Scientists are constantly working to design, synthesize, and evaluate drug molecules with broad-spectrum biological activities in the fields of organic chemistry and pharmaceutical research. The biologically active core skeleton is a key component of chemical drug structures and is widely used in the fields of drug derivation, agrochemical product innovation, compound design, and characterization of structure–activity relationships (SARs) [1,2]. Therefore, the identification and utilization of biologically active core skeletons are of great significance for exploring new drug structures, optimizing drug efficacy, and reducing costs. In recent years, indole has gained increasing attention due to its unique structure, which results in indole derivatives possessing distinctive physiological activities.
Indole (Fig. S1 in Supporting information) is one of the key structural motifs in drug discovery and can serve as a scaffold for a variety of receptor ligands [3-5]. Indole derivatives exhibit unique properties such as mimicking peptide structures and reversible binding to enzymes, which provide significant opportunities for discovering novel drugs with distinct modes of action [6-10]. Indole compounds are widely used in the field of medicine due to their properties such as anti-tumor [11,12], antibacterial [13-16], and antiviral activities [17,18]. In the field of pesticides, indole compounds are mainly applied in plant growth regulation, antiviral [19-21], and antibacterial [22-24] aspects (Fig. S1). From 2010 to 2023, researchers have mainly focused on the antiviral [16,23,24] and antibacterial activities [22,25-29] of indole compounds, with relatively few reports on their plant growth regulating, herbicidal, and insecticidal activities [30].
While there have been reviews on the synthesis methods and biological applications of indole derivatives, they mainly focus on the synthesis of existing varieties and their related properties. As far as we know, a comprehensive summary of the relationship between the structure and biological activity of indole derivatives, as well as their design strategies, has yet to be compiled. Therefore, we have summarized the application of biologically active molecules containing indole structures in pesticides based on design strategies, SARs, and mechanisms of action. It is our hope that this review will provide new insights into the discovery and mechanism of action of novel indole-based pesticides.
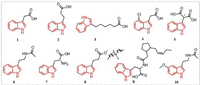
2. Plant growth regulating activityIndole compounds with plant growth regulating activity reported in recent years are mainly derived from natural products. Indole-3-acetic acid (IAA) 1 is the most common indole compound (Fig. 1) with plant growth regulating activity, and it plays an important role in the growth and development of plants [31-34]. Studies have found that IAA can serve as a signal between microbes and plants [35,36]. For example, secretions from roots of Arabidopsis can induce the synthesis of IAA in Falciphora oryza, thereby promoting the growth of lateral roots in Arabidopsis [37]. Research on the mechanism of action has found that IAA regulates the transcription and expression of many genes through the ubiquitin-proteasome system, affecting cell growth and differentiation. The ubiquitination complex is activated in response to the auxin signal receptor transport inhibitor response protein 1 (TIR1), which recruits gene transcription factors for gene transcription. At the same time, the ubiquitination complex degrades the transcriptional repressor protein (Aux/IAA), promoting the transcription of auxin-responsive genes. However, at low concentrations of auxin, the cell tends to suppress the transcription of genes mediated by the Aux/IAA and auxin response factor (ARF) transcription factor complex [38-41]. This is because at low concentrations of auxin, cells need to control gene transcription to maintain normal growth and differentiation. The cell tends to suppress the transcription of genes mediated by the Aux/IAA and ARF transcription factor complex to balance the action of auxin signals, ensuring normal cell growth and development.
|
Download:
|
| Fig. 1. Indole compounds with plant growth-regulating activity. | |
Indole-3-butyric acid (IBA) 2 (Fig. 1) is a plant growth hormone that is structurally similar to IAA. However, the side chain of IBA is longer than that of IAA, and it cannot directly bind to the TIR1-Aux/IAA pocket [42,43]. Instead, IBA needs to be converted to IAA through β-oxidation with the involvement of peroxidases (IBR1, IBR3, IBR10, and ECH2), triggering the auxin signaling cascade [44,45]. Although IBA can mimic the effects of IAA to some extent, whether it should be considered an independent signaling molecule from IAA requires further research and clarification, as its signal transduction mechanism may be different from that of IAA.
A new type of growth hormone, indole-3-hexanoic acid (IHA) 3 (Fig. 1), synthesized from pyridine carboxylic esters, is structurally similar to IAA and IBA. IHA can be recognized directly or indirectly by TIR1 and cause TIR1 to participate in IHA signal reception, showing similar reactions to IAA [46]. Compared to IBA, IHA can induce higher levels of GH3.3 and ACS4, indicating that it has a unique mechanism for regulating plant growth and development. IHA can be further converted to IBA through β-oxidation, thereby affecting plant growth and development. In addition, IHA can trigger feedback inhibition of IBA conversion to IAA, regulating auxin responses. Although further research and identification are needed on the IHA signal mechanism, as a new type of growth hormone, its unique structure and mechanism provide new ideas and possibilities for studying plant growth and development [46].
4-Chloroindole-3-acetic acid (4-Cl-IAA) 4 (Fig. 1) is an IAA analog with a chlorine substitution at the 4-position of the indole ring, originally isolated from immature pea seedlings [47]. Compared to other compounds in pea, 4-Cl-IAA has unique biological activity. It can stimulate gibberellin biosynthesis, inhibit expression of ethylene biosynthesis genes (PsACS4, PsACO2, and PsACO3) in the fruit skin, upregulate expression of ethylene receptor and signal-related genes (PsERS1, PsETR2, PsEBF1, and PsEBF2) in the fruit skin, and promote fruit skin growth [48]. Additionally, 4-Cl-IAA is considered an important signaling molecule for oat floret senescence, although its specific mechanism of action is not yet clear [49]. In terms of molecular structure, the distance between the aromatic ring and the carboxylic acid end of compounds such as IAA, IBA, and 4-Cl-IAA must be within 0.55 Å for optimal plant growth-promoting activity [42,50]. In addition, spatial configuration is also a factor that influences the activity of growth-promoting compounds. For example, indole-3-butyric acid (ISA) 5 (Fig. 1) is another IAA-like compound that shows better growth-promoting effects than IAA or IBA in certain situations. ISA has two enantiomers, and the R-(−)-ISA enantiomer has better plant growth-promoting activity than the S-(+)-ISA enantiomer.
Indole-3-acetamide (IAM) 6 (Fig. 1) is a precursor to IAA biosynthesis and can affect plant growth through two pathways [51]. One pathway is the conversion of IAM to IAA by a specific hydrolytic enzyme (AM1), which promotes plant growth. The other pathway involves the accumulation of IAM in the plant, which promotes the expression of the rate-limiting enzyme NCED3 involved in abscisic acid (ABA) biosynthesis pathway, induces the biosynthesis of ABA, further leading to overexpression of R2R3 MYB transcription factor genes MYB74 either independently or directly induced by ABA, ultimately restricting plant growth but enhancing tolerance to non-biological stress. Another important precursor of plant growth hormone biosynthesis is tryptophan (Trp) 7 (Fig. 1), which is widely present in higher plants. Trp can increase the abundance of Clonostachys rosea metabolites, thereby enhancing C. rosea's ability to promote tomato root growth [52]. Introducing ammonium cations into the structure of tryptophan can yield a class of ionic liquids 8 (Fig. 1) with excellent physical and chemical properties (solubility). Lettuce application can increase biomass by 12%–20% and promote the absorption of some nutrients [53]. In addition, a class of jasmonoyl-L-tryptophan (9) can disrupt the activity of AUX1, leading to the ineffectiveness of IAA, but endogenous 9 plays a small role in regulating plant growth [54]. Tryptophan can also be converted to melatonin (10) (Fig. 1; the biological functions of melatonin are shown in Section S1 in Supporting information) by the action of L-tryptophan decarboxylase (PSID) and serotonin 5-hydroxylase (CYP71P1), thereby participating in and regulating plant growth, promoting post-germination root growth [55-58].
3. Antiviral activityIn agriculture, the reported antiviral activity of indole compounds in recent years has mainly been targeted against tobacco mosaic virus (TMV), cucumber mosaic virus (CMV), potato virus Y (PVY), and others. Two indole-containing compounds discovered in Peganum harmala [59] and Banisteriopsis caapi [60] have been found to possess antiviral activity against TMV. At a concentration of 500 µg/mL, harmine 11 (62.3%, 55.1% and 60.3%) and tetrahydroharmine 12 (Fig. S2 in Supporting information) (64.2%, 57.2% and 59.5%) exhibit significantly higher TMV inactivation, therapeutic, and protective activities than ribavirin (37.4%, 57.2% and 59.5%), respectively. Song et al. used harmine or tetrahydroharmine as lead compounds to synthesize compounds 13, 14, and 15 (Fig. S2) by changing their saturation or introducing bromine and ester groups. These compounds showed better inhibitory activity against TMV than ribavirin. SARs showed that the presence of ester groups was crucial for the antiviral activity of these compounds. For example, replacing the methyl formate ethyl ester structure in compound 12 with an acrylic acid structure significantly reduced its activity. These findings provide valuable guidance for the development of more effective TMV inhibitors.
The introduction of acylhydrazone structure can further enhance the antiviral activity of harmine and tetrahydroharmine derivatives [61-63]. For example, at 50 µmol/L, compounds 16 (76%) and 17 (63%) showed antiviral activity against TMV that was comparable to that of ningnanmycin (NNM) (65%). Mechanistic studies revealed that compounds 16 and 17 (Fig. S2) could delay the replication of TMV in the early stage of infection and maintain their high biological activity against TMV for a longer period of time [64,65]. NK0209 (Fig. S2) is a compound with highly efficient antiviral activity against plant viruses in this class of compounds [66]. At 500 µg/mL, NK0209 exhibited inactivation, therapeutic, and protective activities against TMV (66.9%, 63.2% and 68.1%, respectively), which were superior to those of NNM (57.3%, 54.2% and 55.0%). In the NK0209 molecule, C-1 and C-3 are its two chiral centers, and its four stereoisomers also exhibit superior antiviral activity against TMV compared to NNM. In particular, chiral isomer 18 (1R, 3R) (Fig. S2) showed much higher inactivation, therapeutic, and protective half maximal effective concentration (EC50) values against TMV (127, 108 and 156 µg/mL, respectively) than NNM (348, 392, and 373 µg/mL) [67]. It can activate levels of reactive oxygen species and antioxidants, and induce an increase in salicylic acid content and expression of the responsive gene PR2. In addition, it can weaken the virulence of TMV by directly altering the morphology of viral particles and enhancing the activity of reactive oxygen species enzymes, thereby reducing the production of reactive oxygen species (ROS) induced by TMV during plant infection [65,67].
In 2019–2020, Wei et al. introduced [68] the dithioacetal structure into the indole structure to obtain compounds 21, 22 and 23 (Fig. S3 in Supporting information), which exhibit excellent therapeutic activity against PVY with EC50 values of 282, 217, and 288 µg/mL, respectively, all of which are superior to NNM (468 µg/mL). Compounds 19, 20, 22, 23, 25 and 26 (Fig. S3) exhibited protective EC50 values against TMV of 88.5, 207, 212, and 200 µg/mL, respectively. Mechanism studies have shown that compounds 19, 20, and 24–26 (Fig. S3) have strong interactions with TMV coat protein (CP), thereby inhibiting the assembly of TMV. Compound 20 can increase the expression levels of proteins that regulate malate dehydrogenase (MDH) and the photosynthetic system in chloroplasts, and enhance the activity of defense enzymes superoxide dismutase (SOD), peroxidase (POD), phenylalamine ammonia lyase (PAL), and catalase (CAT), thereby strengthening plant defense responses and increasing resistance to plant viruses such as PVY. SARs have shown that the presence of different thiol compounds affects the antiviral activity of indole derivatives containing dithioacetal moieties against PVY.
Compounds 27 and 28 (Fig. S3) are a class of structurally simple indole derivatives, with an aldehyde group and a carboxyl group present at the 3-position of the indole, respectively. At a concentration of 500 µg/mL, compounds 27 and 28 exhibited inactivation and protective activities against TMV with 77.78%, 86.22%, 77.42% and 89.00%, respectively. Mechanism studies have shown that the application of compounds 27 and 28 can cause TMV particles to break and force the broken particles to aggregate, thereby exerting a preventive effect against TMV. The application of compounds 27 and 28 can increase the transcription levels of non-expressor of PR genes (NPR1), pathogenesis-related protein 1 (PR1), PR2, PR5, and phenylalanine ammonia lyase. They can also upregulate the activities of CAT and POD to reduce damage to membranes caused by peroxides. In addition, compound 28 can increase the activity of PAL and the transcription levels of ICS and PBS3, thereby promoting the accumulation of salicylic acid (SA). Compound 28, on the other hand, only promotes the accumulation of SA through the PAL pathway [19].
3-Hydroxy-2-oxindole is also a class of compounds with good antiviral activity. Compound 29 (Fig. S3) isolated from Strobilanthes cusia showed certain inhibitory activity against TMV. Mechanistic studies revealed that compound 29 was an inducer of systemic acquired resistance in plants via a salicylic acid-mediated signaling pathway. Wei et al. introduced [69] a sulfonamide moiety into the 3-hydroxy-2-oxindole structure to obtain compound 30 (Fig. S3), which exhibited curative and protective activities against PVY of 55.9% and 57.3%, respectively, at a concentration of 500 µg/mL. This was superior to compound 29 (49.5% and 50.5%) and NNM (51.2% and 52.6%). Mechanistic studies revealed that compound 30 could induce the activities of four defense-related enzymes (SOD, POD, PAL, and CAT), upregulate the expression of some genes related to photosynthesis, and inhibit the systemic infection of PVY in tobacco.
Topsentin alkaloids are a group of indole derivatives isolated from marine sponges such as Mediterranean Topsentia genitrix, Carribbean or Korean Spongosorites sp. These compounds contain an imidazole ring in their structure, which can exist in two different levels of saturation [70]. For example, in compounds 31, 32, 33 and 34 (Fig. S3), the substitution of the indole ring at position 7 with bromine or methoxy was found to be favorable for enhancing antiviral activity [71]. Guo et al. used [72] Topsentin alkaloids as lead compounds and synthesized compounds 35 and 36 (Fig. S3) by replacing the imidazole ring with thiazole and oxazole rings. At a concentration of 500 µg/mL, compounds 35 and 36 showed therapeutic, protective, and inactivation activities against TMV of 50%, 53%, 54% and 59%, 52%, 54%, respectively. Studies have shown that compound 36 exerts antiviral effects by aggregating viral particles and restricting their movement in plants, thus slowing down the spread of TMV.
Marine natural product 37 (Fig. S3) belongs to the class of indolopyrroloketone compounds, which differ from topsentin alkaloids only in the structure of the two heterocycles at the 3-position of the indole ring. At a concentration of 500 mg/mL, the therapeutic, protective, and inactivation activities against TMV were 51%, 56% and 53%, respectively. Wang et al. used this compound as a template and introduced a thiourea and a phenyl ring into the pyrroloketone moiety to obtain a class of compounds with good antiviral activity. Compounds 38, 39 and 40 (Fig. S3) exhibit antiviral activity similar to or better than NNM. Mechanistic studies have shown that compound 41 (Fig. S3) has a strong affinity for TMV CP and can interfere with the assembly of TMV CP and RNA by binding to TMV CP. SARs have shown that introducing a phenyl group at the 5- or 6-position of the pyrroloketone moiety can enhance the antiviral activity of these indolopyrroloketone compounds against TMV. In addition, when the substituent on the thiourea moiety is a phenyl group, electron-donating substituents at the ortho position and electron-withdrawing substituents at the para and meta positions are favorable for enhancing the antiviral activity against TMV [73]. Afterwards, Lu et al. synthesized [74] a series of simpler indole compounds 42, 43 and 44 (Fig. S3) as analogues of topsentin alkaloids using gramine as a lead compound. These compounds exhibited better anti-TMV activity than NNM. The assembly of TMV was inhibited by cross-linking its CP.
Some indole compounds containing spirostructures also exhibit good antiviral activity. These compounds generally introduce a cyclic structure at the 3-position of the indole to generate a spirocompound [75]. Compounds 45, 46 and 47 (Fig. S4 in Supporting information) reported [73] by Wang et al. have shown better antiviral activity than ribavirin. SARs have shown that anti-TMV activity can be improved by modifying the skeleton, changing the spatial configuration, and increasing the electron-withdrawing capacity of the substituent on the quinolone benzene ring. Compounds 50–57 (Fig. S4) showed better anti-TMV protection activity than NNM. SARs have shown that the introduction of acyl hydrazide is beneficial in modifying the indole skeleton [75-82].
4. Fungicidal and bactericidal activityIndole-containing compounds with fungicidal and bactericidal activity are mainly derived from natural products. Although many compounds with highly effective antifungal and antibacterial activity have been reported, there are still few studies on the specific mechanism of action of these compounds [83].
In the development of novel indole-based antifungal and antibacterial drugs, the indole-containing β-carboline or tetrahydro-β-carboline structure is often used as the core structure for further modification. Zhang et al. incorporated [84] a urea structure at position 3 of the β-carboline scaffold to obtain compound 58 (Fig. S5). At a concentration of 100 mg/L, this compound showed a 74.66% inhibition rate against Rhizoctonia solani (R. solani), with an EC50 value of 0.131 mmol/L against R. solani.
Although the above two classes of indole compounds exhibit excellent antifungal activity, the poor flexibility of the β-carboline scaffold often results in low solubility of the derived compounds. Xi et al. further modified [85] the C-ring of the β-carboline scaffold by altering saturation and inserting heteroatoms to obtain compound 60 with excellent antifungal activity. This compound showed an EC50 value of 2.35 µg/mL against R. solani. Compounds 59 and 60 (Fig. S5, SARs see Section S2 in Supporting information) have similar mechanisms of action. They can disrupt the mitochondrial membrane potential (MMP) and affect mitochondrial function, leading to accelerated cell death. They can also disrupt cell membrane permeability, cause accumulation of ROS, and interfere with DNA synthesis by inhibiting topoisomerases I and II.
The β-carboline derivatives 61 (Fig. S5, SARs see Section S2), obtained by introducing the acylhydrazone moiety into the β-carboline structure, exhibited broad-spectrum antifungal activity against various plant pathogens. At a concentration of 50 mg/kg, compounds 61 showed inhibitory rates of over 88% against P. piricola, Sclerotinia sclerotiorum (S. sclerotiorum), and Rhizoctonia cerealis (R. cerealis) [86]. In the same year, they introduced an imidazole structure at position 3 of the β-carboline scaffold and obtained compound 62 (Fig. S4), which showed an EC50 value of 4.2 µg/mL against R. solani [87].
Compounds with good Antifungal activity can also be obtained by introducing some heterocyclic rings at the 3-position of the indole ring. For example, indole derivatives 63 (Fig. S5) with a 1,2,4-triazole ring structure exhibited high antifungal activity against Cercospora arachidicola (C. arachidicola). Compounds 64 (Fig. S5) showed activity against C. arachidicola, P. piricola, and R. cerealis, while compound 65 (Fig. S5) exhibited activity against C. arachidicola, R. cerealis, and watermelon anthracnose. The antifungal activity of these compounds was higher than that of carbendazim [88]. Ouyang et al. introduced [89] a disulfide group into the 1,2,4-triazole ring structure to obtain compounds 66–68 (Fig. S5), which exhibited excellent antifungal activity. At a concentration of 50 µg/mL, compound 66 exhibited 81.43% inhibition activity against Burkholderia glumae, while compounds 67 and 68 showed over 80% inhibition activity against Ralstonia solanacearum. Compounds 66 and 68 exhibited inhibition activity of 68.05% and 67.68%, respectively, against Xanthomonas citri. Replacing the 1,2,4-triazole ring with an imidazole ring in such compounds has led to some compounds showing good antifungal activity [89]. For example, compounds 69 and 70 (Fig. S5) exhibited inhibition rates of 88.78% and 100%, respectively, against tobacco bacterial wilt disease. However, the overall antibacterial activity of these compounds decreased significantly.
Pimprinine is a natural indole alkaloid found in Aspergillus fungi [90,91]. Compounds 71–73 (Fig. S5) synthesized based on pimprinine showed inhibition activity of 57%, 67%, and 63%, respectively, against C. arachidicola at a concentration of 50 µg/mL, which were higher than carbendazim (50%) [90]. Gao et al. replaced [91,92] the oxazole ring in the pimprinine structure with an imidazole ring to obtain compounds 74 and 75 (Fig. S5) with good antifungal activity. At 50 µg/mL, they exhibited inhibition rates of 97.5% and 90.3% against Alternaria Leaf Spot and R. solani, respectively. In this class of imidazole-substituted indole compounds, the introduction of halogen atoms on the imidazole ring can further enhance their antifungal activity. Substitution of the oxazole ring with oxadiazole or thiadiazole resulted in a significant reduction or even loss of antibacterial activity for this class of compounds [90].
Nortopsentins are a class of compounds with a bis-indole structure isolated from the sponge Sorites ruetzleri, which exhibit moderate antifungal activity against Candida albicans [93]. Guo et al. replaced [72] the imidazole moiety in bis-indole alkaloids with a thiazole moiety to obtain compounds 76 (Fig. S5) (active against A. solani), 77 (Fig. S5) (active against A. solani and Fusarium graminearum), and 78 (Fig. S5) (active against P. capsica), all of which exhibited higher antifungal activity than that of streptomycin. Introduction of the thiazole ring has a more positive effect on the improvement of antifungal activity compared to the imidazole ring.
In addition to the heterocyclic ring strategy mentioned above, introducing a small, electron-withdrawing substituent at the 4-position of the indole ring can significantly enhance the antifungal activity of indole derivatives. Introducing a small, electron-withdrawing substituent at the 4-position of the indole ring can significantly enhance the antifungal activity of indole compounds, due to the reduced steric hindrance of the substituent. For example, compounds 79 and 80 (Fig. 2) showed 100% inhibition activity against R. solani at a concentration of 10 µg/mL. Their EC50 values against R. solani were 0.62 and 1.25 µg/mL, respectively, which were much better than that of indole (25.56 µg/mL) [94]. Mechanism studies have shown that compound 79 can affect MMP, disrupt normal mitochondrial function, and reduce the DNA content and number of nuclei in R. solani mycelia, thereby interfering with DNA synthesis. Unlike compounds 59 and 60, compound 79 inhibits the accumulation of ROS and has no effect on cell membrane permeability [94].
|
Download:
|
| Fig. 2. Indole compounds with antifungal and antibacterial activity. | |
Some indole derivatives containing a spiral ring structure also exhibit highly efficient antifungal activity [95,96]. The spiroindolinone compounds 81 and 82 (Fig. 2) exhibit good inhibitory effects against S. sclerotiorum and Rhizoctonia cereadis [79,97]. Specifically, compound 83 showed an inhibition rate of 65.7% against R. solani at 50 mg/L. This may be closely related to the presence of fluorine atoms. In addition, some spiroindole compounds have broad-spectrum antifungal activity. For example, compounds 81–83 (Fig. 2) exhibit good inhibitory effects against plant pathogenic fungi such as P. capsica, R. cerealis, and Colletorichum lagenerium [98,99].
In 2022, Li et al. reported [100] a class of indole derivatives containing a pyridine structure linked through diacetyl hydrazide, which exhibit good antibacterial activity against Xanthomonas oryzae pv. oryzicola (Xoc) and X. oryzae pv. oryzae (Xoo). At a concentration of 5 µg/mL, compounds 84–87 (Fig. 2) exhibited inhibition rates of 96.3%, 99.2%, 99.1% and 98.8%, respectively, against Xoo. The EC50 values of compounds 84–87 against Xoo were 5.8, 2.2, 5.6, and 1.0 µg/mL, respectively. SARs have shown that hydrophobic, electrostatic, and steric effects play important roles in the Xoc inhibition activity of this class of compounds. The mechanism of action shows that compound 87 can regulate the conversion of sugar metabolism in rice to produce pyruvic acid, which is further decarboxylated to produce acetyl-coenzyme A (CoA) and then enters the tricarboxylic acid cycle, where NAD+ (oxidation state of NADH) is reduced to nicotinamide adenine dinucleotide (NADH). The NADH generated in this process is then fed into oxidative phosphorylation. The two closely linked pathways ultimately result in the production of usable chemical energy for the plant, thereby enhancing the plant's disease resistance.
5. Herbicidal activityCompared to their applications as antiviral and antifungal agents, indole derivatives are less commonly used as herbicides. The main reported targets of indole-derived herbicides are protoporphyrinogen oxidase (PPO) and p-hydroxyphenylpyruvate dioxygenase (HPPD). Additionally, some indole derivatives can also induce weed death by affecting photosynthesis, thus achieving herbicidal effects.
Thaxtomins is a family of plant toxins with a unique 4-nitroindole and dione-pyridazinone structure. Thaxtomin A (TA) (Fig. S6 in Supporting information), isolated from natural sources, exhibit pre- and post-emergence herbicidal activity of over 60% against Brassica campestris (B. campestris) and Amaranthus retroflexus (A. retroflexus) seedlings [101]. SARs have shown that the presence of a benzyl group has a significant impact on the herbicidal activity. For example, compounds 88, 89 and 90 (Fig. S6) exhibit pre-emergence herbicidal activity (≥85%) against B. campestris and A. retroflexus. Furthermore, the benzyl moiety and hydroxyl groups on the dione-pyridazinone structure have a significant impact on the crop selectivity of this class of compounds. Mechanistic studies have found that compounds TA, 88, 89 and 90 exhibit certain inhibitory activity against PPO [102].
The indole derivative 92 produced by the endophytic fungus Chaetomium globosum from Ginkgo biloba exhibits significant inhibitory activity against carrot seedlings, with an inhibition rate over 60% at a concentration of 50 µg/mL. He et al. treated [103] compound 92 with diethylamine and sulfur trifluoride to obtain a fluorinated indole derivative 93 (Fig. S6), which showed significantly reduced phytotoxicity but increased toxicity against human colon cancer cells.
Indole derivatives 94 and 95 (Fig. S6) substituted at the 8-position with nitro groups reduced the dry biomass of Ipomoea grandifolia and Senna alata weeds by 40%, 37% and 50%. These compounds can block the electron transfer of light and photosystem II (PSII), which further affects the redox process. This leads to a reduction in ATP synthesis and CO2 fixation in plants, thus interfering with plant development [104]. SARs have shown that the presence of strong electron-withdrawing groups, such as NO2, is closely related to herbicidal activity, as the presence of such groups is important for the interaction between the ligand and the binding pocket of protein D1 on PSII [104,105]. However, the unsubstituted tetrahydrocarbazole compound 96 (Fig. S6) showed an inhibition rate of 22% on seed germination and 49.6% on root growth. This may be because the methylene group on the indole structure in compounds 94 and 96 increases their lipophilicity, which promotes their better penetration through the plant cell wall, thus exhibiting better herbicidal activity than compound 95. In addition, electron-withdrawing groups on the aromatic ring of the indole skeleton can also increase the activity of the compound [105]. Dong et al. introduced a pyrimidine ring into the tetrahydrocyclopyrazole structure and obtained a class of compounds with good herbicidal activity, including compound 97 (Fig. S6). At 750 g/ai, compound 97 showed broad-spectrum herbicidal activity. However, the specific mechanism of action is still unknown.
Compound 98 (Fig. S6, SARs see Section S2), which was obtained by introducing a methyl ketone structure at the 3-position of the indole, exhibits strong inhibitory effects on the seed germination and shoot growth of Amaranthus retroflexus. It can completely inhibit the germination of Amaranthus retroflexus seeds at concentrations of 400–800 µmol/L. Mechanism studies have found [106] that compound 98 has a certain inhibitory effect on HPPD, which hinders the conversion of hydroxyphenylpyruvate to urocanic acid, resulting in the inability to synthesize tocopherols and plastoquinones, thus affecting the biosynthesis of carotenoids in the target organism. This promotes the appearance of chlorosis in meristematic and newly formed tissues of the plant, ultimately leading to plant death. The auxin-like α-substituted indole-3-carboxylic acid derivatives 99 and 100 (Fig. S6, SARs see Section S2) showed high inhibition rates of 96% and 95%, respectively, on the root growth of Brassica napus at 100 mg/L, and even at 10 mg/L, they still maintained inhibition rates of 92% and 93%, respectively.
6. Insecticidal activityEvodiamine is a class of indole-containing compounds obtained from the fruit of Tetradium ruticarpum. It exhibits good insecticidal activity against Mythimna separata (M. separata), Plutella xylostella (P. xylostella) and Helicoverpa armigera (H. armigera) [107]. Liu et al. used [108] evodiamine as a lead compound to synthesize compounds 101–103 (Fig. S7 in Supporting information) by introducing fluorine. Compounds 101 and 102 showed insecticidal activity against M. separata at 90% and 80% respectively at a concentration of 2.5 mg/L. Compounds 101–103 showed over 90% insecticidal activity against P. xylostella at a concentration of 1.0 mg/mL. Compound 101 showed 60% insecticidal activity against H. armigera at 50 mg/L. The mechanism of action studies found that compound 101 acts on the endoplasmic reticulum Ca2+ channel protein of insects, releasing stored [Ca2+]i from the endoplasmic reticulum into the cytoplasm.
Indole exhibits insecticidal activity at concentrations greater than 1.0 mmol/L [109]. Lee et al. introduced halogens into the indole ring to obtain compounds 5-iodoindole (104) and 4-fluoroindole (105) (Fig. S7), which showed 100% mortality against Bursaphelenchus xylophilus (B. xylophilus) at a concentration of 1 mmol/L. Especially compound 104 showed significantly better activity than avermectin, with 100% mortality against B. xylophilus at concentrations of 0.1 and 0.5 mmol/L, respectively (compared to avermectin) [110,111]. Mechanism studies have found that 5-iodoindole induces death of nematodes through different mechanisms at different concentrations [112]. At a concentration of 50 mg/L, compound 104 induces rapid death of nematodes by interacting with glutathione S-transferase (GST), leading to GST inactivation, accumulation of ROS, and subsequent death of the nematodes. At a concentration of 20 mg/L, compound 104 induces the formation of several large vacuoles that fuse and gradually lead to death of the nematodes. Cheng et al. introduced the halogenated indole structure into matrine to obtain compound 106 (Fig. S7), which exhibited excellent insecticidal activity against the Spodoptera frugiperda (S. frugiperda). When X is Br, the lethal concentration 50 (LC50) value of compound 106 against the S. frugiperda is 0.00001 mg/mL, while matrine has an LC50 value of only 0.0075 mg/mL.
Indole acylation also yields a class of compounds, bioactive 107 and 108 (Fig. S7, SARs see Section S2), with good insecticidal activity against P. xylostella. Their LC50 values against P. xylostella are 1.00 and 0.42 µg/mg, respectively, which are better than that of Deltamethrin (1.19 µg/mg). Mechanistic studies have shown that compound 66 has minimal negative effects on non-target species such as Apis mellifera. This compound acts by blocking the conversion of indoxacarb (a neurotoxic metabolite) to a more stable compound, which leads to motor dysfunction and paralysis, resulting in the immediate death of P. xylostella [113].
In 2022, Zhang et al. introduced [114] a class of mesoionic pyridine ring structures at the 3-position of the indole ring to obtain compounds 109–111 (Fig. S7), which exhibit excellent insecticidal activity against Sogatella furcifera (S. furcifera) and Aphis craccivora (A. craccivora). Compound 111 (SARs see Section S2), in particular, has LC50 values of 0.86 µg/mL and 0.85 µg/mL against S. furcifera and A. craccivora, respectively, and exhibits better insecticidal activity against A. craccivora than triflupyrad (LC50 = 3.67 µg/mL), which is noteworthy. The mechanism study reveals that compound 111 can inhibit the activity of acetylcholinesterase (AchE) and Ca2+Mg2+-ATPase in S. furcifera, thereby achieving the purpose of pest control.
Indole derivatives containing a spiroring structure also exhibit excellent insecticidal activity [78,115,116]. For example, compound 112 (Fig. S7) has good mortality rates of 100% against M. separata and Culex pipiens pallens (C. pipiens pallens). Spiro indole compounds containing an acylhydrazone fragment, such as compound 113 (Fig. S7), still exhibit a 60% mortality rate against C. pipiens pallens at a concentration of 0.25 mg/kg. Spiro oxindole ethylthiourea compounds 114 and 115 (Fig. S7) exhibit a mortality rate of 100% against C. pipiens pallens larvae at 5 mg/mL, especially compound 114 with an LC50 value of 0.32 mg/mL.
7. Summary and outlookIn recent years, research has highlighted the indole ring as a promising active scaffold with unique structural and property advantages. Its derivatives exhibit broad-spectrum biological activity with diverse modes of action. The biological activity of indole compounds is significantly impacted by the expansion of the indole ring, substituents, and spatial configuration. Indole derivatives with plant growth regulating activity are primarily natural products, which make it difficult to perform structure-activity relationship analysis. For fungicidal, antiviral, and herbicidal activity, introducing structures such as acylhydrazine, pyridine carboxylic acid, acetyl, and methyl into the indole ring can enhance the biological activity of its derivatives. Although indole compounds are not widely used as insecticides, studies have incorporated the indole structure into commercial insecticides, producing unexpected derivatives with good biological activity. Furthermore, compounds with good insecticidal activity have been synthesized by introducing structures such as spirorings, heterocycles, and halogens into the indole ring, providing new avenues for discovering bioactive compounds.
During the period from 2010 to 2020, reports on the biological activity of indole compounds were mainly focused on their antibacterial and antiviral activities. However, since 2020, an increasing number of indole compounds have been studied as potential antiviral agents, which may become a research hotspot in the future. So far, the relationship between the substitution position on the indole ring and its biological activity is not very clear, and the mechanism of action of most indole compounds is also unknown. To some extent, these facts have limited the deep optimization of the substructures of indole compounds. Therefore, future research on indole compounds needs to utilize techniques such as metabolomics, proteomics, photoaffinity probes, and molecular simulation in bioinformatics to clarify the mechanism, targets, and action sites of active compounds, in order to rationally design and optimize indole compounds. It is foreseeable that these technologies will accelerate the development of new indole class pesticides.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe financial support from the National Natural Science Foundation of China (Nos. 32072445 and 21762012), the Program of Introducing Talents to Chinese Universities (No. D20023), the Natural Science research project of Guizhou Education Department (No. KY(2018)009), the Graduate Research Fund in Guizhou Province (No. YJSKYJJ[2021]038) and the specific research fund of The Innovation Platform for Academicians of Hainan Province (No. SQ2020PTZ0009).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109005.
| [1] |
B. Zdrazil, R. Guha, J. Med. Chem. 61 (2018) 4688-4703. DOI:10.1021/acs.jmedchem.7b00954 |
| [2] |
T. Chen, H. Xiong, J.F. Yang, et al., J. Agric. Food Chem. 68 (2020) 9839-9877. DOI:10.1021/acs.jafc.0c03369 |
| [3] |
F.R. De Sá Alves, E.J. Barreiro, C.A. Fraga, Mini Rev. Med. Chem. 9 (2009) 782-793. DOI:10.2174/138955709788452649 |
| [4] |
B.E. Evans, K.E. Rittle, M.G. Bock, et al., J. Med. Chem. 31 (1988) 2235-2246. DOI:10.1021/jm00120a002 |
| [5] |
M.E. Welsch, S.A. Snyder, B.R. Stockwell, Curr. Opin. Chem. Biol. 14 (2010) 347-361. DOI:10.1016/j.cbpa.2010.02.018 |
| [6] |
N.K. Kaushik, N. Kaushik, P. Attri, Molecules 18 (2013) 6620-6662. DOI:10.3390/molecules18066620 |
| [7] |
R.E. Dolle, K.H. Nelson, J. Comb. Chem. 1 (1999) 235-282. DOI:10.1021/cc9900192 |
| [8] |
R.G. Franzén, J. Comb. Chem. 2 (2000) 195-214. DOI:10.1021/cc000002f |
| [9] |
R.E. Dolle, J. Comb. Chem. 3 (2001) 477-517. DOI:10.1021/cc010049g |
| [10] |
T.N. Akhaja, J.P. Raval, Chin. Chem. Lett. 23 (2012) 785-788. DOI:10.1016/j.cclet.2012.05.004 |
| [11] |
Z.N. Xu, Y.Q. Wang, Y.C. Zheng, et al., Org. Chem. Front. 7 (2020) 3709-3714. DOI:10.1039/D0QO01120G |
| [12] |
S.Y. Zhou, G.L. Huang, G.Y. Chen, Bioorg. Med. Chem. Lett. 41 (2021) 128009. DOI:10.1016/j.bmcl.2021.128009 |
| [13] |
A. Hilgeroth, K. Yasrebi, S. Suzen, et al., Med. Chem. 15 (2019) 833-839. DOI:10.2174/1573406415666190208170126 |
| [14] |
Y. Liu, Y. Cui, L.Y. Lu, et al., Arch. Pharm. 353 (2020). |
| [15] |
Q.Q. Tan, Y.Z. Li, T.T. Li, et al., Microporous Mesoporous Mater. 325 (2021) 111342. DOI:10.1016/j.micromeso.2021.111342 |
| [16] |
M. Budovská, R. Michalková, J. Mojžiš, Tetrahedron 143 (2023) 133573. DOI:10.1016/j.tet.2023.133573 |
| [17] |
H.Y. Sun, K.P. Sun, J.Y. Sun, Molecules 28 (2023) 2204. DOI:10.3390/molecules28052204 |
| [18] |
C.L. Wei, L. Zhao, Z.R. Sun, et al., Pestic. Biochem. Physiol. 166 (2020) 104568. DOI:10.1016/j.pestbp.2020.104568 |
| [19] |
Y.B. Sun, H. Wu, W.N. Zhou, et al., Pestic. Biochem. Physiol. 183 (2022) 105077. DOI:10.1016/j.pestbp.2022.105077 |
| [20] |
G.Y. Yang, J.M. Dai, Q.L. Mi, et al., Phytochemistry 198 (2022) 113137. DOI:10.1016/j.phytochem.2022.113137 |
| [21] |
Y.N. Zhu, M.X. Liu, B.B. Cai, et al., Chem. Nat. Compd. 58 (2022) 712-716. DOI:10.1007/s10600-022-03774-y |
| [22] |
X.H. Gao, X.L. Pan, P.Y. Wang, et al., Org. Chem. Front. 9 (2022) 5790-5797. DOI:10.1039/D2QO01091G |
| [23] |
H. Guo, Eur. J. Med. Chem. 164 (2019) 678-688. DOI:10.1016/j.ejmech.2018.12.017 |
| [24] |
H. Wu, M.L. Qin, K. Fan, et al., J. Asian Nat. Prod. Res. 25 (2022) 429-437. |
| [25] |
Y.H. Zhang, L. Li, Y.Q. Li, et al., J. Nat. Prod. 85 (2022) 1880-1885. DOI:10.1021/acs.jnatprod.2c00322 |
| [26] |
X.J. Zhao, L.Z. Zhao, Y. Zhao, et al., Viruses 13 (2021) 1433. DOI:10.3390/v13081433 |
| [27] |
S. Jin, H.K. Do, C. Hwang, et al., Int. J. Environ. Sci. 30 (2021) 369-378. DOI:10.5322/JESI.2021.30.5.369 |
| [28] |
P.D. Miranda de Menezes Neves, B.M. Coelho Ferreira, S. Mohrbacher, et al., Lancet Infect. Dis. 20 (2020) 1215-1215. DOI:10.1016/S1473-3099(20)30323-6 |
| [29] |
Y.H. Xie, Y. Song, Z.W. Cong, Chem. Biodivers. 19 (2022) e202200731. DOI:10.1002/cbdv.202200731 |
| [30] |
L.J. Funkhouser-Jones, R. Xu, G. Wilke, et al., Cell Rep. 42 (2023) 112680. DOI:10.1016/j.celrep.2023.112680 |
| [31] |
D.R. Duca, B.R. Glick, Appl. Microbiol. Biotechnol. 104 (2020) 8607-8619. DOI:10.1007/s00253-020-10869-5 |
| [32] |
K.I. Hayashi, K. Arai, Y. Aoi, et al., Nat. Commun. 12 (2021) 6752. DOI:10.1038/s41467-021-27020-1 |
| [33] |
M.H. Ma, E. Batsaikhan, C.M. Wu, et al., J. Chin. Chem. Soc. 70 (2023) 1200-1207. DOI:10.1002/jccs.202300023 |
| [34] |
Q. Yin, J. Zhang, S. Wang, Hortic. Res. 8 (2021) 229. DOI:10.1038/s41438-021-00658-0 |
| [35] |
S.K. Jaiswal, M. Mohammed, F.Y.I. Ibny, et al., Front. Sustain. Food Syst. 4 (2021) 619676. DOI:10.3389/fsufs.2020.619676 |
| [36] |
K.G. Thanuja, B. Annadurai, S. Thankappan, et al., Arch. Microbiol. 202 (2020) 2739-2749. DOI:10.1007/s00203-020-01983-z |
| [37] |
X. Sun, N. Wang, P. Li, et al., Plant Cell Environ. 43 (2020) 358-373. DOI:10.1111/pce.13667 |
| [38] |
G. Gomes, K. Scortecci, Plant Biol. 23 (2021) 894-904. DOI:10.1111/plb.13303 |
| [39] |
M.R.A. de Figueiredo, L.C. Strader, Trends Biochem. Sci. 47 (2022) 865-874. DOI:10.1016/j.tibs.2022.06.004 |
| [40] |
B. Kloosterman, R.G.F. Visser, C.W.B. Bachem, Plant Physiol. Biochem. 44 (2006) 766-775. DOI:10.1016/j.plaphy.2006.10.026 |
| [41] |
S. Piya, S.K. Shrestha, B. Binder, et al., Front. Plant Sci. 5 (2014) 744. |
| [42] |
S. Damodaran, L.C. Strader, Front. Plant Sci. 10 (2019) 851. DOI:10.3389/fpls.2019.00851 |
| [43] |
H.Q. Dong, M.C. Guo, Y. Liang, et al., Mater. Sci. Eng. 89 (2018) 175-181. DOI:10.1016/j.msec.2018.04.004 |
| [44] |
S. Aihebaier, T. Muhammad, A. Wei, et al., ACS Omega 4 (2019) 16789-16793. DOI:10.1021/acsomega.9b01550 |
| [45] |
L. Fattorini, A. Veloccia, F. Della Rovere, et al., BMC Plant Biol. 17 (2017) 121. DOI:10.1186/s12870-017-1071-x |
| [46] |
R.M. Napier, Auxin receptors and perception, in: E. Zažímalová, J. Petrášek, E. Benková (Eds. ), Auxin and Its Role in Plant Development, Springer Vienna, Vienna, 2014, pp. 101–116.
|
| [47] |
S. Marumo, H. Hattori, H. Abe, et al., Nature 219 (1968) 959-960. |
| [48] |
C.P.A. Jayasinghege, J.A. Ozga, K.D. Waduthanthri, et al., J. Exp. Bot. 68 (2017) 4137-4151. DOI:10.1093/jxb/erx217 |
| [49] |
K. Dziurka, M. Dziurka, M. Warchoł, et al., In Vitro Cell. Dev. Biol. Plant 55 (2019) 221-229. |
| [50] |
X. Cao, H.L. Yang, C.Q. Shang, et al., Int. J. Mol. Sci. 20 (2019) 6343. DOI:10.3390/ijms20246343 |
| [51] |
M.M. Pérez-Alonso, P. Ortiz-García, J. Moya-Cuevas, et al., J. Exp. Bot. 72 (2020) 459-475. |
| [52] |
Z.Y. Han, H. Ghanizadeh, H.T. Zhang, et al., J. Fungi 8 (2022) 1166. DOI:10.3390/jof8111166 |
| [53] |
D. Szymaniak, T. Kleiber, M. Wojcieszak, et al., ChemistrySelect 6 (2021) 5614-5621. DOI:10.1002/slct.202101084 |
| [54] |
P. Staswick, M. Rowe, E.P. Spalding, et al., Front. Plant Sci. 8 (2017) 736. DOI:10.3389/fpls.2017.00736 |
| [55] |
S. Park, K. Back, J. Pineal Res. 53 (2012) 385-389. DOI:10.1111/j.1600-079X.2012.01008.x |
| [56] |
M. Zhang, C.X. Gao, L. Xu, et al., Cells 11 (2022) 3250. DOI:10.3390/cells11203250 |
| [57] |
H.Y. Lee, K. Lee, K. Back, Biomolecules 9 (2019) 712. DOI:10.3390/biom9110712 |
| [58] |
H.B. Zhao, T. Su, L.Q. Huo, et al., J. Pineal Res. 59 (2015) 255-266. DOI:10.1111/jpi.12258 |
| [59] |
C. de Meester, Mutat. Res. 339 (1995) 139-153. DOI:10.1016/0165-1110(95)90008-X |
| [60] |
J.C. Callaway, J. Psychoact. Drugs 37 (2005) 151-155. DOI:10.1080/02791072.2005.10399796 |
| [61] |
Z.Z. Wang, D.D. Xie, X.H. Gan, et al., Bioorg. Med. Chem. Lett. 27 (2017) 4096-4100. DOI:10.1016/j.bmcl.2017.07.038 |
| [62] |
Y.M. Ma, X.A. Liang, Y. Kong, et al., J. Agric. Food Chem. 64 (2016) 6659-6671. DOI:10.1021/acs.jafc.6b01772 |
| [63] |
B.E.M. El-Gendy, M.E. Rateb, Bioorg. Med. Chem. Lett. 25 (2015) 3125-3128. DOI:10.1016/j.bmcl.2015.06.010 |
| [64] |
X.P. Zhang, W.B. Huang, X. Lu, et al., J. Agric. Food Chem. 69 (2021) 7458-7466. DOI:10.1021/acs.jafc.1c00897 |
| [65] |
J.L. Xie, W.T. Xu, H.J. Song, et al., J. Agric. Food Chem. 68 (2020) 5555-5571. DOI:10.1021/acs.jafc.0c00875 |
| [66] |
H.Q. Wang, H.J. Song, J. Agric. Food Chem. 68 (2020) 2631-2638. DOI:10.1021/acs.jafc.9b07694 |
| [67] |
X. Lv, M.T. Yuan, Y.H. Pei, et al., J. Agric. Food Chem. 69 (2021) 4992-5002. DOI:10.1021/acs.jafc.1c00941 |
| [68] |
C.L. Wei, J. Zhang, J. Shi, et al., J. Agric. Food Chem. 67 (2019) 13882-13891. DOI:10.1021/acs.jafc.9b05357 |
| [69] |
C.L. Wei, X. Yang, S.J. Shi, et al., J. Agric. Food Chem. 71 (2023) 267-275. DOI:10.1021/acs.jafc.2c06881 |
| [70] |
K.B. Oh, W. Mar, S. Kim, et al., Bioorg. Med. Chem. Lett. 15 (2005) 4927-4931. DOI:10.1016/j.bmcl.2005.08.021 |
| [71] |
X.F. Ji, Z.W. Wang, J. Dong, et al., J. Agric. Food Chem. 64 (2016) 9143-9151. DOI:10.1021/acs.jafc.6b04020 |
| [72] |
J.C. Guo, Y.A. Hao, X.F. Ji, et al., J. Agric. Food Chem. 67 (2019) 10018-10031. DOI:10.1021/acs.jafc.9b04093 |
| [73] |
T.A. Wang, L. Li, Y.A. Zhou, et al., J. Agric. Food Chem. 69 (2021) 10093-10103. DOI:10.1021/acs.jafc.1c04098 |
| [74] |
A.D. Lu, T.A. Wang, H. Hui, et al., J. Agric. Food Chem. 67 (2019) 2148-2156. DOI:10.1021/acs.jafc.8b06859 |
| [75] |
L.J. Yu, A.L. Dai, W. Zhang, et al., J. Agric. Food Chem. 70 (2022) 10693-10707. DOI:10.1021/acs.jafc.2c02301 |
| [76] |
L.W. Chen, Y.X. Liu, H.J. Song, et al., Mol. Divers. 21 (2017) 61-68. DOI:10.1007/s11030-016-9697-4 |
| [77] |
Q.M. Wang, Q. Wang, Y. Qu, et al., Patent, CN111269237A, 2020.
|
| [78] |
L.W. Chen, Y.K. Hao, H.J. Song, et al., J. Agric. Food Chem. 68 (2020) 10618-10625. DOI:10.1021/acs.jafc.0c04488 |
| [79] |
Q. Wang, H. Song, Q. Wang, Chin. Chem. Lett. 33 (2022) 859-862. DOI:10.1016/j.cclet.2021.08.005 |
| [80] |
Q.M. Wang, H.J. Song, L.W. Chen, C.L. Li, G.Q. Yu, Patent, CN107353292A, 2017.
|
| [81] |
Q.M. Wang, Q. Wang, Y. Qu, Patent, CN111264542A, 2020.
|
| [82] |
F.Z. Xu, Y.Y. Wang, F. He, et al., Green Chem. Lett. Rev. 15 (2022) 139-152. DOI:10.1080/17518253.2021.2023660 |
| [83] |
H. Gadegoni, S. Manda, Chin. Chem. Lett. 24 (2013) 127-130. DOI:10.1016/j.cclet.2013.01.001 |
| [84] |
Z.J. Zhang, Y. Zeng, Z.Y. Jiang, et al., Pest Manag. Sci. 74 (2018) 1736-1746. DOI:10.1002/ps.4873 |
| [85] |
J.M. Xi, Z.J. Zhang, Q. Zhu, G.H. Zhong, Int. J. Mol. Sci. 19 (2018) 4044. DOI:10.3390/ijms19124044 |
| [86] |
Y.X. Liu, H.J. Song, Y.Q. Huang, et al., J. Agric. Food Chem. 62 (2014) 9987-9999. DOI:10.1021/jf503794g |
| [87] |
Z.J. Zhang, Z.Y. Jiang, Q. Zhu, G.H. Zhong, J. Agric. Food Chem. 66 (2018) 9598-9607. DOI:10.1021/acs.jafc.8b02124 |
| [88] |
X.Y. Zhao, A.C. Liao, F. Zhang, et al., J. Heterocycl. Chem. 57 (2020) 761-767. DOI:10.1002/jhet.3817 |
| [89] |
G.P. Ouyang, Z.C. Wang, W.N. Hu, Y.Y. Qi, W. Li, Patent, CN111285860A, 2020.
|
| [90] |
B. Liu, R. Li, Y.A. Li, et al., J. Agric. Food Chem. 67 (2019) 1795-1806. DOI:10.1021/acs.jafc.8b06175 |
| [91] |
Z.Y. Yu, H. Jiang, L. Wang, et al., Front. Chem. 8 (2020) 717. DOI:10.3389/fchem.2020.00717 |
| [92] |
Y. Gao, D.C. Huang, C. Liu, et al., Bioorg. Med. Chem. 35 (2021) 116073. DOI:10.1016/j.bmc.2021.116073 |
| [93] |
K.L. Keel, J.J. Tepe, Org. Lett. 23 (2021) 5368-5372. DOI:10.1021/acs.orglett.1c01681 |
| [94] |
J. Zeng, Z.J. Zhang, Q. Zhu, Z.Y. Jiang, G.H. Zhong, Molecules 25 (2020) 1189. DOI:10.3390/molecules25051189 |
| [95] |
L.W. Chen, J.L. Xie, H.J. Song, et al., J. Agric. Food Chem. 64 (2016) 6508-6516. DOI:10.1021/acs.jafc.6b02683 |
| [96] |
B. Jia, Y.M. Ma, B. Liu, et al., Front. Chem. 7 (2019) 837. DOI:10.3389/fchem.2019.00837 |
| [97] |
Y. Wang, S. Guo, L. Yu, et al., Chin. Chem. Lett. 35 (2024) 108207. DOI:10.1016/j.cclet.2023.108207 |
| [98] |
M. Zhang, L. Chen, J.K. Hong, Y.T. Shen, L.Q. Yang, Patent, CN113735871A, 2021.
|
| [99] |
L.Q. Yang, L. Chen, M. Zhang, M. Xia, T. Zhao, Patent, CN112898311A, 2021.
|
| [100] |
H.D. Li, S. Wu, X. Yang, et al., J. Agric. Food Chem. 70 (2022) 12341-12354. DOI:10.1021/acs.jafc.2c04213 |
| [101] |
R.R. King, L.A. Calhoun, Phytochemistry 70 (2009) 833-841. DOI:10.1016/j.phytochem.2009.04.013 |
| [102] |
H.B. Zhang, Q.P. Wang, X. Ning, H. Hang, et al., J. Agric. Food Chem. 63 (2015) 3734-3741. DOI:10.1021/jf506153t |
| [103] |
H. Li, J. Xiao, Y.Q. Gao, et al., J. Agric. Food Chem. 62 (2014) 3734-3741. DOI:10.1021/jf500390h |
| [104] |
J.M. de Souza, B.R. Fazolo, J.W. Ferreira Lacerda, et al., Photochem. Photobiol. 96 (2020) 1233-1242. DOI:10.1111/php.13295 |
| [105] |
M.C. da Silva Mendes, B.R. Fazolo, J.M. de Souza, et al., Photochem. Photobiol. Sci. 18 (2019) 1350-1358. DOI:10.1039/c8pp00506k |
| [106] |
W. Chotpatiwetchkul, N. Chotsaeng, C. Laosinwattana, P. Charoenying, ACS Omega 7 (2022) 29002-29012. DOI:10.1021/acsomega.2c02704 |
| [107] |
J.B. Liu, Y.B. Shi, W. Wen, et al. Patent, CN113354645, 2021.
|
| [108] |
J.B. Liu, Y.B. Shi, Z.C. Tian, et al., J. Agric. Food Chem. 70 (2022) 5197-5206. DOI:10.1021/acs.jafc.1c08297 |
| [109] |
J.H. Lee, Y.G. Kim, M. Kim, et al., Environ. Microbiol. 19 (2017) 1776-1790. DOI:10.1111/1462-2920.13649 |
| [110] |
S.K. Rajasekharan, J.H. Lee, V. Ravichandran, J. Lee, Sci. Rep. 7 (2017) 6803. DOI:10.1038/s41598-017-07074-2 |
| [111] |
S.K. Rajasekharan, J.H. Lee, V. Ravichandran, et al., Sci. Rep. 9 (2019) 2010. DOI:10.1038/s41598-019-38561-3 |
| [112] |
S.K. Rajasekharan, S. Kim, J.C. Kim, J. Lee, Pestic. Biochem. Physiol. 163 (2020) 76-83. DOI:10.1016/j.pestbp.2019.10.012 |
| [113] |
Â.C. Costa, S.C. Cavalcanti, A.S. Santana, et al., Ecotoxicology 28 (2019) 973-982. DOI:10.1007/s10646-019-02095-1 |
| [114] |
J. Zhang, R.J. Song, S. Wu, et al., J. Agric. Food Chem. 70 (2022) 5349-5356. DOI:10.1021/acs.jafc.2c00838 |
| [115] |
J. Cassayre, L.P. Molleyres, P. Maienfisch, F. Cederbaum, Patent, US8309567, 2012.
|
| [116] |
J. Cassayre, L.P. Molleyres, P. Maienfisch, F. Cederbaum, Patent, US8299058, 2012.
|
 2024, Vol. 35
2024, Vol. 35 

