b Guangxi University of Chinese Medicine, Nanning 530200, China;
c Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
Oral drug delivery is the most convenient way of drug administration because of its simplicity, safety and non-invasiveness, which makes it more easily accepted by patients. Considering patient compliance, oral administration is a reasonable drug delivery route. It is reported that over 60% marketed products are administrated by oral route, showing its immense potential for disease treatment [1-3]. Therefore, the development of efficient oral preparations has always been one of the hot topics in the pharmaceutical field. However, oral drug delivery is not the optimal way from the perspective of bioavailability. For example, the oral bioavailability of albendazole is less than 5% in humans [4], the oral bioavailability of curcumin was only 1% in rats [5], the absolute bioavailability of scutellarin in Beagle dog administered orally was rarely 0.4% [6], and the oral bioavailability is generally low in a variety of ginsenosides (less than 5%) [7]. The drug delivered orally will first enter the gastrointestinal (GI) environment, which has many biological and physical obstacles that prevent the drug from being absorbed into the blood circulation smoothly. The speed and degree of drug absorption will be affected by these barriers, especially for the drugs with poor physicochemical properties (such as low solubility and permeability), resulting in incomplete drug absorption and eventually affecting oral bioavailability [8]. The barriers to oral absorption can be divided into biochemical barriers, mucosal barriers and cellular barriers (Fig. 1) [9]. Specifically, according to the in vivo process of the drug after oral administration, the absorption barriers can be summarized as follows: (1) The stability of the drug in the GI tract. The digestion of pepsin can decompose some acid-labile drugs in the gastric environment with a pH of 1.2–3. The lipase, amylase and biological metabolic enzymes will decompose and digest part of the drug after entering the intestinal tract, which will eventually affect the drug's oral bioavailability. (2) The solubility of the drug at the absorption site. In addition to stability, the drug must exist in the solution in molecular form to be absorbed [10]. The small intestine is the main site of digestion and absorption. Therefore, the poor solubility of the drug in the small intestine, especially the absorption site, will also have a greater impact on the drug's bioavailability. (3) Diffusion barrier on the intestinal epithelial cells, such as the unstirred water layer and mucus layer. The unstirred water layer is close to the mucus and is the main barrier to hindering the absorption of lipid-soluble drugs. The mucus, which covers the surfaces of intestinal epithelial cells, is the main physical barrier for drug absorption by the epithelial cells. The mucus with a complex hydrogel-like structure is mainly composed of proteins (especially mucins) and carbohydrates. It naturally protects intestinal epithelial cells by capturing and removing foreign particles, which makes it one of the absorption obstacles to oral drugs. (4) The small intestinal epithelial cells are another barrier after drugs pass through the mucus layer. Some drugs, such as Biopharmaceutical Classification System (BCS) class III drugs, have poor membrane permeability, which will also affect the absorption of the drug by intestinal cells and ultimately the oral bioavailability of the drug. (5) Intestinal efflux. It is mainly related to the intestinal efflux protein, such as P glycoprotein (P-gp). Some drugs, as the substrates of P-gp, are re-excreted during transport through epithelial cells or even after absorption. This phenomenon of drugs returning to the GI tract is called intestinal efflux, which is also one of the key factors affecting drug oral absorption [11-13].

|
Download:
|
| Fig. 1. Absorption barriers of oral formulations. Figure includes modified templates from Servier Medical Art (smart.servier.com). | |
In the past few decades, researchers have tried to improve the barrier-crossing ability of oral drugs through various pharmaceutical technologies. New dosage forms developed based on excipient and material technologies, such as nanoparticles [14-20], solid dispersion [21-24], nanoemulsion [25-28], polymeric micelles [25-33] and nanocrystals [34-38] have been widely demonstrated to be effective in improving the oral absorption of drugs. As drug vehicles, the encapsulation effect of these microcarriers can mask the unpleasant taste and smell of drugs to improve patient compliance, increase drug solubilization and improve drug stability, which in turn enhances drug survival in the complex GI environment and ultimately increases the drug oral absorption [39,40]. In addition, further surface modification techniques based on these carriers can achieve the active targeting of the drug, thereby reducing the toxic side effects of the drug [41]. The development of new formulation technologies and dosage forms in recent decades has brought new hope for oral drug delivery. Among them, vesicular drug delivery systems have attracted numerous attentions due to their unique nature and drug delivery applications [42]. Vesicles can be defined as a highly ordered spherical assembly composed of one or more concentric bilayers that are formed by the self-assembling of amphiphilic building blocks in water. Liposomes, the first vesicular formulation with vesicle bilayers formed by phospholipids, have been widely used for drug delivery since the first description by Bangham et al. in 1965 [43]. However, with in-depth research, the problems of conventional liposomes have gradually come to the fore, such as low hydrophilic drug encapsulation efficiency, unstable membrane caused drug leakage, short half-life, high cost and poor storage stability [44,45]. Natural lipids, particularly those with aliphatic chains attached to the backbone by means of ester or amide bonds, such as phospholipid, are often subject to the various hydrolytic or lipolytic enzymes, which will cleave off acyl chains, disrupt the lipid bilayer, and eventually result in the leakage of the encapsulated drug [46]. Altering the composition of the vesicle bilayers has been proven to improve the druggability of the vesicles. Therefore, various amphipathic molecules have been employed to form the bilayers, such as fluorinated lipids, ether lipids, synthetic double-chain amphiphiles, as well as single-chain amphiphiles, including nalkylindoles, polyhydroxyl lipids, polyhedral non-ionic surfactants. In addition to bilayer-forming molecules, other functional components were also introduced to further improve the drug delivery efficiency. In summary, researchers have developed a variety of new vesicle drug delivery systems that are morphologically similar to liposomes by changing the composition of the prescription, including niosomes, sphingosomes, bilosomes, chitosomes [47] and some representative deformable nanovesicles such as transfersomes, menthosomes, invasomes, ethosomes and transethosomes [48]. Indeed, some of these novel vesicles can be used as desirable carriers for oral drug delivery, and several successful studies on this concern have been reported recently.
The systematic reviews on the applications of vesicular systems on oral drug delivery is still lack currently, so in this review, we aimed to introduce the concept, related properties, and applications of different vesicular drug delivery systems available for oral drug delivery and analyzed the possible mechanisms by which vesicular carriers can enhance the drug's oral absorption. In addition, we discussed the challenges and possible solutions to enhance the application potential of vesicular drug delivery systems on oral administration. We hope this review will further strengthen our understanding on the related concepts and characteristics of vesicular drug carriers, thereby providing a certain theoretical basis for the applications of this carriers in the field of oral drug delivery and a reference for the design of novel oral formulations.
2. Vesicular drug delivery systems available for oral administrationBy reviewing the application cases of novel vesicle carriers in recent years, it can be found that the vesicular systems currently available for oral drug delivery mainly include liposomes, niosomes, transfersomes, chitosomes, and bilosomes (Fig. 2).

|
Download:
|
| Fig. 2. The structure of vesicular delivery systems available for oral drug delivery. | |
The aforementioned vesicular delivery systems have commonalities in morphology and membrane-forming components, but their features are different. The enclosed vesicle bilayers are the main skeleton components of these five types of vesicular carriers, and all the bilayers are composed of amphiphilic molecules. For the formation mechanism, the self-assembly of amphiphilic molecules in water is mainly affected by the hydrophilic-lipophilic balance (HLB) and the geometric shape of the amphiphilic molecule [49]. Besides, factors such as lipid chain length, chain packing and membrane asymmetry are also related to the formation of vesicles. From the perspective of thermodynamics, the self-assembly of amphiphilic molecules needs to resist the reduction of free energy and negative entropy components, which can only be achieved via favorable enthalpy from intermolecular van der Waals forces, hydrophobic forces and hydrogen bonding [50]. Therefore, the formation of vesicles is closely related to the HLB value of amphiphilic molecules. It has been reported that the sorbitan monostearate surfactants (Span) with an HLB value between 4 and 8 are conducive to vesicle formation [51]. Highly hydrophilic amphiphilic molecules generally cannot form free hydrate units such as vesicles, and these free units tend to aggregate to form a layered structure. Therefore, it is required to select the vesicle-forming molecules with a suitable HLB value, or other substances that can neutralize their hydrophilicity should be added to aid in better vesicle formation. For example, cholesterol is often added to niosomes as a functional ingredient to enhance their tendency to form vesicles. In order to more easily judge the behavior of amphiphilic molecules in water, some researchers have pointed out that the use of nominal geometric parameters for amphiphilic molecules can predict the shape of spontaneously formed associated colloids with considerable certainty. Notably, Israelachvili et al. discussed the critical packing parameter (CPP) in 1976 [52] and it can be defined by Eq. 1.

|
(1) |
where V is the tail volume of the molecules, a0 is the surface area per molecule at the hydrocarbon-water interface, l is the critical span of a fluid molecular chain in an aggregate.
The shape and size of the equilibrium aggregates formed by amphiphilic molecules will change with the CPP value, specifically from spherical micelles (CPP ≤ 1/3) to cylindrical micelles (1/3 ≤ CPP ≤ 1/2), vesicles or planar bilayers (1/2 ≤ CPP ≤ 1), and inverted micelles (CPP > 1). The specific structure can be seen in Fig. 3.

|
Download:
|
| Fig. 3. The relationship between the CPP and the morphology of self-assembled amphiphilic molecules. | |
This is of great significance in explaining the mechanism of vesicle formation, which can help us choose the appropriate vesicle bilayer-forming molecules, addition amounts and hydration conditions. Indeed, these formation theories are suitable for vesicular drug carriers that can be used for oral absorption, and they can guide our vesicle preparation process to a certain extent. On the other hand, since amphiphilic molecules form the bilayer skeleton of vesicles, the stability of vesicular drug delivery systems is related to the physicochemical properties of the loaded drug, amphiphilic molecules, their interaction with each other, vesicle size and other factors in addition to environmental factors such as temperature, pH, oxidation and osmolality [53,54]. Overall, the stability of vesicular drug delivery systems can be greatly enhanced by different formulation designs and surface modification techniques [55]. For drug release from vesicular drug delivery systems, which is closely related to the GI stability of vesicular drug delivery systems, premature rupture of vesicles can lead to drug leakage and release, and there is a risk of rupture of drug-carrying vesicles at any of the barriers mentioned previously [56]. Ideally, if the vesicle can reach the target site such as intestinal epithelial cells in an intact form, the vesicle bilayer can fuse with the cell membrane to release the drug due to the similar membrane composition of the vesicle and the cell membrane. Apart from this, the drug-loaded vesicles can enter the cell through a series of endocytosis (phagocytosis, pinocytosis, caveolin-mediated endocytosis, and clathrin-mediated endocytosis, etc.) as well as the paracellular pathway [57-59]. In addition, sustained and targeted release of drugs can be achieved by surface modification of vesicles [60,61]. The formulation composition, the drug delivery advantages, and some applications of these vesicular drug delivery systems in the oral field are introduced in this section.
2.1. LiposomesLiposomes were discovered and named by Bangham in 1964 when he observed the behavior of phospholipids dispersed in water with an electron microscope [43]. The hydrophobic tails of phospholipids tend to gather together to avoid the water phase, while their hydrophilic heads are exposed to water. A certain number of phospholipid molecules can spontaneously form enclosed vesicles with a bilayer structure in water (Fig. 2) [62]. The specific organization of the lipids depends on their properties, concentration, geometric form, and ambient temperature [63]. Drug molecules can be encapsulated in the hydrophilic inner aqueous phase or the hydrophobic region between the lipid bilayers if they are present during liposome formation. Liposomes can be classified as uni-, oligo- or multilamellar vesicles on the basis of lamellarity and as small, intermediate or large liposomes on the basis of size. The unilamellar vesicles (one lipid bilayer, 50–250 nm) with a large inner aqueous phase are suitable for encapsulating hydrophilic drugs, while the multilamellar vesicles (two or more concentric lipid bilayers, 1–5 µm) are more inclined to encapsulate lipid-soluble drugs. Due to their unique structure and properties, liposomes have been widely used as carriers to efficiently deliver drugs via different routes [42,64].
2.1.1. Compositions and advantages of liposomes as drug carrierLiposomes consist of vesicles enclosed by concentric lipid bilayers with an inner aqueous phase, the bilayers are generally composed of phospholipids and cholesterol (Fig. 2). The phospholipids used in liposomes include natural and/or synthetic phospholipids (phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylserine and phosphatidylinositol). The main structural components of most biological membranes are composed of phosphatidylethanolamine and phosphatidylcholine, also known as lecithin [65]. The characteristics of liposomes, such as encapsulation efficiency, are strictly related to their phospholipid properties because the lipid molecules can affect the surface charge, release, permeability, and biodistribution of liposomes [66]. Cholesterol, the most commonly applied sterol used to increase the stability of liposomes, is not involved in the formation of membrane structures, but it can be embedded between phospholipid molecules to regulate the permeability and fluidity of the membrane, which is essential for maintaining the structural stability of the liposomes [67]. In addition to phospholipid and cholesterol, there are also some other additives, such as polyethylene glycol, modified on the surface of liposomes to realize the long-circulating effect of liposomes.
Liposomes have long been used as drug carriers since they were first reported in the 1960s, and they are considered safe drug delivery vehicles due to their biocompatibility and biodegradability. As drug carriers, liposomes can deliver drugs with different solubility properties because of the unique vesicle structure formed by the amphiphilic molecules. Specifically, the hydrophobic drug will reside in the acyl hydrocarbon chain of the lipid bilayer, and hence the encapsulation efficiency is related to the length and packing density of the acyl chains of the lipids and also to the drug-to-lipid ratio. For the hydrophilic drugs, they will localize in the inner aqueous phase or adjacently near the polar head groups of phospholipids. In addition, the encapsulation effect of liposomes can avoid direct contact between the drug and the unfavorable environment, thus enhancing the stability of the drug before it is absorbed. It can also reduce the exposure of sensitive tissues or organs to toxic drugs and then reduce adverse body reactions after drug administration. Liposomes can be engulfed by macrophages as foreign substances and concentrated in macrophage-rich organs such as the liver, spleen, and lymphatic system. Therefore, passive targeting of drugs can be achieved when liposomes are employed for drug delivery [68,69]. In addition to uptake by macrophages, drug-loaded liposomes can also be adsorbed to the cell surface by nonspecific weak hydrophobic forces, electrostatic forces, and specific interactions with cell surface components. Then the lipid bilayer further inserts into the plasma membrane and fuses with the plasma membrane, simultaneously releasing the contents into the cytoplasm. Therefore, the partition coefficient in oil or water, and the size of the drug molecules, the nature of the lipid bilayer, the density of the liposome surface charge, and the interactions of the drug with the lipid membrane need to be carefully screened in order to deliver the drug efficiently.
2.1.2. Applications of liposomes on oral administrationIn the past few decades, the potential of liposomes as drug carriers has been widely explored in different administration routes. With the development of the preparation process, the first injectable liposome product, AmBisomeⓇ, an amphotericin B-loaded formulation, was approved in 1974 [70]. However, intravenously administered liposomes are rapidly cleared from the blood and eventually accumulate in the tissues or organs of the reticuloendothelial system [71]. Researchers have addressed these issues by surface modification or exploring other drug delivery routes for liposomes. Among them, oral administration has gained the most attention due to its unique characteristics of safety, convenience and easy acceptance by patients. Recently, liposomes have been employed for the oral delivery of a wide range of drugs, including anti-inflammatory, antimicrobial, antiviral drugs, proteins and peptides, etc., and the results indicated that liposomes are able to enhance the intestinal absorption and oral bioavailability of drugs to varying degrees. For one thing, conventional liposomes have been used as carriers to deliver various active ingredients of traditional Chinese medicine, such as baicalein [72], silymarin [73] and breviscapine [74]. The experimental results showed that the oral bioavailability was increased by 4.52, 3.5 and 2.38 times compared with the corresponding raw drug, respectively. For another, Yamazoe et al. significantly improved the oral absorption of fluorescein isothiocyanate dextran by modifying polyethylene glycol (PEG) on the surface of liposomes, and the absorption improvement was related to the PEG concentration [75]. These findings were in good line with daidzein-loaded liposomes with PEG modification [76]. And liposomes can be combined with another functional group, such as vitamin B12, on the basis of PEG modification to further strengthen insulin oral absorption [77]. There are also studies on the preparation of acetic acid transporter-mediated, multifunctional polymer liposomes for oral delivery of docetaxel [78]. In addition, Agardan et al. found that tamoxifen-loaded liposomes containing penetration enhancers have better oral absorption and breast cancer therapeutic effects [79]. Bile salts are another commonly used additive to adapt the liposomes for drug delivery. In recent years, it has also been reported that extracellular adhesion protein derived from Staphylococcus aureus can be used to further improve the oral drug delivery effect of liposomes when coated on the liposome surface [80,81]. The detailed procedures for these studies of liposomes are summarized in Table S1 (Supporting information). It can be found that there are relatively few cases of conventional liposomes for oral drug delivery, and most of the studies prefer to introduce functional components to make liposomes more suitable for oral drug delivery. In summary, common strategies to improve the oral delivery performance of liposomes mainly focus on improving the stability and intestinal absorption of liposomes. Among them, stability enhancement can be achieved by modifying the constituents of the lipid bilayer, surface coating, interior thickening, and other means, such as double-liposomes, which can prevent the destruction by intestinal enzymes due to the outer bilayers serving as a protective coating. For another, intestinal absorption enhancement can be achieved by increasing the adsorption capacity of liposomes on the intestinal mucosal, the addition of absorption enhancers or polymers, and ligand-mediated targeting of intestinal epithelial cells.
2.2. NiosomesWith further research, it was found that the crude phospholipids forming the bilayer of liposome vesicles were difficult to purify and were prone to oxidative deterioration, which reduced membrane fluidity and led to the leakage of encapsulated drugs. The search for other amphiphilic molecules that can replace phospholipids has revealed that many synthetic surfactants are capable of forming vesicles, but the ionic ones are more toxic, so non-ionic surfactants have received more attention. The vesicular carrier formed by the hydration of non-ionic surfactants with cholesterol was first reported by Handjain-Vila et al. in 1979 [82], and "niosomes" were first used to express this vesicular carrier by Azmin et al. in 1985 [83]. Structurally, niosomes consist of an orderly arrangement of nonionic surfactant molecules forming monolayer vesicles with an internal aqueous phase, or multilayer vesicles formed by repeating multiple monolayer vesicles (Fig. 2). The formation mechanism of niosomes is similar to that of liposomes, mainly characterized by the replacement of phospholipids by non-ionic surfactants, which avoids oxidative degradation of phospholipids, resulting in high chemical stability [84].
2.2.1. Composition and advantages of niosomes as drug carrierThe basic components of niosomes are non-ionic surfactants, similar to phospholipids, which are the main molecules that form the bilayer of the vesicles. Non-ionic surfactants exhibit higher stability than cationic, anionic, and amphoteric surfactants due to the fact that the head of the nonionic surfactant molecule does not have any charge [85]. The main molecules that can be used to prepare nonionic surfactant vesicles include fatty acids, amides, amino acids, alkyl esters and alkyl ether surfactants. Depending on the nature of the hydrophilic head group, alkyl ether surfactants can be categorized into alkyl ethers whose hydrophilic head group consists of a repeating glycerol subunit and a related isomer or a larger sugar molecule, and alkyl ethers whose hydrophilic head group consists of a repeating ethylene oxide subunit. The amphiphilic molecules used in the first niosomes were single-chain surfactants such as alkylene oxides [51]. Another important component of niosomes is cholesterol, which is used to regulate the fluidity of the vesicle membrane and affect the drug encapsulation efficiency of niosomes. Usually, the cholesterol is used at a molar mass ratio of 1:1 with the nonionic surfactants. It has been reported that many surfactants form vesicles containing up to 30–50 mol% cholesterol, and there is a strong relationship between the HLB value of the surfactant and the amount of cholesterol added. When the HLB value of nonionic surfactants is increased above 10, the cholesterol concentration has to be increased accordingly to compensate for the effect of larger head groups on CPP, as described previously. In addition, charge-inducing molecules can also be added to niosomes to prevent their aggregation via the electrostatic repulsion effect, thereby further maintaining the stability of niosomes.
Compared to conventional liposomes, niosomes as drug carriers exhibited better chemical stability, permeability and shelf life. Niosomes with no charge are less toxic and more biocompatible, and they do not initiate immunogenic reactions because they can be degraded by biological systems. The functional group on the hydrophilic head makes it easily modified. Similar to liposomes, niosomes can also encapsulate hydrophilic and hydrophobic drugs. In addition, niosomes are able to achieve controlled, sustained, and targeted drug delivery because of their small-sized vesicle drug-loading structure [86]. Recently, there has been an expanding interest in the study of niosomes as drug carriers because they are able to overcome some of the drawbacks of liposomes. The surfactants used in niosomes are easy to derivatize, conferring greater versatility to the vesicular structure, and they are less costly than phospholipids. Drug-loaded niosomes can be administered via intravenous, ocular, nasal, oral, dermal, transdermal, intramuscular, and pulmonary routes [87].
2.2.2. Applications of niosomes on oral administrationNiosomes have been widely used in the oral delivery of various drugs in recent years, mainly antibacterial, antiviral, antitumor, antidiabetic, hypolipidemic, and hypotensive drugs. In contrast to liposomes, conventional niosomes already have good oral drug delivery potential; surface modification and modulation of vesicle bilayer compositions are not common in oral-related niosomes. For the surface modification, there are niosomes coated with chitosan by crosslinking with tripolyphosphate for atorvastatin oral delivery [88]. In addition, some studies have also introduced stearyl amine, a positive charge inducer, to further increase the stability of niosomes and thereby enhance their oral absorption [89]. Currently, the process commonly used to prepare niosomes that are available for oral drug delivery is the film hydration method. And most of the research focuses on screening the amount of the prescription composition of niosomes (such as the non-ionic surfactant and the cholesterol) [83,90-94]. In addition, it has been found that sorbitan monostearate 60, a non-ionic surfactant called Span 60, seems like a common option for most niosomes that can be used for oral drug delivery [95]. The detailed procedures for these studies of niosomes are summarized in Table S2 (Supporting information).
2.3. TransfersomesTransfersomes, the first generation of elastic liposomes, have been developed in order to overcome the poor permeability of liposomes [96]. The term "transfersomes" means carrying body and is derived from a Latin word "transferre" (meaning to carry across) and a Greek word "some" (meaning body). Similar to liposomes, transfersomes can be understood as vesicle carriers with enhanced penetrating ability [97]. Transfersomes were first reported by Cevc et al. in 1992. Deformability is the most important feature of transfersomes, which differs from liposomes in that they are able to change their membrane flexibility and pass through the skin pores spontaneously when they reach the skin pores [98,99], while conventional liposomes can only stay in the upper stratum corneum. Accordingly, transfersomes are also called ultra-flexible vesicles, ultra-deformable vesicles or flexible liposomes. The structure of transfersomes exhibits a bilayer vesicular structure with at least one inner aqueous compartment surrounded by a lipid bilayer containing an edge activator (Fig. 2) [100].
2.3.1. Composition and advantages of transfersomes as drug carrierPhospholipids and surfactants (used as edge activators) are the essential components of transfersomes. Edge activators inserted into the phospholipid bilayer can effectively improve the elasticity and fluidity of transfersomes, making it easier to penetrate the stratum corneum [101]. The compositions of transfersomes are introduced in detail as follows: (1) Phospholipids, an amphiphilic molecule that can be a mixture of lipids, which are the vesicle-forming components that create the lipid bilayer, are the basic and main component of the transfersomes. The phospholipids used to prepare transfersomes are similar to conventional liposomes, such as phosphatidylcholine (PC), hydrogenated lecithin (HPC) and egg yolk lecithin (EPC) [102]. In addition, the cationic lipid DOTAP was also used to prepare cationic transfersomes to enhance the affinity of drugs and skin, and improve the skin retention and penetration capacity of drugs [103]. (2) An edge activator, usually a single-chain surfactant, can be incorporated into the lipid bilayer, the edge activator may position in the lipid bilayer with the head group oriented toward the head group of the phospholipid, and the lipophilic part is arranged parallel to the acyl chains of the phospholipid [104]. The hydrophilic head of the edge activator will flow and gather at the damaged part caused by deformation and then fill the voids in the aqueous solution to maintain membrane integrity. The most commonly used edge activators in transfersomes are single-chain surfactants such as sodium cholate and sodium deoxycholate, as well as nonionic surfactants Tweens and Spans (Tween 20, Tween 60, Tween 80, Span 60, Span 65 and Span 80). In addition, saponins such as dipotassium glycyrrhizinate were also employed as the edge activator, which is one of the significant reasons for the high deformability of the transfersomes [100,102]. (3) About 3%–10% alcohol (ethanol or methanol) as solvent, water, or phosphate buffer (pH 6.5–7) as hydration medium. Cholesterol, a controversial component, was not included in the composition when transfersomes were first reported. As mentioned above, cholesterol can increase the rigidity of lipid bilayers, regulate the stability of liposomes, and reduce drug leakage [105]. For transfersomes, cholesterol is usually added less or not to ensure the fluidity of the lipid bilayer. This fluidity may be associated with the transition of the lipid membrane from a gel state to a liquid-crystalline state. At the pretransition temperature before the phase transition, the phospholipid bilayer is in the corrugated gel phase (lipids will transform into a two-dimensional assembly with periodic fluctuations), which is a critical link before the phase transition. It has been reported that the concentration of cholesterol equal to or less than 5 mol% had no effect on the pretransition of the lipid membrane [106,107]. When the cholesterol concentration is 9 mol% and above, the pretransition process of the lipid membrane cannot be detected, which will affect the phase transition of the lipid bilayer and thus affect the fluidity of the membrane [104].
Transfersomes exhibit a high degree of deformability, which is an important feature that distinguishes them from conventional liposomes, and this property originates from the inhomogeneity of the lipid membrane [108]. Phospholipid and surfactant, two components with different polarities, formed the lipid membrane of the transferosomes. The components with high polarity tend to aggregate in membrane areas with high curvature. For example, the sodium cholate in the transfersomes can accumulate in the high-pressure part to cause deformation, and these molecules have a tendency to form a higher curvature structure. The characteristics of molecular arrangement and aggregation promote the deformation of lipid membranes, thereby enhancing the deformability of the transferosomes [100,109]. In addition, this structure can promote the deformation of transfersomes by reducing the energy expenditure in the process of vesicle formation. The transfersomes will be easy to pass through a channel that is much smaller than their own diameter when they are exposed to extraneous stresses and narrow space. The deformability of transfersomes can be five orders of magnitude greater than that of conventional liposomes [110]. Previous studies have shown that transfersomes with an average particle size of 500 nm can pass through small pores that are 1/5 of their own, and the structure of the transfersomes remains almost unchanged after permeation. The elasticity of the lipid membrane reduces the risk of complete rupture of the vesicle, and the initial structure can be maintained well [111]. In addition, a high degree of hydrophilicity is also one of the properties of transfersomes. Studies have found that the skin permeation of transfersomes is mainly driven by the pressure difference between inward hydration and spontaneous dehydration, which is proportional to the number of transfersomes and the hydrophilicity of the transfersome monomer [112], the skin permeability and its speed are equivalent to pure water. The transfersomes have a tendency to enter the skin along the skin hydration gradient because of their hydrophilicity, and their deformability can ensure smooth penetration. Conventional liposomes usually remain on the skin surface due to their poorer hydrophilicity and deformability until dehydration and fusion [113].
2.3.2. Applications of transfersomes on oral administrationMost relevant research on transfersomes has focused on the field of transdermal drug delivery since their inception. As a transdermal drug carrier, transfersomes can significantly enhance the skin penetration efficiency and bioavailability of loaded drug [114]. However, there have been few studies of transfersomes for oral drug delivery. In addition to the influence of its original application field, another reason is that the formulation composition and concept definition of a new vesicle drug delivery system are easily confused. Nevertheless, some researchers have attempted to prepare transfersomes for oral drug delivery and achieved satisfying results due to their unique formulation compositions and functional properties. The surface modification of transfersomes, such as silica-modified, can further enhance the oral absorption of curcumin compared to conventional transfersomes [115]. And the transfersomes prepared with bile salts or Tween 80 as an edge activator also showed excellent oral delivery of catechins and salmon calcitonin, respectively [116,117]. The detailed procedures of these oral-related studies of transfersomes are summarized in Table S3 (Supporting information).
2.4. ChitosomesPolymers, especially biopolymers, can form a layer around the surface of liposomes after being introduced, which can endow liposomes with different performance according to their different properties [118]. Among these available polymers, chitosan has attracted extensive attention due to its biocompatibility, low toxicity, low immunogenicity, and biodegradability. The positively charged amino groups of chitosan most likely interact with the negatively charged components, so the chitosan can bind to the surface of liposomes through electrostatic interaction, then form a hydrophilic chitosan layer on the surface of liposomes (Fig. 2). Bilosomes were first described by Alamelu et al. in 1991, which refers to liposomes whose surface is modified with chitosan or its derivatives [119,120]. Bilosomes as drug carriers have shown desirable stability, penetration, safety, and drug delivery efficiency.
2.4.1. Compositions and advantages of chitosomes as drug carrierIn addition to phospholipids and cholesterol used to form liposomes, the most important and basic components of chitsomes are chitosan and its derivatives that are modified on the surface. Chitosan, a linear cationic polysaccharide, is obtained by alkaline or enzymatic deacetylation of chitin [121]. The properties of chitosan, a key influence on the drug delivery advantages of chitosomes, are usually related to its molecular weight, degree of deacetylation, and distribution of the acetyl and amino groups along the copolymer chain. For example, the large charge density will lead to the condensation of protonated charges in the chitosan solution and improve its solubility when the degree of deacetylation of chitosan is greater than 75%. It has also been reported that the water solubility of chitosan decreases from 123.2 mg/mL to 0.4 mg/mL as the molecular weight of chitosan increases from 5 kD to 50 kD [122]. On the other hand, the characteristics of chitosan can be further strengthened by changing its structure, making it more suitable for surface modification or realizing other drug delivery functions. According to Karewicz et al., a cationic-hydrophobic chitosan derivative was synthesized by introducing quaternary ammonium salt and N-dodecyl into chitosan, and liposomes modified with this functional cationic hydrophobic polymer have been validated to show that it can markedly improve the structural stability of liposomes by anchoring the liposome bilayer via the long alkyl chain, thereby making it more easily to penetrate the cell membrane and release curcumin in a controlled form [123]. In addition, Yan et al. prepared pH-responsive liposomes by coupling ethylene glycol chitosan to the liposome membrane, which can change from a negative charge to a positive charge in the acidic extracellular tumor microenvironment. The resulting formulation exhibited enhanced cellular uptake, which in turn improved the anticancer efficacy of doxorubicin [124]. It can be seen that the properties of chitosan modified on the surface of liposomes have a significant impact on their drug delivery functions. Therefore, the properties of chitosan and its derivatives should be fully investigated before being added. In addition, chitosomes with different functions can be achieved by changing the structure of chitosan and its derivatives on the liposomes.
Due to the coating effect of the chitosan polymer layer, chitosomes exhibited better mucoadhesive ability, physicochemical stability, and prolonged GI tract residence time compared with conventional liposomes. In addition, by changing the structure of chitosan and its derivatives, chitosomes can be endowed with many functions, such as environment-responsive drug release and targeting. In recent years, chitiosomes have been widely used as a drug in transdermal, ocular, oral, nasal, and intramucosal fields, showing excellent drug delivery potential [125,126].
2.4.2. Applications of chitosomes on oral administrationThe applications of chitosomes in the oral field are mainly focused on the delivery of cardiovascular disease-related drugs. Similar to niosomes, conventional chitosomes already have desirable oral delivery potential. For example, cinnarizine-loaded chitosomes prepared by Oransa et al. improved the bioavailability of drugs via an enhanced mucoadhesive effect, which was in line with a previous finding by Ezzat et al. [127,128] In addition, there are studies that first prepared TPGS-modified liposomes, and then further modified chitosan on their surface to prepare coenzyme Q10-loaded chitosomes, and the results indicated that the systemic exposure of the drug was significantly enhanced after oral administration [129]. Chitosomes can also be used as intermediates, and then they can be further made into fast-dissolving tablets, which can improve the oral bioavailability of rosuvastatin [130]. For the surface modification of chitosomes, some studies have introduced sodium tripolyphosphate into the prescription of chitosomes, which further improves the stability of quercetin in an acidic environment [131]. The detailed studies of chitosomes are summarized in Table S4 (Supporting information).
2.5. BilosomesBilosomes, the nonionic surfactant vesicles incorporating bile salts, are a new vesicular drug carrier first described by Conacher et al. in 2001 [132]. Bilosomes are prepared mainly to further enhance the stability of niosomes in the GI tract. Similar to the niosomes, the structure of bilosomes appears as concentric bilayer vesicles with an inner aqueous phase (Fig. 2). The bile salts act as absorption enhancers and can protect drug-loaded bilosomes from the intestinal media containing bile acids, thereby decreasing premature release of drugs before they reach the absorption sites [133]. With the deepening of research, it has been found that a variety of drugs can be encapsulated in bilosomes, and the drug-loaded bilosomes can be administered in different ways. Therefore, bilosomes as drug carriers can exert the effect of the introduced bile salt molecules, thereby improving drug oral absorption.
2.5.1. Compositions and advantages of bilosomes as drug carrierFor the composition of bilosomes, some previous studies have claimed that the preparations formed by adding bile salts to liposomes are called "bilosomes". However, there are phospholipids in the composition of liposomes, while the bilosomes are vesicles composed of nonionic surfactants and bile salts (bile salts are incorporated into the niosome's membrane), which are considered non-lipoidal biocarriers [134-136]. Thus, this novel nanocarrier can be understood as bile salt-stabilized vesicles. Although bile salts act at the periphery to disrupt vesicle membranes, when incorporated into the vesicle bilayer, bile salts act as stabilizers for vesicle nanocarriers and reduce vesicle disruption by physiological bile salts in the GI tract [137]. In addition, bile salts can act as endogenous surfactants, form mixed micelles with free fatty acids and partially digested lipids to assist the solubilization of lipophilic drugs, and they can also form reverse micelles to improve the penetration ability of hydrophilic drugs through the biological membranes (refer to Section Oral absorption enhancing mechanisms of vesicular drug delivery systems for details). The bile salts commonly used in the preparation of bilosomes include sodium cholate, sodium deoxycholate, sodium chenodeoxycholate and sodium taurocholate, etc. It can be found that the amount of bile salt used in bilosomes is usually not more than 100 mg, while the molar ratio of non-ionic surfactant and cholesterol can be changed according to the actual [138].
In addition to the drug delivery advantages of niosomes, bilosomes as drug carriers can withstand disruption by physiological bile salts in the GI tract, and they are able to enhance the resistance to degradation by digestive enzymes compared to conventional niosomes, thereby improving the structural stability of bilosomes. Bilosomes are generally stable after oral administration, which prevents degradation under highly acidic conditions and enhances the potency of orally administered drugs [139]. Bilosomes can be easily stored after lyophilization (in the case of nanoparticles and microspheres) and do not need strict refrigeration storage conditions.
2.5.2. Applications of bilosomes on oral administrationThe bile salts stabilized and protected the bilosomes and their contents from the harsh environment of the gut, enabling drug delivery via the oral route. Bilosomes exhibit excellent GI stability mainly because of the bile salts incorporated in the formulation, thereby being widely used for oral drug delivery. Bilosomes have been used for oral delivery of a variety of drugs, including vaccines, antitumor, antivirus, antibacterial, antihypertensive, and antidepressant drugs. Among them, the research on bilosomes used for oral delivery of vaccines is relatively extensive. The main reason is that bilosomes do not require the use of live pathogens, and the oral administration of vaccines has better patient compliance. Besides, bilosomes are able to eliminate the cold-chain requirements of preparations such as vaccines, thereby avoiding common problems associated with injections and improving the efficacy of vaccines [140]. For another reason, the addition of absorption enhancers on the basis of bilosomes has also been confirmed to further improve the intestinal absorption of doxorubicin [141]. There are studies that co-load the active ingredients of traditional Chinese medicines to improve the efficacy of drugs through synergistic effects [142]. It can be found that Span 60 is commonly used as the nonionic surfactant to form the vesicle bilayer [143], and the amount of bile salt added is usually not more than 30% [144,145], but the specific amounts are determined according to the properties of the loaded drug and the addition of nonionic surfactant. In summary, bilosomes have shown great potential for efficient oral drug delivery [146-149]. The detailed procedures of these oral-related studies of bilosomes are summarized in Table S5 (Supporting information).
3. Oral absorption enhancing mechanisms of vesicular drug delivery systemsAs mentioned above, vesicular systems have been shown to be useful for oral drug delivery and can significantly enhance the oral absorption of a wide range of drugs. However, the mechanism by which vesicular drug delivery systems enhance oral absorption has not yet been elucidated, and the possible mechanisms by which vesicular drug delivery systems enhance oral absorption can be broadly analyzed on the basis of the oral absorption barriers of the drugs, the prescription composition of the formulation, and the functional characteristics. Before that, we need to understand the general in vivo processes of vesicular carriers and loaded drugs (Fig. 4). Based on the report of He et al. [70], we can hypothesize that orally administered vesicular formulations are partially destroyed upon entry into the acidic environment of the stomach, at which point drug leakage occurs. Thereafter, the still-surviving vesicles are emptied by the stomach into the alkaline intestinal environment, where they are subjected to a second digestion in the intestinal lumen. The surviving vesicles need to pass through the intestinal unstirred water layer and the mucus layer to reach the intestinal epithelium, a process that also involves vesicle rupture and release of the drug. Vesicles that survive this entire digestive process may cross the intestinal epithelium into the bloodstream via the transcellular and paracellular pathways or enter the lymphatic tissue via M-cell uptake. This section summarizes the mechanisms by which vesicular carriers enhance the oral absorption of drugs by analyzing the prescription composition and functional characteristics of different vesicular systems available for oral drug delivery.

|
Download:
|
| Fig. 4. General in vivo processes of oral vesicular drug delivery systems. Figure includes modified templates from Servier Medical Art (smart.servier.com). | |
The stable presence of drugs in the GI tract is a prerequisite for their absorption, and many drugs, such as proteins and peptides, can be degraded and inactivated by a combination of gastric acid, bile salts, proteases and pancreatic lipase [68]. The most intuitive advantage of vesicular carriers for oral delivery is that the bilayer encapsulation can reduce the direct contact between the encapsulated drug and the GI environment to a certain extent and prevent the drug from being digested prematurely [150]. However, it has been reported that the drug-loading structure of vesicular carriers such as liposomes is susceptible to damage in the GI tract. In a gastric juice environment with a low pH, H cations can diffuse into the aqueous phase of liposomes and destabilize them [151]. Therefore, most vesicular drug delivery systems for oral use have been prescribed with functional ingredients to enhance the structural stability of the GI. For example, bile salts added to liposomes, bilosomes and transfersomes play an important role in stabilizing the vesicle structure. In the small intestine, the exogenous bile salt monomers will enter the lipid bilayer of conventional liposomes, and the liposomal vesicles will be induced to micelles as the concentration of bile salts increases, which will affect the structure of liposomes and lead to drug leakage. However, a common edge activator such as sodium cholate can render the transfersomes more resistant to bile salt disruption in the small intestine, mainly due to the repulsion effect of sodium cholate in the lipid bilayer of the transferosomes and the bile salts in the intestinal tract [132]. Hu et al. studied the integrity and stability of liposomes containing sodium glycocholate and conventional liposomes in simulated GI medium as well as isolated rat GI medium. The results showed that there is a significant increase in calcein release in media with low pH or physiological bile salts, and liposomes containing sodium glycocholate showed the greatest drug retention in different simulated release media compared with conventional liposomes [152]. For transfersomes, the hydrophilic head of the edge activator will gather with the hydrophilic head of the phospholipid and quickly fill the broken part of the lipid bilayer to maintain stability as much as possible. The lipid bilayer structure is destroyed at the initial stage, which may be one of the reasons why the transfersomes can maintain the integrity of the lipid bilayer vesicle structure [153]. In addition, the fatty acid radical anions ionized by common anionic surfactants in transfersomes (such as sodium cholate) may hinder the diffusion of H cations into the inner aqueous phase, thereby maintaining the structural stability of transfersomes in acidic environments [154]. Beyond the antagonism effect against exogenous bile salts to maintain the structural stability of the vesicular drug delivery systems, bile salts have also been proven to reduce enzyme degradation of drugs. Niu et al. [155] prepared sodium glycocholate-containing liposomes by reverse-phase evaporation. The results indicated that the presence of sodium glycocholate reduced the enzymatic degradation of loaded insulin by pepsin, trypsin and α-chymotrypsin, which is consistent with previous findings by Yamamoto and Shinichiro et al. [156,157]. In addition to bile salts, other enzyme inhibitors can be added to liposomes to inhibit the enzyme metabolism of protein-based drugs. On the other hand, phospholipids with high phase transition temperatures have been reported to have rigid membranes at physiological temperatures, which help to resist the factors that can destabilize the membrane structure in GI. For chitosomes, the spatial site resistance induced by the polymer coating on the surface or the aqueous layer formed by the polymer may also allow the liposome membrane to be separated from the harsh GI environment, thereby improving the stability of the membrane structure. Recently, selenized liposomes were prepared by deposition of selenium onto the outside and inside of lipid bilayers by the thin film dispersion-in situ reduction technique, which was also shown to significantly enhance the stability of liposomes in the GI tract, providing a novel insight into boosting the stability of liposomes [158]. In short, the vesicular carriers available for oral drug delivery have been designed to enhance vesicle stability in the GI tract to a certain extent by different means.
3.2. Increasing solubilityThere is a relatively immobile water layer with a depth of 100–800 µm close to the surface of the intestinal mucus layer, also called the unstirred water layer or the hydrostatic layer, which is the outermost component of the intestinal pre-epithelial diffusion barrier and mainly affects the intestinal absorption of lipid-soluble drugs [159]. Drugs loaded into vesicular carriers can pass through the unstirred water layer more efficiently. For one thing, lipid-soluble drugs can be encapsulated in the space between vesicle bilayers close to the hydrophobic tails of the amphiphilic molecules; the hydrophilic head of these molecules gathers outside; this amphiphilic drug-loading structure can improve the water solubility of the lipid-soluble drug [160]. In addition, the phospholipids and other amphiphilic molecules in vesicular drug delivery systems can form mixed micelles with the increase of bile salt concentration in the intestinal tract, thus promoting the dissolution of poorly soluble drugs. Tang et al. [161] prepared pueraria flavone-loaded liposomes with the addition of sodium deoxycholate using the reverse-phase evaporation method, and the oral absorption of pueraria flavone was improved due to the enhanced water solubility. In the study of Islam et al., the solubility of ebastine in water could reach 17.9 µg/mL, which increased by up to 751% compared with a pure drug when loaded in the bilosomes prepared by the film hydration method [162]. In addition, it has been reported that sodium taurocholate can interact with drug molecules to inhibit the aggregation and crystallization of drugs, thereby maintaining the stability of the supersaturated system to dissolve more drugs [163]. These findings suggest that vesicular drug carriers have the potential to enhance the solubility of poorly soluble drugs in the intestinal tract, which allows them to better penetrate the unstirred water layer, thus improving the amounts of poorly soluble drugs being absorbed.
3.3. Improving mucus adhesion and penetrationThe mucus layer, another barrier covering the surface of the intestinal epithelial cells, is mainly composed of water (90%–95%), electrolytes, lipids (1%–2%) and mucins (1%–5%). Among them, mucin is the most important functional component of the mucus layer, and the common one is Mucin-2 secreted by intestinal epithelial goblet cells, which determines the most basic and dominant viscoelasticity of the mucus layer with an overall appearance of a highly hydrous gel [164-166]. Previous studies found that the mucus layer can be divided into an inner mucus layer and an outer mucus layer. The inner mucus layer with greater viscoelasticity is continuously secreted by goblet cells and closely connected with intestinal epithelial cells; the outer mucus layer is relatively loose and contains more water, so it appears less viscoelastic. Collectively, the surface of intestinal epithelial cells is covered with a double mucus layer with a dense bottom and a sparse surface. Studies have shown that the hydrophobicity and negative surface charge of the mucus layer are the major factors limiting drug penetration [167,168]. Mucus has a high turnover rate (between 50 and 270 min); therefore, the drug carrier must be able to diffuse over this barrier to prevent quick mucus clearance and reach the intestinal epithelium, in addition to entering the mucus layer [169]. For vesicular drug delivery systems, especially chitosomes, ionic interactions between the positively charged polymers carried on their surface and the negatively charged components of the mucus layer make it easier for chitosomes to adhere to the mucus layer, prolonging the exposure of the vesicles in the small intestine and increasing the chance of absorption [122,170]. The prolonged half-life of the loaded drug observed after oral administration of chitosomes can be attributed to the prolonged drug retention time due to mucosal adhesion. Ezzat et al. enhanced mucosal adhesion by preparing chitosomes loaded with catechin, and the chitosomes exerted a high value of area under the curve (AUC), 1.37-fold higher than uncoated liposomes and 2.12-fold higher than the control solution [129]. Therefore, mucosal adherence ability is supposed to be considered for subsequent oral vesicular drug delivery systems. In addition to adhesion ability, vesicular carriers also need to improve their ability to cross the mucus layer because of the need to overcome the rapid turnover clearance of the mucus layer. Modification of polymers containing PEG chains, such as Pluronic F127, on the surface of vesicular delivery systems has been shown to enhance their mucus-penetrating ability due to the hydrophobic and electrostatic interactions of liposomes modified with Pluronic F127 with mucins being significantly attenuated [171]. For transfersomes and bilosomes, anionic surfactants and bile salts such as sodium cholate have been shown to be effective in reducing the viscosity and elasticity of the mucus layer, which may be helpful for drug penetration [172]. It has been reported that bile salts and lecithin can reduce the viscosity of the mucus layer by 70%–100% under some extreme conditions, such as bile reflux. which may be a commonality of the anionic surfactants added to the vesicular drug delivery systems [173].
3.4. Enhancing intestinal epithelium crossingIn general, the intestinal epithelium, which consists of a variety of cell types, is another barrier to drug absorption under the mucus layer. The intestinal epithelium mainly includes the most abundant absorptive enterocytes, mucus-secreting goblet cells, endocrine cells, and the subepithelial region associated with gut-associated lymphoid tissue (GALT). This region is called Peyer's patches, which are rich in dendritic cells, lymphocytes, and macrophages [174,175]. In addition, the patches are covered by follicle-associated epithelium (FAE) with a different cellular pattern. FAE is devoid of goblet and endocrine cells but contains enterocytes and M cells, which have shorter microvilli, less mucus, and less lysosomal activity. Therefore, FAE shows better transcytosis potential, and M cells are the main route for introducing foreign substances into lymphoid tissues [176]. Drugs are mainly transported through the epithelium via the transcellular and paracellular routes. The transcellular routes mainly include passive diffusion (lipid-soluble small molecules are usually absorbed by passive diffusion) and energy-dependent uptake (pinocytosis and endocytosis) [151,177]. The paracellular pathway is applicable to hydrophilic molecules that cannot diffuse across epithelial cells [178]. However, for the paracellular pathway, the plasma membranes between adjacent epithelial cells are tightly bound to form an important junctional complex consisting of transmembrane protein families (occludin and claudin) and peripheral membrane protein families (ZO proteins), etc., which plays an important role in the paracellular transport barrier and maintains cell polarity [179].
For the transcellular routes, vesicular drug delivery systems such as liposomes, chitosomes, and transefersomes have good compatibility between their lipid bilayers and cell membranes; they are able to naturally fuse with the cell membranes and thus facilitate the cellular uptake of the drug, wherein the chitosomes, due to the positively charged chitosan coating on their surfaces, have an enhanced ability to adhere to the cell surfaces, further resulting in the fusion of vesicles in contact with the cell membranes [120,180]. On the other hand, drug-loaded vesicular carriers containing bile salts can combine with organic cations to form a lipophilic ion-pair complex, thereby enhancing the membrane permeability of hydrophilic drugs. Song et al. prepared salmon calcitonin-loaded transfersomes with sodium cholate as the edge activator, which has been proven to enhance calcitonin oral absorption by forming the lipophilic complex [117]. Furthermore, bile salts can change the distribution of lipids and proteins in the cell membrane at a lower concentration, reduce the order of the arrangement of phospholipid molecules in the cell membrane, thus increasing the cell membrane permeability, which is also conducive to the transmembrane transport of hydrophilic drugs. Notably, bile salts such as sodium cholate at high concentrations may directly combine with membrane phospholipids to cause membrane dissolution [181]. For another, bile salts are usually highly soluble in the bilayers of vesicular drug systems, which can form reverse micelles under a series of digestions in the GI tract. The inside of the reverse micelles is hydrophilic, and the outside is lipophilic, which may facilitate the penetration of hydrophilic drugs through the cell membrane [182]. Apart from the bile salts, nonionic surfactants used in niosomes, bilosomes and transfersomes are have also been reported to enhance the transcellular transport of drugs. Deshmukh et al. have prepared cromolyn-loaded proliposomal bead formulations coated with phospholipid-cholesterol-surfactant (Tween 80) systems, and further research found that it can significantly enhance the transport of cromolyn, especially when the concentration of the surfactant is low. And there is no evidence of damage to Caco-2 monolayers (e.g., a marked decrease in transmembrane electrical resistance (TEER) value), which suggests that the surfactants may enhance drug transport via enhanced transcellular pathways rather than tight junction modulation or cellular disruption [183]. The possible reason for this is that surfactants can modulate the fluidity of cell membranes, and the ability of Tween 80 and Cremophor EL to fluidize cellular phospholipid bilayers has been reported [184]. In addition, receptor-mediated endocytosis (clathrin-mediated endocytosis or caveolae-mediated endocytosis) is also one of the common mechanisms by which vesicular carriers enhance the transcellular routes. Receptor-mediated endocytosis or liposome accumulation at the site of uptake can be accomplished through the modification of targeting ligands on the surface of the vesicle, and the commonly used ligands are folic acid, exogenous lectin, biotin and mannose [70].
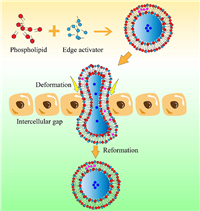
For the paracellular routes, bile salts, a functional ingredient commonly used in vesicular drug delivery systems, can bind Ca2+, affect the distribution and function of myosin, and open the tight junction between intestinal epithelial cells. At the same time, the increase of ions passing through the cell bypass will lead to a decrease in the TEER of the Caco-2 cell monolayer model [185], The specific mechanism is that sodium cholate produced a change in the distribution of ZO-1, claudin-1, occludin and E-cadherin, the normal distribution of the proteins was interrupted, and the proteins detached from the cytomembrane and entered the cytoplasm, which is consistent with the findings of Wang et al. [186]. In addition, some researchers have prepared insulin-loaded sodium cholate liposomes that can significantly reduce the TEER value of the Caco-2 cell monolayer model, and the TEER value gradually returned to normal after removing the liposomes, which suggests that the effect of sodium cholate on tight junctions is reversible [187]. In addition, a number of studies have demonstrated that surfactants commonly used in vesicular drug carriers are also capable of opening or modulating tight junctions and facilitating the paracellular transport of drugs [188-190]. As the classical deformable vesicles, transfersomes can more easily cross tight junctions between cells that have been opened compared to other oral vesicle systems due to their high degree of deformability (Fig. 5). It has been confirmed that the transfersomes can pass through a gap whose size is 1/10–1/5 of its own, and the drug-loading structure hardly changes after permeation [111,191]. This may allow more drugs to penetrate the intestinal epithelial cells because of their greater structural stability, thereby enhancing their drug intestinal absorption.
|
Download:
|
| Fig. 5. Deformable vesicle carriers such as transfersomes utilize deformability through intercellular tight junctions. | |
Some drugs can gain access to the systemic circulation via intestinal lymphatic routes in addition to being absorbed by the mesenteric blood capillaries [192]. In general, drugs mainly enter the blood circulation through the hepatic portal vein because the flow rate of blood is about 500 times faster than that of the lymphatic fluid in the intestinal lymphatic vessels [193]. However, drug-containing chylomicrons can enter the mesenteric lymphatics, move to the thoracic duct, and subsequently enter the systemic circulation at the junction of the left jugular vein and the left subclavian vein, which can effectively avoid hepatic first-pass metabolism, and thus improve drug bioavailability [194,195]. As mentioned above, drugs loaded into vesicular delivery systems can self-assemble into a drug-containing mixed micelle that can freely diffuse into the unstirred water layer after a series of digestions. Generally, micelles cannot be absorbed in a complete form, and most of them are absorbed in the form of free fatty acids, monoglycerides, and drugs. Thereafter, long-chain fatty acids are re-esterified in the smooth endoplasmic reticulum of small intestinal epithelial cells to form triglycerides, which will combine with the initial lipoprotein produced by the rough endoplasmic reticulum, and then the nucleus expands to form chylomicrons that can selectively enter the capillary lymphatics after being secreted from cells [196]. The lymphatic absorption of drugs can be enhanced if the drug is combined with the triglycerides in the chylomicron nucleus. For vesicular drug delivery systems, most of the lipophilic groups of the surfactants used in niosomes, transfersomes and bilosomes are long-chain fatty acids. This may provide more raw materials for the production of chylomicrons, and also increase the combination of drugs with chylomicrons. In addition, uptake through M cells in the intestinal epithelium is one of the mechanisms that enhances the lymphatic absorption of drugs [197]. It has been reported that M cells are able to transport a variety of particles from the intestinal lumen to the lower lymphoid tissues, including vesicles that survive a series of digestive processes. Since M cells do not secrete mucus, they are least protected by mucus above them (Fig. 4) and have more access to drug-loaded vesicles. It has also been investigated to enhance drug lymphatic uptake by modifying lectins on the vesicle surface to target M cells [198]. The enhanced lymphatic absorption has a positive significance for improving the drug's oral bioavailability.
3.6. Reducing intestinal effluxThere is still a risk of being pumped back into the intestinal lumen after the drug is absorbed by intestinal epithelial cells, which is known as the efflux effect of the intestine [199]. The transmembrane protein P-glycoprotein (P-gp), located at the top of intestinal epithelial cells, is a typical efflux drug pump that can energy-dependently efflux drugs back to the intestinal lumen by hydrolyzing ATP, which can reduce the oral bioavailability of some drugs that can be considered substrates of P-gp [200,201,175]. For vesicular drug delivery systems such as liposomes, bilosomes, and transfersomes, the bile salts incorporated in the vesicles, such as sodium cholate, have been shown to inhibit the efflux effect of P-gp and enhance the drug's intestinal absorption [202]. In addition, some nonionic surfactants that are commonly used in niosomes or as edge activators in transfersomes also have the effect of inhibiting P-gp, especially the polysorbate surfactants. Previous studies explored the effects of a series of surfactants on the intracellular accumulation and intestinal absorption of epirubicin via the Caco-2 cell model and the eversion intestinal sac method (rat jejunum and ileum intestine). The results indicated that Tween 20 and Tween 80 can significantly increase the basolateral uptake of Caco-2 cells and the serosal absorption of epirubicin in rat jejunum and ileum [203]. The specific mechanism is that these surfactants inhibit intestinal P-gp, multidrug resistance-related protein families or other transport proteins, thereby reducing the intestinal efflux effect, and the optimal hydrophilic-lipophilic balance value of surfactants with suitable hydrocarbon chains and polar groups is the key factor for inhibiting the intestinal efflux.
4. Challenges hindering vesicular drug delivery systems for oral administrationClinical application is the ultimate goal for the exploration of vesicular drug carriers. Indeed, vesicular drug delivery systems have been well studied and have shown promising results in the field of oral delivery during the last decades. However, the clinical translation of vesicular drugs is not optimistic. We summarized the typical vesicular carriers related product that have been approved in Table S6 (Supporting information). It can be found that the only vesicular drug delivery system that has been marketed for clinical treatment is liposomes. Moreover, these liposome-based formulations are mostly focused on intravenous injection, and there are few vesicular drug delivery formulations that have been approved for oral drug delivery, which indicates that the vesicular carriers available for oral drug delivery still face great challenges. An analysis of this situation reveals that, similar to a lot of nanocarrier-based drug delivery systems, the main challenges facing the vesicular carrier are safety, unspecified in vivo fate, and scale-up production, which are also unavoidable problems during the process of moving drug formulations towards clinical applications.
4.1. SafetyFor vesicular carriers applied to oral drug delivery, formulation safety is mainly related to the toxicity of the prescription compositions in the GI tract. Phospholipids, the necessary material for the bilayer formation of liposomes and chitosomes, have been widely reported to have good biocompatibility and biodegradability [204,205]. It was noted that no significant toxicity was observed in acute, chronic, reproductive, and mutagenic toxicity tests following all routes of highly purified phosphatidylcholine. In addition, fully saturated natural phosphatidylcholine is also safe when employed for oral drug delivery, but high-dose administration for several weeks can cause elevations in plasma cholesterol [206]. Chitosan and bile salts, the two essential functional components of chitosomes and bilosomes, have also been extensively proved to be relatively safe and usually nontoxic at routine doses [207,208]. These two components will not be the toxicity source of the vesicular drug delivery system in general. However, surfactants used in vesicular drug delivery systems are most likely to lead to safety issues. Nonionic surfactants can be added as vesicle bilayer-forming molecules in niosomes and bilosomes, and can also be incorporated into transfersomes as edge activators, showing a wide range of applications in vesicular drug delivery systems. Therefore, the analysis of surfactant toxicity is of great significance to the safety assessment of vesicular drug carriers. It has been claimed that anionic surfactants are less toxic to humans with increasing molecular weight, which may be related to the lower adsorption of anionic surfactants in the intestine. Therefore, it is unlikely that anionic surfactants will have acute toxic effects, but chronic effects are more likely, as daily intake in humans can be as high as 5 mg per person [209]. Several cytotoxicity tests reveal the toxicity of surfactants in the order of cationic, anionic, amphoteric, and nonionic [210]. Furthermore, it has been suggested that surfactants could damage the cell membranes, thus causing structural and consequent permeability changes in epidermis [211]. Ujhelyi et al. found that polysorbate 20, 60 and 80 were relatively toxic among the nonionic surfactants using the Caco-2 monolayer model. In the case of caprylocaproyl polyoxyl-8 glycerides, a kind of nonionic oil-in-water surfactant, the degree of esterification and the lack of a sorbose component reduced its cytotoxicity. If the hydrophyl head is changed from polyethylene glycol to propylene glycol, the main determinants of cytotoxicity are monoester content and carbon chain length [212]. In addition, the correlation between surfactant molecular structure and cytotoxicity suggests that the size of the polyethyleneoxide moiety is more important than the size of the hydrocarbon chain. More specifically, the presence of very long polyethyleneoxide groups (> 30 units) was found to result in reduced cytotoxicity [213]. Therefore, we can screen the structural characteristics of the surfactants used in prescriptions via the above aspects to reduce the potential toxicity of the formulations on the premise of ensuring their functions. In addition, choosing safer surfactants as bilayer-forming molecules is a favorable option. Dodecyl maltoside (DDM), a type of alkyl polyglycoside surfactants (green nonionic surfactants with high efficiency, low toxicity, and biodegradable advantages), has been approved by the Food and Drug Administration (FDA) as an excipient for the marketable formulation VALTOCOⓇ in 2020, confirming the safety of DDM [214]. Another safe natural surfactant is betaine, which, as a mild surfactant, also has anti-inflammatory properties and may serve as a molecular alternative for vesicle bilayer formation [215,216]. Cholesterol, the common vesicular bilayer membrane modulator, has a certain toxic effect in vivo. Kim et al. pointed out that high levels of cholesterol are cytotoxic and need to be strictly controlled [217]. These findings are similar to the results of the study by Yosie et al., which mentioned that cholesterol would cause toxicity in the rat's liver if more than 3% of cholesterol was used in the diet [218]. Although the amount of cholesterol added is usually not too high in vesicular drug carriers, it is important to pay attention to cholesterol during the evaluation of its safety and to screen the amount of cholesterol in the prescription, thereby reducing its impact on the GI tract. At present, most of the research tends to modify the functional groups on the surface of the vesicle system to make it more suitable for drug delivery; therefore, it is necessary for the safety evaluation of the introduced functional materials. For example, polyethylene glycol (PEG)-modified liposomes with stealth function can avoid premature destruction of liposomes by phagocytosis by increasing the repulsion between liposomes and serum. Although PEG has been reported to have good biocompatibility, but with the deepening of the study still found that its absorption into the bloodstream will induce immune reactions, and repeated administration of PEGylated liposomes will lead to severe accelerated blood clearance. Further, it has also been reported that PEG may also cause pseudoallergy related to complement activation [219,220]. And polylactic acid-hydroxyacetic acid copolymer (PLGA), a European Medicines Agency (EMA) and FDA-approved drug and medical device, which still hydrolyzes in vivo to produce lactic acid that can lower the pH of the surrounding environment and eventually cause an amelioration of the inflammatory response [221]. Therefore, for the vesicular carriers available for oral drug delivery with the plan of surface modification, a comprehensive safety evaluation of the modifying materials is required, and it should be eliminated to use the materials that have been reported to have serious adverse effects.
4.2. Unspecified in vivo fateOver the past decades, although there has been a great deal of research on the effectiveness, safety, stability and manufacturability of vesicular drug delivery systems, the in vivo fate and underlying mechanisms of these drug carriers remain less explored, limiting progress toward commercialization [222]. In addition, the unspecified in vivo fate affects the judgment of the effectiveness of the formulation, which largely hinders the application of vesicular carriers for oral drug delivery and increases the resistance to clinical translation of these formulations. This is consistent with Ren et al.'s review of vesicular drug delivery systems, where they state that in vivo fate affects the performance of vesicular formulations and that the most important factors impacting the efficiency of oral drug delivery are premature release and structural stability, which largely depend on the properties of both payloads and vehicles used [223]. According to the size, composition and drug-loading form of the vesicular drug delivery systems, they can be classified as nano-carrier drugs [224]. Indeed, the in vivo fate of these nano-carrier drugs for oral use has been surrounded by controversy for several years. Some researchers argue that the enhanced oral absorption is attributable to the vesicles crossing the intestinal epithelium as a whole, while others insist that these drug-loaded vesicles are absorbed in the GI tract after a series of digestive processes to form other drug-loaded carriers (e.g., mixed micelles) that differ from the initial form [225]. Therefore, clarifying the in vivo fate of the vesicular drug delivery system is of great significance in explaining the mechanisms by which the formulation enhances drug absorption, as well as the safety and stability evaluation. Initially, in vivo analysis of drugs was performed by conventional liquid chromatography and liquid chromatography-mass spectrometry (LC-MS/MS) techniques. By determining the amount of drug in plasma or tissue, the possible in vivo processes of orally administered drugs were inferred. However, these methods do not accurately determine the form in which the drug is present, and therefore require pre-treatment of plasma/tissue samples prior to determination. For example, Su et al. analyzed the pharmacokinetic behavior of amphotericin B liposomes by separating carrier-bound amphotericin B and freely released amphotericin B from plasma by solid-phase extraction columns, and then determining the drug content using LC-MS/MS, respectively [226]. Although these analytical methods can determine to some extent how much drug is absorbed, the in vivo fate of the drug prior to absorption is still unknown. In order to elucidate this issue, various biosensing tools such as fluorescent imaging, computed tomography, and magnetic resonance imaging have been employed to explore the in vivo fate of vesicular drug delivery systems [227-229]. Further, the fluorescent-based tracer technology has been widely studied due to its flexible controllability, negligible toxicity and superior photostability [230]. Tang et al. reported novel fluorophores with aggregation-induced emission (AIE) properties [231]. And when fluorescein in solution reaches high localized concentrations and forms aggregates, both conventional fluorescein fluorophores and novel fluorescent nanomaterials may suffer a strong π-π stack, leading to a significant decrease in fluorescence intensity in the process called "aggregation-induced quenching" (ACQ) [232]. Gratifyingly, researchers have developed a series of fluorescent probes based on the principles of AIE and ACQ for exploring the in vivo fate of nano-carrier drugs and have made promising progress in recent years. Wu et al. developed a series of near-infrared ACQ fluorescent probes with sensitive water quenching properties, effectively eliminating the interference of free probes on the signals from labeled intact particles, thereby realizing the accurate resolution of the in vivo spatiotemporal fate of nano-carrier drugs. These novel probes have been successfully applied to exploring the in vivo fate of various nanocarriers via different administration routes, including nanoemulsion [233], injectable fat emulsions [234], polymeric nanoparticles [235,236], solid lipid nanoparticles [237], ultrafine drug particles [238], nanosuspensions [239] and polymer micelles [240,241], etc. The schematic of the ACQ effect (Fig. 6A) and some results of the in vivo fate studies of the above nanocarriers are summarized in Fig. 6. Hu et al. found that the water-quenching dyes (P2) was able to report the in vivo fate of lipid nanocarriers more accurately compared to the conventional non-water-quenching dyes (DiR), suggesting that water-quenching dyes can serve as better tracer molecules (Fig. 6B) [242]. In addition, Wu et al. proposed the concept of the absolute aggregation-caused quenching effect for the first time based on these studies [243]. Using aza-BODIPY, the classical ACQ probe, as the parent, by enhancing its hydrophobicity, planarity and rigidity, they synthesized the novel fluorescent probes FD-B21 and FD-C7 [244], which exhibited better near-infrared emission, high quantum yield, photostability, water sensitivity, and negligible re-illumination compared with conventional probes, thereby enhancing the accuracy of these probes in exploring the in vivo fate of the nanocarriers. The specific mechanism can be explained using the example of polymer micelles. The ACQ probe possesses a strong and stable fluorescence signal when the water-quenched probe is encapsulated in the hydrophobic core of the polymer micelles. Once the micelles are depolymerized or degraded, the probe is released and then aggregates in water, and its fluorescence is rapidly quenched due to the ACQ effect. Thus, the interference of the free probe signal can be completely eliminated, and the signal can be tracked in real time from the intact polymer micelle particle. The final experimental results support integral polymeric micelles across the enteric epithelia, but the total amount may be limited (Fig. 6D) [240]. This result is similar to a previous report that bilosomes surviving the GI environment can be absorbed as intact vesicles (Fig. 6C) [155,187]. On the other hand, benefiting from these fluorescent probe technologies, researchers can conduct more in-depth studies, such as using the fluorescent resonance energy transfer (FRET) probes (DiO and DiI) to investigate the effect of nanoparticle shape on cellular uptake, and the results demonstrated that more nanorods were internalized into Caco-2 cells than nanospheres (Fig. 6E) [245].

|
Download:
|
| Fig. 6. (A) The schematic diagram of the principle of water quenching ACQ fluorescent probe. (B) Comparison of in vitro and in vivo tracing of water-quenched ACQ fluorescent probes and conventional probes. Reproduced with permission [242]. Copyright 2015, Elsevier Science Ltd. (C) Fluorescence images of bilosomes and conventional liposomes in the intestine. Reproduced with permission [187]. Copyright 2014, Elsevier Science Ltd. (D) Cumulative trans-monolayer transport of P2-labeled polymer micelles in Caco-2, Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B monolayers. Reproduced with permission [240]. Copyright 2020, Elsevier Science Ltd. (E) Differences in the exocytosis of nanorods and nanospheres by Caco-2 cells with prolonged incubation time. C0–C3 are images of cellular uptake, exocytosis of nanospheres at 15 min, 30 min and 2 h, respectively; D0–D3 are for nanorods. Reproduced with permission [245]. Copyright 2018, Elsevier Science Ltd. | |
The suitability of the preparation process of the vesicular oral drug delivery systems for scale-up production is also critical for successful clinical translation. The main factors surrounding the scale-up production of formulations are the scalability of the manufacturing process and the reliability as well as reproducibility of the final product [246]. Currently, most of the conventional preparation methods for vesicular drug carriers, such as thin film dispersion, reversed-phase evaporation, complex emulsion, and solvent injection, are only suitable for small-scale production, while their direct application to scale-up production may cause problems such as uneven particle size distribution, poor reproducibility between batches, high production costs and organic solvent residues. As mentioned above, although some vesicle-based formulations have been marketed, the batch-to-batch variation affects the clinical application of the drug. A continuous high-pressure homogenization device has been developed for the scale-up production of liposomes encapsulating plasmid DNA to overcome the problem of particle size inhomogeneity. However, drug leakage under high pressure is serious, and the application of this method in large-scale production is not successful due to the complicated production process and high cost [247-249]. Analysis of marketed vesicle-based formulations reveals that they are all parenteral routes of administration, which make it easier to achieve clinically effective doses, while higher doses and longer courses of treatment are usually required for oral administration. Therefore, vesicular drug delivery systems usually incorporate some functional ingredients through various formulation techniques in order to achieve better drug delivery efficacy, thereby fulfilling the requirements of oral drug delivery. However, the complexity of the preparation process will increase the cost of production, raise the requirements for the equipment and sites, and ultimately increase the difficulty of drug feasibility assessment. Moreover, the cumbersome preparation methods will also complicate the pharmacokinetic, pharmacodynamic, and toxicological evaluation of the formulations after oral administration, which is a serious impediment to preparation and process optimization [250-252]. Accordingly, it is supposed to minimize the introduction of non-essential functional materials in future formulation design on the premise of guaranteeing the effectiveness of vesicle-based drug delivery systems. For example, Arikayce™, a liposome of butylcarbamazine approved in 2018, has a prescription composition of only dipalmitic acid phosphatidylcholine and cholesterol. This formulation enhances the efficacy of the drug in lung disease while reducing its toxicity, which indicates that a simple formulation design can also efficiently improve the clinical translation of the drug to a great extent. For the issue of reproducibility in scale-up production, a thorough understanding of the formulation key components and their interactions is required, which is helpful to define the critical characteristics of the product. These characteristics can be utilized as evaluation metrics in the early development of vesicular drug delivery systems. For example, the effect of organic solvents, ultrasound, high-speed homogenization, emulsification, evaporation of organic solvents, and other steps commonly used in preparation on the final formulation. Consideration should be given at the "small scale" stage as to which methods will be beneficial in scaling up the product formulation, and early identification of these characteristics will also assist in the selection of appropriate scale-up production methods to establish critical process steps and analytical criteria to ensure product reproducibility [253]. Encouragingly, various preparation methods have been optimized and improved to meet the requirements of scale-up production. Roces et al. prepared PEGylated liposome empty shells encapsulating ammonium sulfate via the microfluidic method, and then doxorubicin was actively loaded into these pre-formed liposome shells. The functionalized liposomes obtained by this method can overcome the tediousness of multi-container and batch-processing-based technology [254]. Further, the microfluidic velocity does not affect the properties of the liposomal product, thus allowing high speeds in scale-up production, and the liposomes prepared in large batches by this method have a consistent release profile with that of the marketed product, indicating that the method was successful in realizing vesicular drug delivery for scale-up production. In order to reduce production costs and increase safety, studies have successfully developed suitable methods for the scale-up production of conventional liposomes without the need for many manufacturing steps or the use of organic solvents [255,256].
5. Conclusions and outlookDue to their unique drug-loading structures and functional characteristics, vesicular drug delivery systems have attracted wide attention in the field of drug delivery with different administration routes. Summarizing the relevant studies, it was found that liposomes, niosomes, transfersomes, chitosomes, and bilosomes are the most promising carriers that can enhance oral absorption, exhibiting desirable performance as oral drug carriers in the last decades. This review describes the formation, prescription composition, and drug delivery advantages of these vesicular drug delivery systems. Further, the possible mechanisms for enhancing the oral absorption of drugs are also analyzed based on the research examples. Although these vesicles-based drug carriers demonstrate a favorable potential for oral drug delivery, their clinical translation remains unimpressive. According to the analysis, it was found that these carriers still face pressing issues such as safety, unspecified in vivo fate, and scale-up production. In particular, the elucidation of the in vivo spatiotemporal fate of the formulation is crucial to explaining the oral drug delivery advantages of the dosage form. Various fluorescent-based probes as tracing tools have been developed for exploration of drug in vivo fate in recent years, which, to a certain extent, unveil the in vivo fate of vesicular drug formulations. Indeed, there are still undefined mechanisms of oral drug digestion and absorption due to the complex organismal environment. It is important to note that future research should not only focus on regulating the composition of the vesicle bilayer or modifying the surface of vesicles with polymers or ligands to optimize the in vivo fate of vesicles-based drug carriers, but also on the principles of simplicity and effectiveness in the design of vesicular drug prescriptions and preparation processes, which can minimize the resistance to final review of drug formulations as well as the possibility of scale-up production. We believed that in the future, with the development of multidisciplinary cross-fertilization, the improvement of basic theories, and the breakthroughs of technological bottlenecks, there will be a more comprehensive and in-depth understanding of vesicular drug delivery systems and their application principles, which will guide researchers to overcome the existing challenges and achieve long-term development in the field of oral drug delivery.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was funded by the National Natural Science Foundation of China (No. 81960717), the project of academic and technical leaders in major disciplines in Jiangxi Province (No. 20212BCJL23060), the Guangxi science and technology base and talent project (No. Guike AD20238058), the Jiangxi University of Chinese Medicine science and technology innovation team development program (Nos. CXTD-22004, CXTD-22008) and the PhD startup foundation of the Affiliated Hospital of Jiangxi University of Chinese Medicine (No. 23KYQDZJ02).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109129.
| [1] |
Z. Vinarov, B. Abrahamsson, P. Artursson, et al., Adv. Drug Deliv. Rev. 171 (2021) 289-331. DOI:10.1016/j.addr.2021.02.001 |
| [2] |
L. Gao, G. Liu, J. Ma, et al., Pharm. Res. 30 (2013) 307-324. DOI:10.1007/s11095-012-0889-z |
| [3] |
R.P. Dixit, S.P. Puthli, J. Control. Release 139 (2009) 94-107. DOI:10.1016/j.jconrel.2009.06.014 |
| [4] |
Z. Liang, M. Chen, Y. Yan, D. Chen, S. Xie, Nanomaterials 12 (2022) 3032. DOI:10.3390/nano12173032 |
| [5] |
Y. Huang, S. Deng, X. Luo, et al., Int. J. Nanomedicine (2019) 9721-9730. |
| [6] |
L.H. Qian, N.G. Li, Y.P. Tang, et al., Int. J. Mol. Sci. 12 (2011) 8208-8216. DOI:10.3390/ijms12118208 |
| [7] |
S. Jin, C.H. Lee, D.Y. Lim, et al., Pharmaceutics 13 (2021) 1022. DOI:10.3390/pharmaceutics13071022 |
| [8] |
Y. Lu, K. Park, Int. J. Pharm. 453 (2013) 198-214. DOI:10.1016/j.ijpharm.2012.08.042 |
| [9] |
T.D. Brown, K.A. Whitehead, S. Mitragotri, Nat. Rev. Mater. 5 (2020) 127-148. |
| [10] |
F. Zhao, X. Sun, W. Lu, et al., Drug Deliv. 27 (2020) 216-227. DOI:10.1080/10717544.2020.1716879 |
| [11] |
V. Mishra, P. Nayak, N. Yadav, et al., Expert Opin. Drug Deliv. 18 (2021) 315-332. DOI:10.1080/17425247.2021.1856073 |
| [12] |
J.A. Finbloom, F. Sousa, M.M. Stevens, T.A. Desai, Adv. Drug Deliv. Rev. 167 (2020) 89-108. DOI:10.1016/j.addr.2020.06.007 |
| [13] |
S. Barua, S. Mitragotri, Nano Today 9 (2014) 223-243. DOI:10.1016/j.nantod.2014.04.008 |
| [14] |
L. Zou, Z. Zhang, J. Feng, et al., J. Pharm. Sci. 111 (2022) 1776-1784. DOI:10.1016/j.xphs.2022.03.013 |
| [15] |
Y. Pan, Y.J. Li, H.Y. Zhao, et al., Int. J. Pharm. 249 (2002) 139-147. DOI:10.1016/S0378-5173(02)00486-6 |
| [16] |
X. Xie, Q. Tao, Y. Zou, et al., J. Agric. Food Chem. 59 (2011) 9280-9289. DOI:10.1021/jf202135j |
| [17] |
H. Xing, H. Wang, B. Wu, X. Zhang, Curr. Pharm. Des. 23 (2017) 6705-6713. |
| [18] |
R. Liu, C. Luo, Z. Pang, et al., Chin. Chem. Lett. 34 (2023) 107518. DOI:10.1016/j.cclet.2022.05.032 |
| [19] |
J. Yuan, M. Guo, S. Zhao, et al., Chin. Chem. Lett. 34 (2023) 107943. DOI:10.1016/j.cclet.2022.107943 |
| [20] |
Y. Cui, T. Zhu, X. Zhang, et al., Chin. Chem. Lett. 33 (2022) 4617-4622. DOI:10.1016/j.cclet.2022.03.077 |
| [21] |
S.A. Fouad, F.A. Malaak, M.A. El-Nabarawi, Abu Zeid, PLoS One 15 (2020) e0244646. DOI:10.1371/journal.pone.0244646 |
| [22] |
M.A. Shaker, H.M. Elbadawy, M.A. Shaker, Int. J. Pharm. 574 (2020) 118891. DOI:10.1016/j.ijpharm.2019.118891 |
| [23] |
Y. Hou, H. Wang, F. Zhang, et al., Int. J. Nanomedicine (2019) 557-571. |
| [24] |
X. Zhang, H. Xing, Y. Zhao, Z. Ma, Pharmaceutics 10 (2018) 74. DOI:10.3390/pharmaceutics10030074 |
| [25] |
L. Zou, W. Ding, Q. Huang, et al., Biol. Pharm. Bull. 45 (2022) 1106-1115. DOI:10.1248/bpb.b22-00154 |
| [26] |
F. Gao, Z. Zhang, H. Bu, et al., J. Control. Release 149 (2011) 168-174. DOI:10.1016/j.jconrel.2010.10.013 |
| [27] |
F. Zeng, D. Wang, Y. Tian, et al., J. Pharm. Sci. 110 (2021) 2555-2561. DOI:10.1016/j.xphs.2021.02.030 |
| [28] |
M. Zhang, Q. Cao, Y. Yuan, et al., Chin. Chem. Lett. 35 (2024) 108710. DOI:10.1016/j.cclet.2023.108710 |
| [29] |
Q. Liu, M. Cheng, J. Liang, et al., J. Biomed. Nanotechnol. 16 (2020) 1160-1168. DOI:10.1166/jbn.2020.2956 |
| [30] |
L. Tu, G. Wang, N. Qi, et al., Int. J. Pharm. 578 (2020) 119105. DOI:10.1016/j.ijpharm.2020.119105 |
| [31] |
T. Yang, J. Feng, Q. Zhang, et al., Drug Deliv. 27 (2020) 575-584. DOI:10.1080/10717544.2020.1748762 |
| [32] |
C. Zhang, J. Li, M. Xiao, et al., Chin. Chem. Lett. 33 (2022) 4924-4929. DOI:10.1016/j.cclet.2022.03.110 |
| [33] |
I. Zoya, H. He, L. Wang, et al., Chin. Chem. Lett. 32 (2021) 1545-1549. DOI:10.1016/j.cclet.2020.09.038 |
| [34] |
W. Liu, M. Cheng, Z. Lu, et al., Drug Deliv. 29 (2022) 3432-3442. DOI:10.1080/10717544.2022.2149894 |
| [35] |
M. Cheng, F. Yuan, J. Liu, et al., AAPS PharmSciTech 21 (2020) 90. DOI:10.1208/s12249-019-1616-4 |
| [36] |
S. Han, X. Li, C. Zhou, et al., ACS Appl. Bio. Mater. 3 (2020) 4684-4695. DOI:10.1021/acsabm.0c00599 |
| [37] |
W. Liu, M. Cheng, F. Yuan, et al., J. Drug Deliv. Sci. Technol. 79 (2023) 104006. DOI:10.1016/j.jddst.2022.104006 |
| [38] |
K.T. Magar, G.F. Boafo, M. Zoulikha, et al., Chin. Chem. Lett. 34 (2023) 107453. DOI:10.1016/j.cclet.2022.04.051 |
| [39] |
R. Lombardo, T. Musumeci, C. Carbone, R. Pignatello, Pharm. Dev. Technol. 26 (2021) 824-845. DOI:10.1080/10837450.2021.1950186 |
| [40] |
E. Damiani, C. Puglia, J. Pharm. Sci. 108 (2019) 3769-3780. DOI:10.1016/j.xphs.2019.09.009 |
| [41] |
X. Zhang, W. Wu, Drug Discov. Today 19 (2014) 898-904. DOI:10.1016/j.drudis.2014.03.001 |
| [42] |
K.T. Magar, G.F. Boafo, X. Li, Z. Chen, W. He, Chin. Chem. Lett. 33 (2022) 587-596. DOI:10.1016/j.cclet.2021.08.020 |
| [43] |
A.D. Bangham, R.W. Horne, J. Mol. Biol. 8 (1964) 660-668. DOI:10.1016/S0022-2836(64)80115-7 |
| [44] |
A. Sharma, U.S. Sharma, Int. J. Pharm. 154 (1997) 123-140. DOI:10.1016/S0378-5173(97)00135-X |
| [45] |
J.Y. Kim, J.K. Kim, J.S. Park, Y. Byun, C.K. Kim, Biomaterials 30 (2009) 5751-5756. DOI:10.1016/j.biomaterials.2009.07.021 |
| [46] |
S. Shailesh, S. Neelam, K. Sandeep, G.D. Gupta, J. Pharm. Res. 2 (2009) 1163-1167. |
| [47] |
S. Kamboj, V. Saini, N. Magon, S. Bala, V. Jhawat, J. Drug Deliv. 5 (2013) 121-130. |
| [48] |
D. Nayak, V.K. Tippavajhala, Iran J. Pharm. Res. 20 (2021) 186-205. |
| [49] |
I. Pflueger, C. Charrat, C.O. Mellet, et al., Org. Biomol. Chem. 14 (2016) 10037-10049. DOI:10.1039/C6OB01882C |
| [50] |
C. Marianecci, L. Di Marzio, F. Rinaldi, et al., Adv. Colloid Interface Sci. 205 (2014) 187-206. DOI:10.1016/j.cis.2013.11.018 |
| [51] |
I.F. Uchegbu, A.T. Florence, Adv. Colloid Interface Sci. 58 (1995) 1-55. DOI:10.1016/0001-8686(95)00242-I |
| [52] |
J.N. Israelachvili, D.J. Mitchell, B.W. Ninham, J. Chem. Soc., Faraday Trans. 2 72 (1976) 1525-1568. DOI:10.1039/f29767201525 |
| [53] |
M.P. Nikolova, E.M. Kumar, M.S. Chavali, Pharmaceutics 14 (2022) 2195. DOI:10.3390/pharmaceutics14102195 |
| [54] |
A. Figueroa-Robles, M. Antunes-Ricardo, D. Guajardo-Flores, Int. J. Pharm. 593 (2021) 120125. DOI:10.1016/j.ijpharm.2020.120125 |
| [55] |
J. Yang, C. Jia, J. Yang, Int. J. Med. Sci. 18 (2021) 2943. DOI:10.7150/ijms.60874 |
| [56] |
J. Zhou, Y. Zhang, R. Wang, Giant 12 (2022) 100126. DOI:10.1016/j.giant.2022.100126 |
| [57] |
H.C. Verdera, J.J. Gitz-Francois, R.M. Schiffelers, P. Vader, J. Control. Release 266 (2017) 100-108. DOI:10.1016/j.jconrel.2017.09.019 |
| [58] |
K. O'Brien, S. Ughetto, S. Mahjoum, A.V. Nair, X.O. Breakefield, Cell Rep. 39 (2022) 110651. DOI:10.1016/j.celrep.2022.110651 |
| [59] |
S.A. Ejazi, R. Louisthelmy, K. Maisel, ACS Nano 17 (2023) 13044-13061. DOI:10.1021/acsnano.3c02403 |
| [60] |
V. De Leo, F. Milano, A. Agostiano, L. Catucci, Polymers 13 (2021) 1027. DOI:10.3390/polym13071027 |
| [61] |
N.T.T. Le, V.D. Cao, T.N.Q. Nguyen, et al., Int. J. Mol. Sci. 20 (2019) 4706. DOI:10.3390/ijms20194706 |
| [62] |
G. Bozzuto, A. Molinari, Int. J. Nanomedicine 10 (2015) 975-999. |
| [63] |
V.A. Frolov, A.V. Shnyrova, J. Zimmerberg, Cold Spring Harbor Perspect. Biol. 3 (2011) a004747. |
| [64] |
L. Zheng, B. Hu, D. Zhao, et al., Chin. Chem. Lett. 35 (2024) 108647. DOI:10.1016/j.cclet.2023.108647 |
| [65] |
A. Laouini, C. Jaafar-Maalej, I. Limayem-Blouza, et al., J. Colloid Sci. Biotechnol. 1 (2012) 147-168. DOI:10.1166/jcsb.2012.1020 |
| [66] |
B. Maherani, E. Arab-Tehrany, M.R. Mozafari, C. Gaiani, M. Linder, Curr. Nanosci. 7 (2011) 436-452. DOI:10.2174/157341311795542453 |
| [67] |
P. Nakhaei, R. Margiana, D.O. Bokov, et al., Front. Bioeng. Biotechnol. 9 (2021) 748. |
| [68] |
T.X. Nguyen, L. Huang, M. Gauthier, G. Yang, Q. Wang, Nanomedicine 11 (2016) 1169-1185. DOI:10.2217/nnm.16.9 |
| [69] |
R. Banerjee, J. Biomater. Appl. 16 (2001) 3-21. DOI:10.1106/RA7U-1V9C-RV7C-8QXL |
| [70] |
H. He, Y. Lu, J. Qi, et al., Acta Pharm. Sin. B 9 (2019) 36-48. DOI:10.1016/j.apsb.2018.06.005 |
| [71] |
T. Lian, R.J.Y. Ho, J. Pharm. Sci. 90 (2001) 667-680. DOI:10.1002/jps.1023 |
| [72] |
J. Liang, W. Wu, Q. Liu, S. Chen, Drug Deliv. 20 (2013) 319-323. DOI:10.3109/10717544.2013.834420 |
| [73] |
N. Kumar, A. Rai, N.D. Reddy, et al., Pharmacol. Rep. 66 (2014) 788-798. DOI:10.1016/j.pharep.2014.04.007 |
| [74] |
Z. Song, J. Yin, P. Xiao, et al., Pharmaceutics 13 (2021) 132. DOI:10.3390/pharmaceutics13020132 |
| [75] |
E. Yamazoe, J.Y. Fang, K. Tahara, Int. J. Pharm. 593 (2021) 120148. DOI:10.1016/j.ijpharm.2020.120148 |
| [76] |
Q. Wang, W. Liu, J. Wang, H. Liu, Y. Chen, Nanoscale Res. Lett. 14 (2019) 321. DOI:10.1186/s11671-019-3164-y |
| [77] |
S. Sarhadi, S.A. Moosavian, M. Mashreghi, et al., J. Drug Deliv. Sci. Technol. 69 (2022) 103141. DOI:10.1016/j.jddst.2022.103141 |
| [78] |
X. Guo, J. Zhang, Q. Cai, et al., Colloids Surf. B: Biointerfaces 198 (2021) 111499. DOI:10.1016/j.colsurfb.2020.111499 |
| [79] |
N.B.M. Agardan, Z. Degim, S. Yilmaz, L. Altıntas, T. Topal, J. Drug Deliv. Sci. Technol. 57 (2020) 101612. DOI:10.1016/j.jddst.2020.101612 |
| [80] |
C. Yi, M. Fu, X. Cao, et al., J. Agric. Food Chem. 61 (2013) 5961-5971. DOI:10.1021/jf3055278 |
| [81] |
S. Menina, J. Eisenbeis, M.A.M. Kamal, et al., Adv. Healthc. Mater. 8 (2019) 1900564. DOI:10.1002/adhm.201900564 |
| [82] |
R.M. Handjani-Vila, A. Ribier, B. Rondot, G. Vanlerberghie, Int. J. Cosmet. Sci. 1 (1979) 303-314. DOI:10.1111/j.1467-2494.1979.tb00224.x |
| [83] |
M.N. Azmin, A.T. Florence, R.M. Handjani-Vila, et al., J. Pharm. Pharmacol. 37 (1985) 237-242. |
| [84] |
X. Zhou, Y. Hao, L. Yuan, et al., Chin. Chem. Lett. 29 (2018) 1713-1724. DOI:10.1016/j.cclet.2018.10.037 |
| [85] |
S. Moghassemi, A. Hadjizadeh, J. Control. Release 185 (2014) 22-36. DOI:10.1016/j.jconrel.2014.04.015 |
| [86] |
P. Bhardwaj, P. Tripathi, R. Gupta, S. Pandey, J. Drug Deliv. Sci. Technol. 56 (2020) 101581. DOI:10.1016/j.jddst.2020.101581 |
| [87] |
M. Moghtaderi, K. Sedaghatnia, M. Bourbour, et al., Med. Oncol. 39 (2022) 240. DOI:10.1007/s12032-022-01836-3 |
| [88] |
N.D. Fayed, A.E. Goda, E.A. Essa, G.M. El Maghraby, J. Drug Deliv. Sci. Technol. 66 (2021) 102866. DOI:10.1016/j.jddst.2021.102866 |
| [89] |
M. Yaghoobian, A. Haeri, N. Bolourchian, S. Shahhosseni, S. Dadashzadeh, Int. J. Nanomedicine 15 (2020) 8767-8781. DOI:10.2147/IJN.S261932 |
| [90] |
S. Bansal, G. Aggarwal, P. Chandel, S.L. Harikumar, J. Pharm. Bioallied Sci. 5 (2013) 318. DOI:10.4103/0975-7406.120080 |
| [91] |
P.S. Jadon, V. Gajbhiye, R.S. Jadon, K.R. Gajbhiye, N. Ganesh, AAPS PharmSciTech 10 (2009) 1186-1192. DOI:10.1208/s12249-009-9325-z |
| [92] |
S. Kamboj, V. Saini, S. Bala, Sci. World J. 2014 (2014) 959741. |
| [93] |
A. Ahad, M. Raish, F.I. Al-Jenoobi, A.M. Al-Mohizea, Curr. Drug Deliv. 15 (2018) 260-266. DOI:10.2174/1567201814666170518131934 |
| [94] |
I.A. Attia, S.A. El-Gizawy, M.A. Fouda, A.M. Donia, AAPS PharmSciTech 8 (2007) 206-212. DOI:10.1208/pt0804106 |
| [95] |
A.A. Sultan, S.A. El-Gizawy, M.A. Osman, G.M. El Maghraby, J. Liposome Res. 28 (2018) 209-217. DOI:10.1080/08982104.2017.1343835 |
| [96] |
T. Amnuaikit, T. Limsuwan, P. Khongkow, P. Boonme, Asian J. Pharm. Sci. 13 (2018) 472-484. DOI:10.1016/j.ajps.2018.02.004 |
| [97] |
A. Kumar, J. Drug Delivery Ther. 8 (2018) 100-104. DOI:10.22270/jddt.v8i5-s.1981 |
| [98] |
M.E. Planas, P. Gonzalez, L. Rodriguez, S. Sanchez, G. Cevc, Anesth. Analg. 75 (1992) 615-621. |
| [99] |
S. Rai, V. Pandey, G. Rai, Nano. Rev. Exp. 8 (2017) 1325708. DOI:10.1080/20022727.2017.1325708 |
| [100] |
S.A.T. Opatha, V. Titapiwatanakun, R. Chutoprapat, Pharmaceutics 12 (2020) 855. DOI:10.3390/pharmaceutics12090855 |
| [101] |
H.A.E. Benson, Expert Opin. Drug Deliv. 3 (2006) 727-737. DOI:10.1517/17425247.3.6.727 |
| [102] |
T. Jiang, T. Wang, T. Li, et al., ACS Nano 12 (2018) 9693-9701. DOI:10.1021/acsnano.8b03800 |
| [103] |
S. Mahor, A. Rawat, P.K. Dubey, et al., Int. J. Pharm. 340 (2007) 13-19. DOI:10.1016/j.ijpharm.2007.03.006 |
| [104] |
G.M. El Maghraby, A.C. Williams, B.W. Barry, Int. J. Pharm. 276 (2004) 143-161. DOI:10.1016/j.ijpharm.2004.02.024 |
| [105] |
L.G. Hermida, M. Sabes-Xamani, R. Barnadas-Rodriguez, J. Liposome Res. 19 (2009) 207-219. DOI:10.1080/08982100902740847 |
| [106] |
T.P. McMullen, R.N. Lewis, R.N. McElhaney, Biochemistry 32 (1993) 516-522. DOI:10.1021/bi00053a016 |
| [107] |
T.P. McMullen, R.N. McElhaney, Biochim. Biophys. Acta 1234 (1995) 90-98. DOI:10.1016/0005-2736(94)00266-R |
| [108] |
H. Natsheh, E. Touitou, Molecules 25 (2020) 2959. DOI:10.3390/molecules25132959 |
| [109] |
G. Cevc, A. Schätzlein, G. Blume, J. Control. Release 36 (1995) 3-16. DOI:10.1016/0168-3659(95)00056-E |
| [110] |
G. Cevc, A.G. Schätzlein, H. Richardsen, U. Vierl, Langmuir 19 (2003) 10753-10763. DOI:10.1021/la026585n |
| [111] |
V. Garg, H. Singh, S. Bimbrawh, et al., Curr. Drug Deliv. 14 (2017) 613-633. |
| [112] |
A.Y. Pawar, Asian J. Pharm. 10 (201) S425-S436. |
| [113] |
M.Jose Morilla, E.Lilia Romero, Curr. Pharm. Des. 22 (2016) 1118-1134. DOI:10.2174/1381612822666151216145737 |
| [114] |
M.W. Akram, H. Jamshaid, F.U. Rehman, et al., AAPS PharmSciTech 23 (2022) 7. |
| [115] |
C. Li, Y. Zhang, T. Su, et al., Int. J. Nanomedicine 7 (2012) 5995-6002. |
| [116] |
Y.B. Huang, M.J. Tsai, P.C. Wu, et al., J. Drug Target. 19 (2011) 709-718. DOI:10.3109/1061186X.2010.551402 |
| [117] |
K.H. Song, S.J. Chung, C.K. Shim, J. Control. Release 106 (2005) 298-308. DOI:10.1016/j.jconrel.2005.05.016 |
| [118] |
M.M. Seyedabadi, H. Rostami, S.M. Jafari, M. Fathi, Food Biosci. 40 (2021) 100857. DOI:10.1016/j.fbio.2020.100857 |
| [119] |
S. Alamelu, K.P. Rao, J. Microencapsul. 8 (1991) 505-519. DOI:10.3109/02652049109021874 |
| [120] |
B.S. Esposto, P. Jauregi, D.R. Tapia-Blacido, M. Martelli-Tosi, Trends Food Sci. Technol. 108 (2021) 40-48. DOI:10.1016/j.tifs.2020.12.003 |
| [121] |
M. Dash, F. Chiellini, R.M. Ottenbrite, E. Chiellini, Prog. Polym. Sci. 36 (2011) 981-1014. DOI:10.1016/j.progpolymsci.2011.02.001 |
| [122] |
C. Sebaaly, A. Trifan, E. Sieniawska, H. Greige-Gerges, Processes 9 (2021) 445. DOI:10.3390/pr9030445 |
| [123] |
A. Karewicz, D. Bielska, A. Loboda, et al., Colloids Surf. B: Biointerfaces 109 (2013) 307-316. DOI:10.1016/j.colsurfb.2013.03.059 |
| [124] |
L. Yan, S.H. Crayton, J.P. Thawani, et al., Small 11 (2015) 4870-4874. DOI:10.1002/smll.201501412 |
| [125] |
M. Manconi, S. Mura, M.L. Manca, et al., Int. J. Pharm. 392 (2010) 92-100. DOI:10.1016/j.ijpharm.2010.03.038 |
| [126] |
O. Mertins, R. Dimova, Langmuir 27 (2011) 5506-5515. DOI:10.1021/la200553t |
| [127] |
H.A. Oransa, M.F. Boughdady, H.M. El-Sabbagh, Int. J. Nanomedicine (2022) 5641-5660. |
| [128] |
H.M. Ezzat, Y.S.R. Elnaggar, O.Y. Abdallah, Int. J. Pharm. 565 (2019) 488-498. DOI:10.1016/j.ijpharm.2019.05.034 |
| [129] |
Y. Shao, L. Yang, H.K. Han, Eur. J. Pharm. Biopharm. 89 (2015) 339-346. DOI:10.1016/j.ejpb.2014.12.026 |
| [130] |
T.A. Ahmed, H. Elimam, A.O. Alrifai, et al., Int. J. Pharm. 588 (2020) 119791. DOI:10.1016/j.ijpharm.2020.119791 |
| [131] |
C. Caddeo, O. Díez-Sales, R. Pons, et al., J. Colloid Interface Sci. 461 (2016) 69-78. DOI:10.1016/j.jcis.2015.09.013 |
| [132] |
M. Conacher, J. Alexander, J.M. Brewer, Vaccine 19 (2001) 2965-2974. DOI:10.1016/S0264-410X(00)00537-5 |
| [133] |
S. Bhairy, S. Pitchika, S. Maurya, J. Patil, Indo Am. J. Pharm. Res. 10 (2020) 1326-1344. |
| [134] |
H. Alghurabi, T. Tagami, K. Ogawa, T. Ozeki, Polymers 14 (2022) 2693. DOI:10.3390/polym14132693 |
| [135] |
M.A. El-Nabarawi, R.N. Shamma, F. Farouk, S.M. Nasralla, J. Liposome Res. 30 (2020) 1-11. DOI:10.1080/08982104.2019.1577256 |
| [136] |
T. Rajput, M.K. Chauhan, J. Drug Delivery Ther. 7 (2017) 4-16. |
| [137] |
J. Ahmad, M. Singhal, S. Amin, et al., Curr. Pharm. Des. 23 (2017) 1575-1588. DOI:10.2174/1381612823666170124111142 |
| [138] |
A. Shukla, V. Mishra, P. Kesharwani, Drug Discov. Today 21 (2016) 888-899. DOI:10.1016/j.drudis.2016.03.013 |
| [139] |
J.S. Wilkhu, S.E. McNeil, D.E. Anderson, Y. Perrie, J. Drug Target. 21 (2013) 291-299. DOI:10.3109/1061186X.2012.747528 |
| [140] |
N. Marasini, M. Skwarczynski, I. Toth, Expert Rev. Vaccines 13 (2014) 1361-1376. DOI:10.1586/14760584.2014.936852 |
| [141] |
A.A. Sultan, G.A. Saad, G.M. El Maghraby, Int. J. Pharm. 630 (2023) 122427. DOI:10.1016/j.ijpharm.2022.122427 |
| [142] |
Y. Chen, Z. Jiang, J. Xu, et al., J. Nanobiotechnol. 19 (2021) 230. DOI:10.1186/s12951-021-00979-1 |
| [143] |
A. Ismail, M. Teiama, B. Magdy, W. Sakran, AAPS PharmSciTech 23 (2022) 188. DOI:10.1208/s12249-022-02339-0 |
| [144] |
G. Arzani, A. Haeri, M. Daeihamed, H. Bakhtiari-Kaboutaraki, S. Dadashzadeh, Int. J. Nanomedicine 10 (2015) 4797-4813. |
| [145] |
Y.S.R. Elnaggar, S. Omran, H.A. Hazzah, O.Y. Abdallah, Int. J. Pharm. 564 (2019) 410-425. DOI:10.1016/j.ijpharm.2019.04.069 |
| [146] |
P. Guan, Y. Lu, J. Qi, W. Wu, Colloids Surf. B: Biointerfaces 144 (2016) 143-151. DOI:10.1016/j.colsurfb.2016.04.006 |
| [147] |
M.H. Elkomy, H.M. Eid, M. Elmowafy, et al., Drug Deliv. 29 (2022) 2694-2704. DOI:10.1080/10717544.2022.2110997 |
| [148] |
M. Joseph Naguib, A. Moustafa Kamel, A. Thabet Negmeldin, A.H. Elshafeey, I. Elsayed, Drug Deliv. 27 (2020) 996-1009. DOI:10.1080/10717544.2020.1787557 |
| [149] |
Z. Saifi, M. Rizwanullah, S.R. Mir, S. Amin, J. Drug Deliv. Sci. Technol. 57 (2020) 101634. DOI:10.1016/j.jddst.2020.101634 |
| [150] |
M.K. Lee, Pharmaceutics 12 (2020) 264. DOI:10.3390/pharmaceutics12030264 |
| [151] |
E. Roger, F. Lagarce, E. Garcion, J.P. Benoit, Nanomedicine 5 (2010) 287-306. DOI:10.2217/nnm.09.110 |
| [152] |
S. Hu, M. Niu, F. Hu, et al., Int. J. Pharm. 441 (2013) 693-700. DOI:10.1016/j.ijpharm.2012.10.025 |
| [153] |
R. Bnyan, I. Khan, T. Ehtezazi, et al., J. Pharm. Sci. 107 (2018) 1237-1246. DOI:10.1016/j.xphs.2018.01.005 |
| [154] |
C.A. Bunton, K. Ohmenzetter, L. Sepulveda, J. Phys. Chem. 81 (1977) 2000-2004. DOI:10.1021/j100536a009 |
| [155] |
M. Niu, Y. Lu, L. Hovgaard, W. Wu, Int. J. Nanomedicine 6 (2011) 1155-1166. DOI:10.2217/nnm.11.116 |
| [156] |
A. Yamamoto, E. Hayakawa, V.H.L. Lee, Life Sci. 47 (1990) 2465-2474. DOI:10.1016/0024-3205(90)90492-A |
| [157] |
H. Shinichiro, Y. Takatsuka, M. Hiroyuki, Int. J. Pharm. 9 (1981) 173-184. DOI:10.1016/0378-5173(81)90010-7 |
| [158] |
M. Zhu, S. Zhu, Q. Liu, et al., Chin. Chem. Lett. 34 (2023) 107482. DOI:10.1016/j.cclet.2022.04.080 |
| [159] |
A. Farhadi, A.L.I. Banan, J. Fields, A.L.I. Keshavarzian, J. Gastroenterol. Hepatol. 18 (2003) 479-497. DOI:10.1046/j.1440-1746.2003.03032.x |
| [160] |
A.R. Mohammed, N. Weston, A.G.A. Coombes, M. Fitzgerald, Y. Perrie, Int. J. Pharm. 285 (2004) 23-34. DOI:10.1016/j.ijpharm.2004.07.010 |
| [161] |
M. Tang, Z. Gui, X. Liang, et al., Pharm. Dev. Technol. 26 (2021) 1051-1060. DOI:10.1080/10837450.2021.1980010 |
| [162] |
N. Islam, A.F. Zahoor, H.K. Syed, et al., Pak. J. Pharm. Sci. 33 (2020) 2301-2306. |
| [163] |
J. Lu, J.D. Ormes, M. Lowinger, et al., Cryst. Growth Des. 17 (2017) 2782-2791. DOI:10.1021/acs.cgd.7b00237 |
| [164] |
P. Dharmani, V. Srivastava, V. Kissoon-Singh, K. Chadee, J. Innate Immun. 1 (2009) 123-135. DOI:10.1159/000163037 |
| [165] |
R. Bansil, B.S. Turner, Adv. Drug Deliv. Rev. 124 (2018) 3-15. DOI:10.1016/j.addr.2017.09.023 |
| [166] |
S. Chen, H. Zhu, Y. Luo, J. Mater. Chem. B 10 (2022) 7328-7348. DOI:10.1039/D2TB00874B |
| [167] |
L.M. Ensign, R. Cone, J. Hanes, Adv. Drug Deliv. Rev. 64 (2012) 557-570. DOI:10.1016/j.addr.2011.12.009 |
| [168] |
M.E.V. Johansson, D. Ambort, T. Pelaseyed, et al., Cell Mol. Life Sci. 68 (2011) 3635-3641. DOI:10.1007/s00018-011-0822-3 |
| [169] |
S.K. Lai, Y.Y. Wang, J. Hanes, Adv. Drug Deliv. Rev. 61 (2009) 158-171. DOI:10.1016/j.addr.2008.11.002 |
| [170] |
S. Mizrahy, D. Peer, Chem. Soc. Rev. 41 (2012) 2623-2640. DOI:10.1039/C1CS15239D |
| [171] |
D. Chen, D. Xia, X. Li, et al., Int. J. Pharm. 449 (2013) 1-9. DOI:10.1016/j.ijpharm.2013.04.002 |
| [172] |
S. Jin, S. Fu, J. Han, et al., J. Drug Target. 20 (2012) 615-622. DOI:10.3109/1061186X.2012.702770 |
| [173] |
G.P. Martin, C. Marriott, I.W. Kellaway, Gut 19 (1978) 103-107. DOI:10.1136/gut.19.2.103 |
| [174] |
I.N. Farstad, T.S. Halstensen, B. Lien, et al., Immunology 89 (1996) 227-237. DOI:10.1046/j.1365-2567.1996.d01-727.x |
| [175] |
M. Sun, H. Hu, L. Sun, Z. Fan, Chin. Chem. Lett. 31 (2020) 1729-1736. DOI:10.1016/j.cclet.2020.02.035 |
| [176] |
T. Landsverk, Acta Vet. Scand. 22 (1981) 198-210. DOI:10.1186/BF03547509 |
| [177] |
M. Ghadiri, P.M. Young, D. Traini, Pharmaceutics 11 (2019) 113. DOI:10.3390/pharmaceutics11030113 |
| [178] |
A.L. Daugherty, R.J. Mrsny, Pharm. Sci. Technol. Today 2 (1999) 144-151. DOI:10.1016/S1461-5347(99)00142-X |
| [179] |
B.R. Tash, M.C. Bewley, M. Russo, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 10855-10860. DOI:10.1073/pnas.1121390109 |
| [180] |
S. Kataria, P. Sandhu, A. Bilandi, M. Akanksha, B. Kapoor, Int. J. Res. Ayurveda Pharm. 2 (2011) 1534-1538. |
| [181] |
G.S. Gordon, A.C. Moses, R.D. Silver, J.S. Flier, M.C. Carey, Proc. Natl. Acad. Sci. U. S. A. 82 (1985) 7419-7423. DOI:10.1073/pnas.82.21.7419 |
| [182] |
A. Yamamoto, T. Uchiyama, R. Nishikawa, T. Fujita, S. Muranishi, J. Pharm. Pharmacol. 48 (1996) 1285-1289. |
| [183] |
D.D. Deshmukh, W.R. Ravis, G.V. Betageri, Int. J. Pharm. 358 (2008) 128-136. DOI:10.1016/j.ijpharm.2008.02.026 |
| [184] |
B.D. Rege, J.P.Y. Kao, J.E. Polli, Eur. J. Pharm. Sci. 16 (2002) 237-246. DOI:10.1016/S0928-0987(02)00055-6 |
| [185] |
Q. Yu, Z. Wang, P. Li, Q. Yang, Drug Dev. Ind. Pharm. 39 (2013) 587-592. DOI:10.3109/03639045.2012.692376 |
| [186] |
C.Y. Wang, M. Sun, Z. Fan, J.Z. Du, Chin. J. Polym. Sci. 40 (2022) 1154-1164. DOI:10.1007/s10118-022-2726-0 |
| [187] |
M. Niu, Y. Tan, P. Guan, et al., Int. J. Pharm. 460 (2014) 119-130. DOI:10.1016/j.ijpharm.2013.11.028 |
| [188] |
M. Xian, P. Wawrzyniak, B. Rückert, et al., J. Allergy Clin. Immunol. 138 (2016) 890-893. |
| [189] |
Y. Mine, J.W. Zhang, Int. Arch. Allergy Immunol. 130 (2003) 135-142. DOI:10.1159/000069009 |
| [190] |
H. Lin, M. Gebhardt, S. Bian, et al., Int. J. Pharm. 330 (2007) 23-31. DOI:10.1016/j.ijpharm.2006.08.043 |
| [191] |
M. Kateh Shamshiri, A.A. Momtazi-Borojeni, M. Khodabandeh Shahraky, F. Rahimi, J. Cell Biochem. 120 (2019) 9023-9033. DOI:10.1002/jcb.28176 |
| [192] |
T. Zietek, W.A.D. Boomgaarden, E. Rath, Pharmaceutics 13 (2021) 1280. DOI:10.3390/pharmaceutics13081280 |
| [193] |
N.L. Trevaskis, L.M. Kaminskas, C.J.H. Porter, Nat. Rev. Drug Discov. 14 (2015) 781-803. DOI:10.1038/nrd4608 |
| [194] |
W.N.A. Charman, V.J. Stella, Int. J. Pharm. 34 (1986) 175-178. DOI:10.1016/0378-5173(86)90027-X |
| [195] |
H. Kim, Y. Kim, J. Lee, Asian J. Pharm. Sci. 8 (2013) 96-103. DOI:10.1016/j.ajps.2013.07.012 |
| [196] |
I. Niot, H. Poirier, T.T.T. Tran, P. Besnard, Prog. Lipid Res. 48 (2009) 101-115. DOI:10.1016/j.plipres.2009.01.001 |
| [197] |
Z. Zhang, Y. Lu, J. Qi, W. Wu, Acta Pharm. Sin. B 11 (2021) 2449-2468. DOI:10.1016/j.apsb.2020.12.022 |
| [198] |
N. Zhang, Q.N. Ping, G.H. Huang, W.F. Xu, Int. J. Pharm. 294 (2005) 247-259. DOI:10.1016/j.ijpharm.2005.01.018 |
| [199] |
M.J. Rein, M. Renouf, C. Cruz-Hernandez, et al., Br. J. Clin. Pharmacol. 75 (2013) 588-602. DOI:10.1111/j.1365-2125.2012.04425.x |
| [200] |
K. Pleban, S. Kopp, E. Csaszar, et al., Mol. Pharmacol. 67 (2005) 365-374. DOI:10.1124/mol.104.006973 |
| [201] |
S. Dey, J. Patel, B.S. Anand, et al., Invest. Ophthalmol. Visual Sci. 44 (2003) 2909-2918. DOI:10.1167/iovs.02-1142 |
| [202] |
L. Yang, J.P. Fawcett, J. Ostergaard, H. Zhang, I.G. Tucker, Mol. Pharm. 9 (2012) 29-36. DOI:10.1021/mp200201y |
| [203] |
Y.l. Lo, J. Control. Release 90 (2003) 37-48. DOI:10.1016/S0168-3659(03)00163-9 |
| [204] |
F. Hao, Y. He, Y. Sun, et al., Saudi J. Biol. Sci. 23 (2016) S113-S125. DOI:10.1016/j.sjbs.2015.09.024 |
| [205] |
W. Dong, J. Ye, J. Zhou, et al., Acta Pharm. Sin B 10 (2020) 1576-1585. DOI:10.1016/j.apsb.2019.10.002 |
| [206] |
M.J. Parnham, H. Wetzig, Chem. Phys. Lipids 64 (1993) 263-274. DOI:10.1016/0009-3084(93)90070-J |
| [207] |
Z.L. Kong, H.P. Kuo, A. Johnson, L.C. Wu, K.L.B. Chang, Int J. Mol. Sci. 20 (2019) 2918. DOI:10.3390/ijms20122918 |
| [208] |
H. Abbas, Y.A. El-Feky, M.M. Al-Sawahli, et al., Drug Deliv. 29 (2022) 714-727. DOI:10.1080/10717544.2022.2044938 |
| [209] |
S. Rebello, A.K. Asok, S. Mundayoor, M.S. Jisha, Environ. Chem. Lett. 12 (2014) 275-287. DOI:10.1007/s10311-014-0466-2 |
| [210] |
R.L. Grant, C. Yao, D. Gabaldon, D. Acosta, Toxicology 76 (1992) 153-176. DOI:10.1016/0300-483X(92)90162-8 |
| [211] |
A.T. Florence, J.M.N. Gillan, Pestic. Sci. 6 (1975) 429-439. DOI:10.1002/ps.2780060411 |
| [212] |
Z. Ujhelyi, F. Fenyvesi, J. Varadi, et al., Eur. J. Pharm. Sci. 47 (2012) 564-573. DOI:10.1016/j.ejps.2012.07.005 |
| [213] |
K. Ekelund, K. Osth, C. Pahlstorp, et al., J. Pharm. Sci. 94 (2005) 730-744. DOI:10.1002/jps.20283 |
| [214] |
X.F. Li, Z.W. Zhang, X.X. Hong, et al., Acta Pharm. Sin. 56 (2021) 1591-1598. |
| [215] |
J.E. Hunter, J.F. Fowler Jr, J. Surfactants Deterg. 1 (1998) 235-239. DOI:10.1007/s11743-998-0025-3 |
| [216] |
J.M. Yang, R. Zhou, M. Zhang, H.R. Tan, J.Q. Yu, Molecules 23 (2018) 1274. DOI:10.3390/molecules23061274 |
| [217] |
S. Kim, M. Lee, D.N. Dhanasekaran, Y.S. Song, BMC Cancer 18 (2018) 1232. DOI:10.1186/s12885-018-5152-5 |
| [218] |
Y. Andriani, T.S. Tengku-Muhammad, H. Mohamad, et al., Molecules 20 (2015) 4410-4429. DOI:10.3390/molecules20034410 |
| [219] |
M.D. McSweeney, Z.C. Versfeld, D.M. Carpenter, S.K. Lai, Clin. Transl. Sci. 11 (2018) 162-165. DOI:10.1111/cts.12537 |
| [220] |
H. Schellekens, W.E. Hennink, V. Brinks, Pharm. Res. 30 (2013) 1729-1734. DOI:10.1007/s11095-013-1067-7 |
| [221] |
Y. Shen, T. Tu, B. Yi, et al., Acta Biomater. 97 (2019) 200-215. DOI:10.1016/j.actbio.2019.08.014 |
| [222] |
W. Wu, T. Li, Adv. Drug Deliv. Rev. 143 (2019) 1-2. DOI:10.1016/j.addr.2019.08.003 |
| [223] |
Y. Ren, L. Nie, S. Zhu, X. Zhang, Int. J. Nanomedicine (2022) 4861-4877. |
| [224] |
K. Xu, S. Li, Y. Zhou, et al., Pharmaceutics 15 (2023) 1064. DOI:10.3390/pharmaceutics15041064 |
| [225] |
J. Qi, J. Zhuang, Y. Lu, et al., Drug Discov. Today 22 (2017) 166-172. DOI:10.1016/j.drudis.2016.09.024 |
| [226] |
C. Su, H. Yang, H. Sun, et al., J. Pharm. Biomed. Anal. 158 (2018) 288-293. DOI:10.1016/j.jpba.2018.06.014 |
| [227] |
S. Hirsjarvi, L. Sancey, S. Dufort, et al., Int. J. Pharm. 453 (2013) 594-600. DOI:10.1016/j.ijpharm.2013.05.057 |
| [228] |
D. Yue, M. Wang, F. Deng, et al., Chin. Chem. Lett. 29 (2018) 648-656. DOI:10.1016/j.cclet.2018.01.046 |
| [229] |
Z. Xu, J. Chen, L.L. Hu, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. DOI:10.1016/j.cclet.2017.07.018 |
| [230] |
Y. Wang, Y. Zhang, J. Wang, X.J. Liang, Adv. Drug Deliv. Rev. 143 (2019) 161-176. DOI:10.1016/j.addr.2018.12.004 |
| [231] |
J. Luo, Z. Xie, J.W.Y. Lam, et al., Chem. Commun. (2001) 1740-1741. |
| [232] |
M. Noh, T. Kim, H. Lee, et al., Colloids Surf. A: Physicochem. Eng. Asp. 359 (2010) 39-44. DOI:10.1016/j.colsurfa.2010.01.059 |
| [233] |
W. Fan, Z. Yu, H. Peng, et al., Int. J. Pharm. 586 (2020) 119551. DOI:10.1016/j.ijpharm.2020.119551 |
| [234] |
J. Yang, Z. Dong, W. Liu, et al., Chin. Chem. Lett. 31 (2020) 875-879. DOI:10.1016/j.cclet.2019.07.016 |
| [235] |
Y. Li, C. Wang, S. Zong, et al., J. Biomed. Nanotechnol. 15 (2019) 686-702. DOI:10.1166/jbn.2019.2724 |
| [236] |
H. He, S. Jiang, Y. Xie, et al., Nanoscale Horiz. 3 (2018) 397-407. DOI:10.1039/C8NH00010G |
| [237] |
X. Hu, W. Fan, Z. Yu, et al., Nanoscale 8 (2016) 7024-7035. DOI:10.1039/C5NR07474F |
| [238] |
Y. Xie, B. Shi, F. Xia, et al., J. Control. Release 270 (2018) 65-75. DOI:10.1016/j.jconrel.2017.11.046 |
| [239] |
D. Liu, B. Wan, J. Qi, et al., Chin. Chem. Lett. 29 (2018) 1834-1838. DOI:10.1016/j.cclet.2018.11.015 |
| [240] |
H. He, L. Wang, Y. Ma, et al., J. Control. Release 327 (2020) 725-736. DOI:10.1016/j.jconrel.2020.09.024 |
| [241] |
H. He, J. Zhang, Y. Xie, et al., Mol. Pharm. 13 (2016) 4013-4019. DOI:10.1021/acs.molpharmaceut.6b00705 |
| [242] |
X. Hu, J. Zhang, Z. Yu, et al., Nanomedicine 11 (2015) 1939-1948. DOI:10.1016/j.nano.2015.06.013 |
| [243] |
H. He, C. Liu, J. Ming, et al., Aggregate 3 (2022) e163. DOI:10.1002/agt2.163 |
| [244] |
Y. Cai, X. Ji, Y. Zhang, et al., Aggregate 4 (2023) e277. DOI:10.1002/agt2.277 |
| [245] |
J. Zhuang, D. Wang, D. Li, et al., Chin. Chem. Lett. 29 (2018) 1815-1818. DOI:10.1016/j.cclet.2018.10.012 |
| [246] |
A. Narang, R.K. Chang, M.A. Hussain, J. Pharm. Sci. 102 (2013) 3867-3882. DOI:10.1002/jps.23691 |
| [247] |
D. Shukla, S. Chakraborty, S. Singh, B. Mishra, Expert Opin. Drug Deliv. 8 (2011) 207-224. DOI:10.1517/17425247.2011.547469 |
| [248] |
T. Schneider, A. Sachse, G. Robling, M. Brandl, Drug Dev. Ind. Pharm. 20 (1994) 2787-2807. DOI:10.3109/03639049409042681 |
| [249] |
E. Pupo, A. Padron, E. Santana, et al., J. Control. Release 104 (2005) 379-396. DOI:10.1016/j.jconrel.2005.02.001 |
| [250] |
S. Tinkle, S.E. McNeil, S. Muhlebach, et al., Ann. N. Y. Acad. Sci. 1313 (2014) 35-56. DOI:10.1111/nyas.12403 |
| [251] |
Y. Wang, C. Wang, K. Li, et al., J. Control. Release 330 (2021) 618-640. DOI:10.1016/j.jconrel.2021.01.002 |
| [252] |
N. Filipczak, J. Pan, S.S.K. Yalamarty, V.P. Torchilin, Adv. Drug Deliv. Rev. 156 (2020) 4-22. DOI:10.1016/j.addr.2020.06.022 |
| [253] |
N. Desai, AAPS J. 14 (2012) 282-295. DOI:10.1208/s12248-012-9339-4 |
| [254] |
C.B. Roces, E.C. Port, N.N. Daskalakis, et al., Int. J. Pharm. 586 (2020) 119566. DOI:10.1016/j.ijpharm.2020.119566 |
| [255] |
C. Jaafar-Maalej, A. Elaissari, H. Fessi, Expert Opin. Drug Deliv. 9 (2012) 1111-1127. DOI:10.1517/17425247.2012.702751 |
| [256] |
J.C. Kraft, J.P. Freeling, Z. Wang, R.J.Y. Ho, J. Pharm. Sci. 103 (2014) 29-52. DOI:10.1002/jps.23773 |
 2024, Vol. 35
2024, Vol. 35 

