. Nanotechnol. 13 (2018) 746-754. DOI:10.1038/s41565-018-0146-7 |
| [19] |
H. Yu, Y. Yang, T. Jiang, et al., ACS Appl. Mater. Interfaces 11 (2019) 27536-27547. DOI:10.1021/acsami.9b07804 |
| [20] |
S. Mitragotri, P.A. Burke, R. Langer, Nat. Rev. Drug Discov. 13 (2014) 655-672. DOI:10.1038/nrd4363 |
| [21] |
L. Padrela, M.A. Rodrigues, A. Duarte, et al., Adv. Drug Deliv. Rev. 131 (2018) 22-78. DOI:10.1016/j.addr.2018.07.010 |
| [22] |
W. He, X. Xing, X. Wang, et al., Adv. Funct. Mater. 30 (2020) 1910566. DOI:10.1002/adfm.201910566 |
| [23] |
B. Liu, F. Jiang, J. Sun, et al., J. Mater. Chem. B 9 (2021) 7007-7022. DOI:10.1039/D1TB00725D |
| [24] |
M. Maeki, N. Kimura, Y. Sato, et al., Adv. Drug Deliv. Rev. 128 (2018) 84-100. DOI:10.1016/j.addr.2018.03.008 |
| [25] |
M. Sun, H. Hu, L. Sun, et al., Chin. Chem. Lett. 31 (2020) 1729-1736. DOI:10.1016/j.cclet.2020.02.035 |
| [26] |
Z.M. Wu, L. Zhou, X.D. Guo, et al., Int. J. Pharm. 425 (2012) 1-8. DOI:10.1016/j.ijpharm.2011.12.055 |
| [27] |
S. Yu, X. Xu, J. Feng, et al., Int. J. Pharm. 560 (2019) 282-293. DOI:10.1016/j.ijpharm.2019.02.012 |
| [28] |
M. Zhang, Z. Dai, S. Theivendran, et al., Nano Today 36 (2021) 101035. DOI:10.1016/j.nantod.2020.101035 |
| [29] |
K. Byrappa, S. Ohara, T. Adschiri, Adv. Drug Deliv. Rev. 60 (2008) 299-327. DOI:10.1016/j.addr.2007.09.001 |
| [30] |
H. Okamoto, K. Danjo, Adv. Drug Deliv. Rev. 60 (2008) 433-446. DOI:10.1016/j.addr.2007.02.002 |
| [31] |
W.C. Tsai, Y. Wang, Prog. Polym. Sci. 98 (2019) 101161. DOI:10.1016/j.progpolymsci.2019.101161 |
| [32] |
J. Jung, M. Perrut, J. Supercrit. Fluids 20 (2001) 179-219. DOI:10.1016/S0896-8446(01)00064-X |
| [33] |
H. Chen, H. Cheng, Q. Dai, et al., J. Control. Relea. 323 (2020) 635-643. DOI:10.1016/j.jconrel.2020.04.021 |
| [34] |
P. He, E. Ren, B. Chen, et al., Theranostics 12 (2022) 1769-1782. DOI:10.7150/thno.68456 |
| [35] |
Y. Zhang, H. Cheng, H. Chen, et al., Eur. J. Nucl. Med. Mol. Imag. 49 (2022) 2605-2617. DOI:10.1007/s00259-021-05654-z |
| [36] |
P. He, Y. Xiong, J. Ye, et al., J. Nanobiotechnol. 20 (2022) 250. DOI:10.1186/s12951-022-01467-w |
| [37] |
Y. Peng, P. He, X. Gao, et al., Front. Bioeng. Biotechnol. 10 (2022) 952194. DOI:10.3389/fbioe.2022.952194 |
| [38] |
X. Shi, D. Xu, H. Cheng, et al., Acc. Mater. Res. 4 (2023) 251-263. DOI:10.1021/accountsmr.2c00195 |
| [39] |
R.K. Kankala, Y.S. Zhang, S.B. Wang, et al., Adv. Healthc. Mater. 6 (2017) 1700433. DOI:10.1002/adhm.201700433 |
| [40] |
R.E. Sievers, B.P. Quinn, S.P. Cape, et al., J. Supercrit. Fluid. 42 (2007) 385-391. DOI:10.1016/j.supflu.2007.03.001 |
| [41] |
Z. Du, Y.X. Guan, S.J. Yao, et al., Int. J. Pharm. 421 (2011) 258-268. DOI:10.1016/j.ijpharm.2011.10.002 |
| [42] |
E. Reverchon, A. Antonacci, Ind. Eng. Chem. Res. 45 (2006) 5722-5728. DOI:10.1021/ie060233k |
| [43] |
P.Y. Xu, R.K. Kankala, Y.W. Li, et al., Regen. Biomater. 9 (2022) rbac072. DOI:10.1093/rb/rbac072 |
| [44] |
B.Q. Chen, R.K. Kankala, G.Y. He, et al., ACS Biomater. Sci. Eng. 4 (2018) 3487-3497. DOI:10.1021/acsbiomaterials.8b00705 |
| [45] |
M. Karimi, H. Kamali, M. Mohammadi, et al., J. Drug Deliv. Sci. Technol. 69 (2022) 103186. DOI:10.1016/j.jddst.2022.103186 |
| [46] |
A.S. Zarena, K.U. Sankar, Ther. Deliv. 2 (2011) 259-277. DOI:10.4155/tde.10.82 |
| [47] |
M.M. Duarte, I.V. Silva, A.R. Eisenhut, et al., Mater. Horiz. 9 (2022) 864-891. DOI:10.1039/D1MH01720A |
| [48] |
S. Bai, Y. Zhang, D. Li, et al., Nano Today 36 (2021) 101038. DOI:10.1016/j.nantod.2020.101038 |
| [49] |
M. Cocero, A. Martin, Recent Patent. Eng. 2 (2008) 9-20. DOI:10.2174/187221208783478561 |
| [50] |
I. Pasquali, R. Bettini, F. Giordano, Adv. Drug Deliv. Rev. 60 (2008) 399-410. DOI:10.1016/j.addr.2007.08.030 |
| [51] |
P.G. Debenedetti, J.W. Tom, X. Kwauk, et al., Fluid Phase Equilib. 82 (1993) 311-321. DOI:10.1016/0378-3812(93)87155-T |
| [52] |
A. Montes, R. Merino, D.M. De los Santos, et al., J.CO2 Utilizat. 21 (2017) 169-176. DOI:10.1016/j.jcou.2017.07.009 |
| [53] |
A. Jash, A. Krueger, S.S.H. Rizvi, Green Chem. 24 (2022) 5326-5337. DOI:10.1039/D2GC00877G |
| [54] |
S.D. Yeo, G.B. Lim, P.G. Debendetti, et al., Biotech. Bioeng. 41 (1993) 341-346. DOI:10.1002/bit.260410308 |
| [55] |
P.M. Gallagher, M.P. Coffey, V.J. Krukonis, et al., J. Supercrit. Fluid. 5 (1992) 130-142. DOI:10.1016/0896-8446(92)90030-N |
| [56] |
Y. Kang, G. Yin, P. Ouyang, et al., J. Colloid Interface Sci. 322 (2008) 87-94. DOI:10.1016/j.jcis.2008.02.031 |
| [57] |
G. Liu, Q. Lin, Y. Huang, et al., J. CO2 Utilizat. 20 (2017) 43-51. DOI:10.1016/j.jcou.2017.04.015 |
| [58] |
L.Y. Lee, C.H. Wang, K.A. Smith, J. Control. Relea. 125 (2008) 96-106. DOI:10.1016/j.jconrel.2007.10.002 |
| [59] |
O. Nuchuchua, M.R. Nejadnik, S.C. Goulooze, et al., J. Supercrit. Fluid. 128 (2017) 244-262. DOI:10.1016/j.supflu.2017.06.002 |
| [60] |
A.R.C. Duarte, J.F. Mano, R.L. Reis, Int. Mater. Rev. 54 (2013) 214-222. |
| [61] |
W.Z. He, Q.L. Suo, Z.H. Jiang, et al., J. Supercrit. Fluid. 31 (2004) 101-110. DOI:10.1016/j.supflu.2004.01.009 |
| [62] |
K. Kaga, M. Honda, T. Adachi, et al., J. Supercrit. Fluid. 136 (2018) 44-51. DOI:10.1016/j.supflu.2018.02.008 |
| [63] |
B. Xue, J. Huang, H. Zhang, et al., Artif. Cells Nanomed. Biotechnol. 48 (2020) 1135-1143. DOI:10.1080/21691401.2020.1815755 |
| [64] |
R.E. Sievers, Aerosol Sci. Technol. 30 (1999) 3-15. |
| [65] |
R.E. Sievers, P.D. Milewski, S.P. Sellers, et al., Ind. Eng. Chem. Res. 39 (2000) 4831-4836. DOI:10.1021/ie000190m |
| [66] |
R.E. Sievers, E.T.S. Huang, J.A. Villa, et al., Pure Appl. Chem. 73 (2001) 1299-1303. DOI:10.1351/pac200173081299 |
| [67] |
E.T.S. Huang, H. Chang, C.D. Liang, R.E. Sievers, Fine particle pharmaceutical manufacturing using dense carbon dioxide mixed with aqueous or alcoholic solutions, in: A.S. Gopalan, C.M. Wai, H.K. Jacobs (Eds. ), Supercritical Carbon Dioxide: Separations and Processes, American Chemical Society, Washington D. C, 2003, pp. 324–338.
|
| [68] |
E. Reverchon, Ind. Eng. Chem. Res. 41 (2002) 2405-2411. DOI:10.1021/ie010943k |
| [69] |
E. Reverchon, G.Della Porta, J. Supercrit. Fluid. 26 (2003) 243-252. DOI:10.1016/S0896-8446(02)00162-6 |
| [70] |
M.Q. Cai, Y.X. Guan, S.J. Yao, et al., J. Supercrit. Fluid. 43 (2008) 524-534. DOI:10.1016/j.supflu.2007.07.008 |
| [71] |
M. van de Weert, L. Jorgensen, E.Horn Moeller, et al., Expert Opin. Drug Deliv. 2 (2005) 1029-1037. DOI:10.1517/17425247.2.6.1029 |
| [72] |
A. Varanko, S. Saha, A. Chilkoti, Adv. Drug Deliv. Rev. 156 (2020) 133-187. DOI:10.1016/j.addr.2020.08.008 |
| [73] |
L. Wang, N. Wang, W. Zhang, et al., Signal Transduct. Target. Ther. 7 (2022) 48. DOI:10.1038/s41392-022-00904-4 |
| [74] |
J. Wu, J.K. Sahoo, Y. Li, et al., J. Control. Relea. 345 (2022) 176-189. DOI:10.1016/j.jconrel.2022.02.011 |
| [75] |
R. Langer, Science 249 (1990) 1527-1533. DOI:10.1126/science.2218494 |
| [76] |
D.R. Owens, B. Zinman, G. Bolli, Diabet. Med. 20 (2003) 886-898. DOI:10.1046/j.1464-5491.2003.01076.x |
| [77] |
A. Hou, L. Li, Y. Huang, et al., Drug Deliv. Transl. Res. 8 (2018) 693-701. DOI:10.1007/s13346-018-0515-7 |
| [78] |
J.S. Patton, P.R. Byron, Nat. Rev. Drug Discov. 6 (2007) 67-74. DOI:10.1038/nrd2153 |
| [79] |
J.S. Patton, Proc. Am. Thorac. Soc. 1 (2004) 338-344. DOI:10.1513/pats.200409-049TA |
| [80] |
N. Benowitz, R.P. Forsyth, K.L. Melmon, et al., Clin. Pharm. Ther. 16 (1974) 87-98. DOI:10.1002/cpt1974161part187 |
| [81] |
A. Singh, R. Malviya, P.K. Sharma, Curr. Drug Ther. 6 (2011) 137-151. DOI:10.2174/157488511795304930 |
| [82] |
R.K. Wolff, J. Aerosol. Med. 11 (1998) 197-219. DOI:10.1089/jam.1998.11.197 |
| [83] |
A. Tandya, F. Dehghani, N.R. Foster, J. Supercrit. Fluid. 37 (2006) 272-278. DOI:10.1016/j.supflu.2005.10.004 |
| [84] |
Q. Zhang, C. Ou, S. Ye, et al., J. Microencapsul. 34 (2017) 687-698. DOI:10.1080/02652048.2017.1376001 |
| [85] |
P. Caliceti, S. Salmaso, N. Elvassore, et al., J. Control. Relea. 94 (2004) 195-205. DOI:10.1016/j.jconrel.2003.10.015 |
| [86] |
C.W. Shields, L.L.W. Wang, M.A. Evans, et al., Adv. Mater. 32 (2019) 1901633. |
| [87] |
D.J. Irvine, E.L. Dane, Nat. Rev. Immunol. 20 (2020) 321-334. DOI:10.1038/s41577-019-0269-6 |
| [88] |
M. Hua, X. Hua, Nano Micro Lett. 6 (2013) 20-23. |
| [89] |
Y. Li, X. Zhang, X. Liu, et al., Chem. Sci. 12 (2021) 3130-3145. DOI:10.1039/D0SC06557A |
| [90] |
B.Q. Chen, R.K. Kankala, A.Z. Chen, et al., Int. J. Nanomed. 12 (2017) 1877-1890. DOI:10.2147/IJN.S129526 |
| [91] |
J.L. Burger, S.P. Cape, C.S. Braun, et al., J. Aerosol Med. Pulm. Drug Deliv. 21 (2008) 25-34. DOI:10.1089/jamp.2007.0658 |
| [92] |
S.P. Cape, J.A. Villa, E.T. Huang, et al., Pharm. Res. 25 (2008) 1967-1990. DOI:10.1007/s11095-008-9575-6 |
| [93] |
Z. Du, C. Tang, Y.X. Guan, et al., Int. J. Pharm. 454 (2013) 174-182. DOI:10.1016/j.ijpharm.2013.07.001 |
| [94] |
Y.B. Shen, Z. Du, C. Tang, et al., Int. J. Pharm. 505 (2016) 223-233. DOI:10.1016/j.ijpharm.2016.03.053 |
| [95] |
Y. Ren, L. Cao, M. You, et al., TrAC Trend. Analyt. Chem. 157 (2022) 116774. DOI:10.1016/j.trac.2022.116774 |
| [96] |
H. Kim, I.H. Jeong, Y.K. Choi, et al., Adv. Healthc. Mater. 12 (2023) e2202358. DOI:10.1002/adhm.202202358 |
| [97] |
M. Kubczak, S. Michlewska, M. Bryszewska, et al., Adv. Drug Deliv. Rev. 179 (2021) 114038. DOI:10.1016/j.addr.2021.114038 |
| [98] |
T. Ito, T. Okuda, R. Takayama, et al., J. Pharm. Sci. 108 (2019) 2661-2667. DOI:10.1016/j.xphs.2019.03.029 |
| [99] |
A. Langford, B. Bhatnagar, R. Walters, et al., Dry. Technol. 36 (2017) 677-684. |
| [100] |
F. Emami, A. Vatanara, E.J. Park, et al., Pharmaceutics 10 (2018) 131. DOI:10.3390/pharmaceutics10030131 |
| [101] |
S. Bhatta, T. Stevanovic Janezic, C. Ratti, Foods 9 (2020) 87. DOI:10.3390/foods9010087 |
| [102] |
M. Bjelosevic, A. Zvonar Pobirk, O. Planinsek, et al., Int. J. Pharm. 576 (2020) 119029. DOI:10.1016/j.ijpharm.2020.119029 |
| [103] |
M. Tservistas, M.S. Levy, M.Y.A. Lo-Yim, et al., Biotechnol. Bioeng. 72 (2001) 12-18. DOI:10.1002/1097-0290(20010105)72:1<12::AID-BIT2>3.0.CO;2-Z |
| [104] |
H. Okamoto, S. Nishida, H. Todo, et al., J. Pharm. Sci. 92 (2003) 371-380. DOI:10.1002/jps.10285 |
| [105] |
H. Okamoto, K. Shiraki, R. Yasuda, et al., J. Control. Relea. 150 (2011) 187-195. DOI:10.1016/j.jconrel.2010.12.006 |
| [106] |
T. Okuda, D. Kito, A. Oiwa, et al., Biol. Pharm. Bull. 36 (2013) 1183-1191. DOI:10.1248/bpb.b13-00167 |
| [107] |
A.Z. Chen, Y.Q. Kang, S.B. Wang, et al., J. Mater. Chem. B 3 (2015) 6439-6447. DOI:10.1039/C5TB00715A |
| [108] |
P.Y. Xu, R.K. Kankala, Y.J. Pan, et al., Int. J. Nanomed. 13 (2018) 4685-4698. DOI:10.2147/IJN.S169399 |
| [109] |
R.K. Kankala, X.F. Lin, H.F. Song, et al., ACS Biomater. Sci. Eng. 4 (2018) 4225-4235. DOI:10.1021/acsbiomaterials.8b00992 |
| [110] |
S. Gao, G. Tang, D. Hua, et al., J. Mater. Chem. B 7 (2019) 709-729. DOI:10.1039/C8TB02491J |
| [111] |
A.C. Borges, C. Eyholzer, F. Duc, et al., Acta Biomater. 7 (2011) 3412-3421. DOI:10.1016/j.actbio.2011.05.029 |
| [112] |
Z. Cheng, Z. Ye, A. Natan, et al., ACS Appl. Mater. Interfaces 11 (2019) 42486-42495. DOI:10.1021/acsami.9b15234 |
| [113] |
B. Yang, W. Yuan, ACS Appl. Mater. Interface. 11 (2019) 40620-40628. DOI:10.1021/acsami.9b14040 |
| [114] |
X. Li, Y. Tang, J. Zhu, et al., Small 16 (2020) e2001935. DOI:10.1002/smll.202001935 |
| [115] |
K. Wu, L. Zhang, Y. Yuan, et al., Adv. Mater. 32 (2020) e2002292. DOI:10.1002/adma.202002292 |
| [116] |
M.F. Bostanudin, A. Lalatsa, D.C. Gorecki, et al., Carbohydr. Polym. 236 (2020) 116060. DOI:10.1016/j.carbpol.2020.116060 |
| [117] |
R. Parhi, Environ. Chem. Lett. 18 (2020) 577-594. DOI:10.1007/s10311-020-00963-5 |
| [118] |
Y. Zhao, B. Yan, Z. Wang, et al., Mini Rev. Med. Chem. 20 (2020) 96-106. DOI:10.2174/1389557519666190913151632 |
| [119] |
S.C. Richardson, H.V. Kolbe, R. Duncan, Int. J. Pharm. 178 (1999) 231-243. DOI:10.1016/S0378-5173(98)00378-0 |
| [120] |
T. Kean, M. Thanou, Adv. Drug Deliv. Rev. 62 (2010) 3-11. DOI:10.1016/j.addr.2009.09.004 |
| [121] |
M. Cheng, J. Deng, F. Yang, et al., Biomaterials 24 (2003) 2871-2880. DOI:10.1016/S0142-9612(03)00117-0 |
| [122] |
E.I. Rabea, M.E. Badawy, C.V. Stevens, et al., Biomacromolecules 4 (2003) 1457-1465. DOI:10.1021/bm034130m |
| [123] |
K.W. Kim, R.L. Thomas, Food Chem. 101 (2007) 308-313. DOI:10.1016/j.foodchem.2006.01.038 |
| [124] |
S.M. Ghoreishi, S. Komeili, J. Supercrit. Fluid. 50 (2009) 183-192. DOI:10.1016/j.supflu.2009.05.007 |
| [125] |
S.M. Ghoreishi, A. Hedayati, M. Kordnejad, J. Supercrit. Fluid. 111 (2016) 162-170. DOI:10.1016/j.supflu.2016.01.005 |
| [126] |
H.H. Peng, Z.D. Wang, Y.X. Guan, et al., J. CO2 Utiliz. 46 (2021) 101486. DOI:10.1016/j.jcou.2021.101486 |
| [127] |
J. Zhao, Y. Li, D. He, et al., ACS Appl. Mater. Interface. 12 (2020) 16159-16167. DOI:10.1021/acsami.0c02788 |
| [128] |
X. Li, R. Xing, C. Xu, et al., Carbohydr. Polym. 264 (2021) 118050. DOI:10.1016/j.carbpol.2021.118050 |
| [129] |
K. Song, Y. Hao, Y. Liu, et al., Carbohydr. Polym. 300 (2023) 120272. DOI:10.1016/j.carbpol.2022.120272 |
| [130] |
N. Gorshkova, O. Brovko, I. Palamarchuk, et al., Polym. Adv. Technol. 32 (2021) 3474-3482. DOI:10.1002/pat.5358 |
| [131] |
H.H. Peng, D.X. Hong, Y.X. Guan, et al., Int. J. Pharm. 558 (2019) 82-90. DOI:10.1016/j.ijpharm.2018.12.077 |
| [132] |
N. Jovanovic, A. Bouchard, M. Sutter, et al., Int. J. Pharm. 346 (2008) 102-108. DOI:10.1016/j.ijpharm.2007.06.013 |
| [133] |
N. Jovanovic, A. Bouchard, G.W. Hofland, et al., Pharm. Res. 21 (2004) 1955-1969. DOI:10.1023/B:PHAM.0000048185.09483.e7 |
| [134] |
T. Mizuno, K. Mohri, S. Nasu, et al., J. Control. Relea. 134 (2009) 149-154. DOI:10.1016/j.jconrel.2008.11.018 |
| [135] |
A. O'Sullivan, K.M. Ryan, L. Padrela, J. Supercrit. Fluid. 187 (2022) 105645. DOI:10.1016/j.supflu.2022.105645 |
| [136] |
Y. Sun, Curr. Pharm. Des. 20 (2014) 349-368. DOI:10.2174/13816128113199990404 |
 2024, Vol. 35
2024, Vol. 35 

