b Hubei Industry Technology Research Institute of Intelligent Health, Xianning 437100, China;
c School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China
Novel coronavirus disease 2019 (COVID-19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1-5]. It has become a worldwide public health disease seriously endangering human life and health, and is one of the most urgent public health problems facing the world at present. Since the outbreak of COVID-19 epidemic, people have been actively looking for the effective therapeutic pharmaceuticals, mainly including three drug development strategies: new use of old medicines [6,7], small molecular drug development [8], and antibody medicines [9]. Among them, small-molecular drugs have become the focus of anti-COVID-19 drug research and development because they can block the replication of virus in cells, have the prominent advantages of conservative target and high stability.
During the past three years, a series of small molecules have been declared to be effective for COVID-19 curing [9-15]. Indeed, no matter how effective they are, the COVID-19 epidemic has promoted public awareness of these drug molecules, and this can be taken as a way of science popularization of pharmaceutical chemistry. It also shows the significances of synthetic organic chemistry and provides additional interest and driving forces for graduate students learning the organic chemistry knowledges [16,17]. Thus, this review aims to introduce the molecules for COVID-19 treatment on their chemical structures, synthetic methods as well as their effects. It may provide sufficient information for new comers in the field learning the synthetic skills in combination with the pharmaceutical synthesis practice. This article is divided into sections according to the molecules, i.e., each section discusses a molecule. Based on the current status of research and development of small molecule drug development against COVID-19, we selected eleven "star" drugs that have been on the market or are in clinical trials as representatives, and reviewed their chemical synthesis routes, in order to provide some reference for the research and development of small molecule drugs against COVID-19.
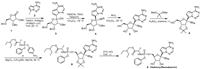
2. Veklury/RemdesivirVeklury/Remdesivir is an aminophosphate prodrug developed by Gilead Sciences Inc. It can be synthesized from 2,3,5-tribenzyloxy-D-ribosyl-1,4-lactone 1 (Scheme 1) [18]. In the process, the glycosylation reaction of 1 with 7-iodopyrrolo[2,1-f][1,2,4]triazin-4-amine 2 affords the intermediate 3, which was then treated by TMSCN/TMSOTf at −78 ℃ to give the intermediate 4 via the cyanation reaction. Treating 4 by BCl3 at −20 ℃ led to 5, the debenzylation product, which then reacted with 2,2-dimethoxypropane in the presence of sulfuric acid to give the intermediate 6. The transesterification reaction of 2-ethylbutyl 6 with ((S)-(4-nitrophenoxy)(phenoxy)phosphoryl)-L-alaninate 7 led to 8, which was then converted to Veklury/Remdesivir molecule via the deprotection reaction under acid conditions.
|
Download:
|
| Scheme 1. Synthetic route for Veklury/Remdesivir. | |
Veklury/Remdesivir was developed by Gilead to fight against the Ebola virus [19]. The triphosphate mimetic nucleotide of Veklury/Remdesivir can be covalently linked to the replicating RNA, thereby preventing further SARS-CoV-2 RNA synthesis [20]. Veklury/Remdesivir was approved by the U.S. Food and Drug Administration (FDA) for emergency treatment of severe COVID-19 patients in May 2020, and was officially launched in October 2020, becoming the first COVID-19 treatment drug officially approved in the United States and the first small molecule anti COVID-19 drug approved for marketing in the world. However, the World Health Organization (WHO) issued a statement saying that no matter how serious the condition of COVID-19 in-patients is, it is not recommended to use the antiviral drug Remdesivir for treatment, because there is no evidence that the drug can improve the survival rate of patients or reduce the demand for ventilators [21]. In addition, due to the limitation of Veklury/Remdesivir's own chemical structure, its physical and chemical properties are poor for oral use, and can only be used by intravenous drip, which limits its early use and large-scale applications.
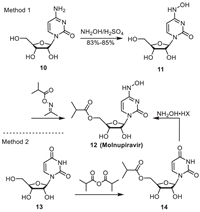
3. MolnupiravirMolnupiravir is the world's first oral anti COVID-19 medicine developed by Merck Sharp & Dohme Ltd. (MSD), which is a nucleoside analogue. According to the category of starting materials, the synthesis routes can be divided into three major categories: cytidine [22-25], uridine [26,27], and ribose [28]. Considering factors such as raw material sources and production costs, the route for obtaining the target compound from cytidine through hydroxyamination reaction and selective esterification catalyzed by enzyme Novozyme 435 is relatively optimal [22]. In method 1 (Scheme 2), the hydroxylation reaction of 10 led to the intermediate 11. Catalyzed by enzymes, the esterification reaction of 11 afforded Molnupiravir. The overall yield of this synthetic route is high (59%), and the employed amount of commercial enzyme Novozyme 435 is low (only 20%), without the need for column chromatography purification. The operation is simple and efficient, and is suitable for industrial scale-up production.
|
Download:
|
| Scheme 2. Synthetic route for Molnupiravir. | |
In method 2, uridine 13 was acylated with isobutyric anhydride under acidic conditions to obtain the intermediate 14. Then, the intermediate 14 reacted with hydroxylamine hydrochloride or hydroxylamine sulfate in the presence of HMDS/TMSOTf to give Molnupiravir [29]. This production process is low cost, high yield (up to 92%) and thereby enhancing industrial production efficiency. Moreover, the enzymatic synthesis of Molnupiravir is quite interesting, as it leads to less reaction steps compared to chemical synthesis, resulting in higher yields and greener and environmentally friendly conditions [22].
At the beginning of the COVID-19 pandemic, Molnupiravir was in preclinical development for seasonal influenza [30]. Molnupiravir was approved for marketing by the UK Medicines and Healthcare Products Administration in November 2021, and has since received emergency use authorization from the US FDA. The results of the Phase III clinical trial (MOVe-OUT) for non-hospitalized patients with mild and moderate symptoms showed that, compared with the control group, the relative risk of clinical or death of non-hospitalized patients with COVID-19 could be reduced by 30% [31]. However, the mid-term results of Phase II/III clinical trials for moderate and severe cases showed no significant benefits for hospitalized patients, so MSD has terminated its Phase II/III clinical trial (MOVe-IN) for hospitalized patients.
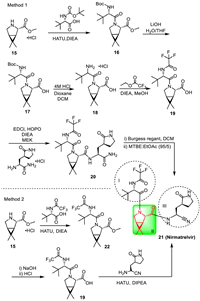
4. PaxlovidPaxlovid is an oral small molecule COVID-19 treatment drug developed by Pfizer, which is a combination drug composed of key components Nirmatrelvir (PF-07321332) and low-dose Ritonavir. Nirmatrelvir can prevent SARS-CoV-2 coronavirus replication by inhibiting 3-chymotrypsin-like protease (3CLpro) [32]. The molecule of Nirmatrelvir includes three segments (Scheme 3). Synthesis of segment II is the most important work, which can be achieved via two methods: chiral resolution and enzyme catalysis, of which the enzyme catalysis method has high yield and good stereoselectivity [33-38]. The splicing of three fragments mainly involves two paths (Scheme 3). In method 1, azabicyclic 15 and N-(tert-butoxycarbonyl)-L-tert-leucine were subjected to HATU condensation to obtain amide compound 16, followed by the hydrolysis of methyl ester group under alkaline conditions to give 17. The deprotection of 17 with hydrochloric acid led to the hydrochloride salt of 18. The reaction of 18 with trifluoroacetic acid ethyl ester afforded trifluoro-acetyl compound 19, which was then converted to 20 via condensation. Dehydration of the amide moiety of 20 afforded Nirmatrelvir as the final product (Scheme 3) [32]. This route is the original route announced by Pfizer, with a total yield of 48%. In the method, the introduction of trifluoro-acetyl and the dehydration amide moiety were completed after the condensation reaction.
|
Download:
|
| Scheme 3. Synthetic routes for Nirmatrelvir. | |
In Method 2, the reaction of azabicyclic 15 with (S)−3,3-dimethyl-2-(2,2,2-trifluoroacetamido)butanoic acid initially gave the intermediate 22. Hydration of 22 under alkaline conditions led to intermediate 19. The condensation reaction of 19 with (2R)−2-amino-3-(2-oxopyrrolidin-3-yl)propanenitrile led to Nirmatrelvir (Scheme 3). This method is more concise and the total yield of Nirmatrelvir can be enhanced to 60% [39]. In the method, the introduction of trifluoroacetyl groups and the dehydration step of amides in this route are completed before the condensation reaction.
The data of the Phase II/III clinical trial (EPIC—HR) of non-hospitalized patients with serious disease risk showed that compared with the control group, Paxlovid could reduce the hospitalization or mortality rate of non-hospitalized patients with COVID-19 by 89% [40]. On December 22, 2021, the U.S. FDA approved Paxlovid emergency use authorization for the treatment of mild to moderate COVID-19 in adults and children (over 12 years old, weighing at least 40 kg or about 88 pounds); On February 11, 2022, China conditionally approved the import registration of Paxlovid. On March 15, 2022, the National Health Commission of China issued the Diagnosis and Treatment Plan for COVID-19 (Ninth Edition), which included Paxlovid in the diagnosis and treatment plan; In the WHO updated treatment guidelines for COVID-19 in 2020, Paxlovid is recommended for mild patients [41].
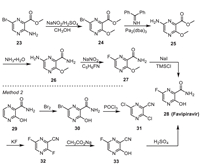
5. Favipiravir/AviganFavipiravir is an RNA dependent RNA polymerase (RdRp) inhibitor developed by Fuji Pharma Co., Ltd., which has strong anti RNA virus activity. One of the synthetic routes of the molecule employs pyrazine derivative 23 as the starting material (Scheme 4, method 1) [42]. In the method, 23 was initially converted into 24 via the classic diazotization method. Catalyzed by Pd2(dba)3, the reaction of 24 with diphenylmethanimine led to intermediate 25, which led to 26 via ammonolysis reaction. Replacing the amino group on the pyrazine ring of 26 with fluoro via the diazotization method could produce 27, which then led to Favipiravir after demethylation. This route has a high cost and requires multi-step column chromatography purification, resulting in low overall yield and is unfavourable for industrial application. Another synthetic route starts with 3-hydroxypyrazine-2-carboxamide 29 (Scheme 4, method 2) [43]. In the method, the bromination reaction of 29 led to 30, which was then converted to 31 by treating with POCl3. Reaction of 31 with KF afforded the fluorine intermediate 32, which was converted into 33 after hydrolysis in the presence of CH3CO2Na. Hydrolysis of the nitrile moiety of 33 with H2SO4 produced the final product Favipiravir. This method avoids the generation of the explosive diazonium salt and is safe for operation in industrial grade production.
|
Download:
|
| Scheme 4. Synthetic routes for Favipiravir. | |
In March 2014, Favipiravir was approved for listing in Japan. It is effective for non-severe COVID-19 patients. However, because the inflammatory reaction caused by COVID-19 pneumonia due to immunity may inhibit the effect of the medicine, high dosage intake is necessary [44,45]. Although Favipiravir did not cause any fatal adverse reactions or clinically significant improvements, more investigations are required to clarify the long-term effects of the medicine on patients [46].
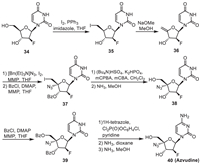
6. AzvudineAzvudine was originally a novel nucleoside reverse transcriptase inhibitor with antiviral activity against human immunodeficiency virus (HIV). It was also employed for COVID-19 treatment. The molecule was synthesized by using furan derivative 34 as the starting material (Scheme 5) [47-51]. In the method, the iodization reaction of 34 with I2/PPh3 in the presence of imidazole in THF initially led to the intermediate 35, which then led to the terminal alkene 36 via the elimination of HI by treating with NaOMe in MeOH. Addition of [Bn(Et)3N]N3 to the terminal C=C bond of 36 and the subsequent protection of its hydroxyl by benzoyl (Bz) led to the intermediate 37, which then led to the BzO-protected intermediate 39 through 38. Modification of the nitrogen heterocycle of 39 and the subsequent deprotection of hydroxyl afforded Azvudine as the final product.
|
Download:
|
| Scheme 5. Synthetic route for Azvudine. | |
The clinical trial results show that Azvudine has the advantages of low dosage, good effect, high safety, strong targeting and long-term effect in the treatment of COVID-19. The daily dose of 5 mg per person shows good therapeutic effect on COVID-19 patients, and has obvious inhibitory effect on COVID-19 variants [52-54]. On August 9, 2022, Azvudine was included in China's Diagnosis and Treatment Plan for COVID-19 (Ninth Edition) as a therapeutic drug.
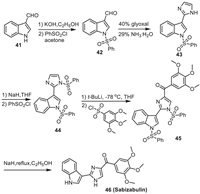
7. SabizabulinSabizabulin (VERU-111) is an oral small molecule tubulin inhibitor developed by Veru. It has potential anti-tumor, antiviral and anti-inflammatory activities and is a new COVID-19 candidate drug. The molecule can be synthesized from 1H-indole-3-carbaldehyde 41 as being illustrated in Scheme 6 [55]. The reaction of 41 with PhSO2Cl initially afforded the intermediate 42. Treating 42 in glyoxal/NH3 solution could form an imidazole cycle on its aldehyde moiety to produce 43. Protection of the N—H of 43 by PhSO2Cl led to 44, in which the C—H adjacent to nitrogen could be converted into the carbon anion by t-BuLi, and the subsequent reaction with 3,4,5-trimethoxybenzenesulfonyl chloride led to intermediate 45. Refluxing 45 with NaH in ethanol produced Sabizabulin.
|
Download:
|
| Scheme 6. Synthetic route for Sabizabulin. | |
On April 11, 2022, Veru announced the planned mid-term positive efficacy and safety results of the COVID-19 clinical trial, which evaluated the comparison of 9 mg of the medicine to placebo in 150 high-risk hospitalized COVID-19 patients with acute respiratory distress syndrome (ARDS). Compared to placebo, Sabizabulin reduced the number of deaths by 55%. In June 2022, Veru submitted an emergency use authorization application to the FDA [56-58]. On November 9, 2022, Veru announced that the FDA had reviewed Sabizabulin's emergency use authorization for moderate to severe COVID-19 inpatients with respiratory distress syndrome and acute high risk.
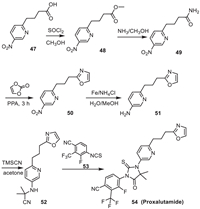
8. ProxalutamideProxalutamide is a small molecule androgen receptor (AR) antagonist jointly developed by Kintor Pharmaceutica and China Medical University. It was synthesized from 4-(5-nitropyridin-2-yl)butanoic acid 47 (Scheme 7) [59]. In the process, 47 was initially converted into the amide 49 via the intermediate ester 48. Reaction of 49 with 1,3-dioxol-2-one formed the oxazole moiety to give the intermediate 50. Reduction of the nitro group of 50 led to 51, which was then converted into 52 via the reaction with TMSCN and acetone. Cyclization reaction of 52 with 53 produced Proxalutamide.
|
Download:
|
| Scheme 7. Synthetic route for Proxalutamide. | |
Proxalutamide can prevent the invasion of COVID-19 by down regulating the expression levels of ACE2 and TMPRSS2 in host cells, play an antiviral role in the early stage of COVID-19 infection, and effectively improve the clinical symptoms of moderate and severe patients in the middle and late stages of COVID-19 infection [60]. In September 2021, Proxalutamide was approved by China National Drug Administration to conduct a Phase III clinical trial. The clinical trial results of Proxalutamide in the treatment of patients with mild to moderate symptoms show that it can 100% reduce the risk of hospitalization in male patients and 90% reduce the risk of hospitalization in female patients [61,62]. The clinical trial results in the treatment of patients with severe COVID-19 showed that Proxalutamide could reduce the death risk of patients with severe COVID-19 by 92%, and shorten the average hospital stay by 9 days [63].
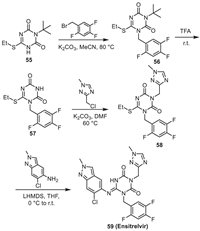
9. EnsitrelvirEnsitrelvir is a kind of non-peptide, non-covalent small molecule 3CLpro inhibitor jointly developed by Shionogi and Hokkaido University. Its synthetic route is illustrated in Scheme 8 [64]. The nucleophilic substitution reaction of 3-(tert-butyl)−6-(ethylthio)−1,3,5-triazine-2,4(1H, 3H)-dione 55 with 1-(bromomethyl)−2,4,5-trifluorobenzene led to the intermediate 56, which could afford 57 after de-protection. The nucleophilic reaction of the imide site in 57 with 3-(chloromethyl)−1-methyl-1H-1,2,4-triazole led to 58, which reacted with 6-chloro-2-methyl-2H-indazol-5-amine to produce Ensitrelvir.
|
Download:
|
| Scheme 8. Synthetic route for Ensitrelvir. | |
On February 25, 2022, Shionogi submitted a manufacturing and sales application to the Ministry of Health, Labour and Welfare of Japan [65]. On March 16, 2022, FDA approved its clinical trial application for a global multicenter Phase III clinical study called SCORPIO—HR (NCT05305547) [66]. On July 4, 2022, Shionogi submitted the relevant preparation materials for the new drug marketing license application to the Centre for Drug Evaluation (NMPA) of the State Drug Administration of China [67]. On September 29, 2022, Shionogi declared that the clinical trial results of COVID-19 patients with mild/moderate symptoms showed that Ensitrelvir could significantly shorten the time required for recovery of patients with Omicron compared with the control drug. However, the results of Phase II/III clinical trial in Part 2b for asymptomatic/mild COVID-19 infected patients showed that compared to the control group, the proportion of virus titer positive patients decreased by about 90% [65,66,68].
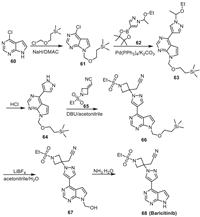
10. BaricitinibBaricitinib is a tyrosine protein kinase JAK1/2 inhibitor developed by Lilly and Incyte [69]. It was synthesized via the method described in Scheme 9 [70]. First, the NH moiety in 4-chloro-7H-pyrrolo[2,3-d]pyrimidine 60 was protected by using (2-(chloromethoxy)ethyl)trimethylsilane via the nucleophilic reaction to produce the intermediate 61. Suzuki coupling reaction of 61 with 62 produced the intermediate 63, which was then treated by HCl to afford 64. Nucleophilic addition of NH in the 1H-pyrazole moiety of 64 to the C = C adjacent to CN in 65 led to the intermediate 66. Treating 66 with LiBF4 removed the silicon moiety and gave 67, which was then converted into Baricitinib via the deprotection reaction under pH 9–10 conditions with NH3.H2O.
|
Download:
|
| Scheme 9. Synthetic route for Baricitinib. | |
Baricitinib is now the only JAK inhibitor that has been approved by FDA for COVID-19 treatment. The results of the clinical trials (ACTT-2, COV-BARRIER) indicate that Baricitinib can reduce the rate of death of the hospital patients, including HFNC or non-ambulance patients [71,72]. On November 19, 2020, Baricitinib was approved by FDA to be used in combination with Remdesivir for the treatment of adult hospitalized COVID-19 patients who need supplemental oxygen, non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO). It is better than Remdesivir alone in accelerating the improvement of clinical status of COVID-2019 patients, and can shorten the recovery time [73]. In May 2022, FDA approved Baricitinib for the treatment of adult hospitalized patients with COVID-19 who need auxiliary oxygen supply, non-invasive/invasive mechanical ventilation and ECMO [74].
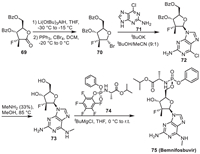
11. BemnifosbuvirBemnifosbuvir (AT-527) is an oral nucleoside based anti-COVID-19 drug jointly developed by Atea Pharmaceuticals and Roche. Scheme 10 is the synthetic route of this molecule [75]. The starting molecule 69 was converted into 70 via the reduction of carbonyl by Li(OtBu)3AlH and the subsequent bromination by CBr4. Promoted by tBuOK base, the nucleophilic substitution reaction of 70 with 71 led to the intermediate 72, which could produce 73 after deprotection of the hydroxyls. The transesterification reaction of 74 with 73 finally afforded Bemnifosbuvir. The total yield of this synthetic route is 61%.
|
Download:
|
| Scheme 10. Synthetic route for Bemnifosbuvir. | |
Previously, Bemnifosbuvir is an oral purine nucleotide prodrug used in the treatment of hepatitis C (HCV) and has now entered the COVID-19 clinical trial Phase III study [76]. Bemnifosbuvir has a unique mechanism of action, with dual targets of chain termination (RdRp) and nucleotide transferase (NiRAN) inhibition, demonstrating good safety and tolerance, as well as low-risk drug interactions, and has great potential as a drug for treating COVID-19 [77,78].
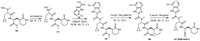
12. PBI-0451PBI-0451 is a peptide like covalent inhibitor developed by Pardes Biosciences [79]. The synthetic route of PBI-0451 is illustrated by Scheme 11 [80]. Deprotection reaction of amine in 76 with HCl led to the related salt 77. Condensation of 77 with carboxylic acid 78 led to the amide 79, which was converted into 80 after the ammonolysis reaction. Dehydration of the terminal amide in 80 with Burgess reagent produced PBI-0451.
|
Download:
|
| Scheme 11. Synthetic route for PBI-0451. | |
On February 3, 2022, the drug application of PBI-0451 was approved by FDA [81]. The results of non-clinical and Phase I clinical trials indicate that PBI-0451 has good tolerance and oral bioavailability [82]. In September 2022, Pardes Biosciences launched a Phase II clinical trial, and the results are expected to be announced in the first quarter of 2023 [83].
13. Conclusions and perspectiveSince the end of 2019, the epidemic of COVID-19 has deeply affected the global economic and political pattern. At present, COVID-19 is still prevalent worldwide, posing a serious threat to human life. As of June 7, 2023, there have been more than 767 million confirmed cases and more than 6.94 million deaths worldwide [84]. In the face of the constantly mutating COVID-19, there is an urgent need for effective therapeutic drugs to ensure the safety of human life. That is to say, COVID-19 epidemic situation does not stop, and new version viruses continue emerging recently. Therefore, developing new medicines is still a meaningful task. As is well known, vaccines, antibodies, and small molecule drugs are the key to overcoming the epidemic. Among them, small molecule drugs have the advantages of convenient administration, low production cost, easy storage and transportation, and have become a hot spot in the field of drug development against COVID-19.
In the future, with the rational application of new technologies and strategies such as artificial intelligence and structure-based drug design, it will certainly promote the discovery of more candidate small molecule drugs specifically against COVID-19. In addition to drug therapy, the authors believe that it is also important to suppress the spread of COVID-19 and reduce its harm by improving public immunity. For example, literature has reported a direct relationship between selenium deficiency and severe damage caused by COVID-19 [85]. Therefore, consuming sufficient selenium from foods may be a good method for preventing the disease [86-88]. The related investigations are ongoing in our laboratory [89].
Declaration of competing interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank Natural Science Foundation of Hubei Province (No. 2022CFB008), the Open Fund Project of Hubei Key Laboratory of Radiation Chemistry and Functional Materials (No. 2021KF07), the fund of the joint-laboratory of Shanghai Dingya Pharmaceutical Chemical Technology Co., Ltd. with Yangzhou University (2022–2027) and Priority Academic Program Development of Jiangsu Higher Education Institution for support.
| [1] |
W.J. Wiersinga, A. Rhodes, A.C. Cheng, S.J. Peacock, H.C. Prescott, JAMA 324 (2020) 782-793. DOI:10.1001/jama.2020.12839 |
| [2] |
X. Xu, P. Chen, J. Wang, et al., Sci. China Life Sci. 63 (2020) 457-460. DOI:10.1007/s11427-020-1637-5 |
| [3] |
M. Hoenigl, D. Abramovitz, R.E. Flores Ortega, N.K. Martin, N. Reau, Clin. Infect. Dis. 75 (2022) e955-e961. DOI:10.1093/cid/ciac175 |
| [4] |
J. Zhang, B. Xie, K. Hashimoto, Brain Behav. Immun. 87 (2020) 59-73. DOI:10.1016/j.bbi.2020.04.046 |
| [5] |
T.C. Liu, S.M. Yoo, M.S. Sim, et al., JAMA Netw. Open 6 (2023) e2311974. DOI:10.1001/jamanetworkopen.2023.11974 |
| [6] |
J.M. Perry, F. Tao, A. Roy, et al., Nat. Cell Biol. 22 (2020) 689-700. DOI:10.1038/s41556-020-0507-y |
| [7] |
N. Mast, P. Verwilst, C.J. Wilkey, F.P. Guengerich, I.A. Pikuleva, J. Med. Chem. 63 (2020) 6477-6488. DOI:10.1021/acs.jmedchem.9b01383 |
| [8] |
H. Beck, M. Härter, B. Haß, C. Schmeck, L. Baerfacker, Drug Discov. Today 27 (2022) 1560-1574. DOI:10.1016/j.drudis.2022.02.015 |
| [9] |
Y. Yang, L. Du, Sig. Transduct, Target. Ther. 6 (2021) 95. |
| [10] |
S. Lei, X. Chen, J. Wu, X. Duan, K. Men, Sig. Transduct. Target. Ther. 7 (2022) 387. DOI:10.1038/s41392-022-01249-8 |
| [11] |
D. Tian, Y. Liu, C. Liang, et al., Biomed. Pharmacother. 137 (2021) 111313. DOI:10.1016/j.biopha.2021.111313 |
| [12] |
W.S. Ho, R. Zhang, Y.L. Tan, C.L.L. Chai, Pharmacol. Res. 179 (2022) 106201. DOI:10.1016/j.phrs.2022.106201 |
| [13] |
D. Li, J. Hu, D. Li, et al., Top. Curr. Chem. 379 (2021) 4. DOI:10.1007/s41061-020-00318-2 |
| [14] |
L. Zhong, Z. Zhang, X. Peng, J. Zou, S. Yang, Precis. Clin. Med. 5 (2022) pbac024. DOI:10.1093/pcmedi/pbac024 |
| [15] |
A.C. Puhl, T.R. Lane, F. Urbina, S. Ekins, Front. Drug Discov. 2 (2022) 837587. DOI:10.3389/fddsv.2022.837587 |
| [16] |
D.C. Blakemore, L. Castro, I. Churcher, et al., Nat. Chem. 10 (2018) 383-394. DOI:10.1038/s41557-018-0021-z |
| [17] |
K.R. Campos, P.J. Coleman, J.C. Alvarez, et al., Science 363 (2019) eaat0805. DOI:10.1126/science.aat0805 |
| [18] |
D. Siegel, H.C. Hui, E. Doerffler, et al., J. Med. Chem. 60 (2017) 1648-1661. DOI:10.1021/acs.jmedchem.6b01594 |
| [19] |
M.G. Santoro, E. Carafoli, Biochem. Biophys. Res. Commun. 538 (2021) 145-150. DOI:10.1016/j.bbrc.2020.11.043 |
| [20] |
W. Yin, C. Mao, X. Luan, et al., Science 368 (2020) 1499-1504. |
| [21] |
W HO, BMJ 371 (2020) m4475. DOI:10.1136/bmj.m4475 |
| [22] |
D.J. Paymode, N. Vasudevan, S. Ahmad, et al., Org. Process Res. Dev. 25 (2021) 1822-1830. DOI:10.1021/acs.oprd.1c00033 |
| [23] |
V. Gopalsamuthiram, C. Williams, J. Noble, et al., Synlett 32 (2021) 326-328. DOI:10.1055/a-1275-2848 |
| [24] |
G.P. Ahlqvist, C.P. McGeough, C. Senanayake, et al., ACS Omega 6 (2021) 10396-10402. DOI:10.1021/acsomega.1c00772 |
| [25] |
T. Hu, Y. Xie, Y. Liu, et al., ChemRxiv (2021), doi: 10.26434/chemrxiv.14208206.v1.
|
| [26] |
G.R. Painter, D.B. Guthrie, G.R. Bluemling, et al., Patent: WO2019113462A1, 2019.
|
| [27] |
A. Steiner, D. Znidar, S.B. Ötvös, et al., Eur. J. Org. Chem. 2020 (2020) 6736-6739. DOI:10.1002/ejoc.202001340 |
| [28] |
T. Benkovics, J.A. Mcintosh, S.M. Silverman, et al., ChemRxiv (2020), doi: 10.26434/chemrxiv.13472373.v1.
|
| [29] |
H. Chen, Y. Lin, Z. Wang, et al., Patent: CN114560894B, 2023.
|
| [30] |
S.M.R. Hashemian, M.H. Pourhanifeh, M.R. Hamblin, M.K. Shahrzad, H. Mirzaei, Biomed. Pharmacother. 146 (2022) 112517. DOI:10.1016/j.biopha.2021.112517 |
| [31] |
A. Jayk Bernal, M.M. Gomes da Silva, D.B. Musungaie, et al., New Engl. J. Med. 386 (2021) 509-520. |
| [32] |
S. Venkatraman, S.L. Bogen, A. Arasappan, et al., J. Med. Chem. 49 (2006) 6074-6086. DOI:10.1021/jm060325b |
| [33] |
R. Zhang, A. Mamai, J.S. Madalengoitia, J. Org. Chem. 64 (1999) 547-555. DOI:10.1021/jo9816109 |
| [34] |
J. Park, A. Sudhakar, G.S. Wong, et al., Patent: WO2004113295A1, 2004.
|
| [35] |
G. Wu, F.X. Chen, P. Rashatasakhon, et al., Patent: WO2007075790A1, 2007.
|
| [36] |
H. Togo, S. Iida, Synlett 2006 (2006) 2159-2175. DOI:10.1055/s-2006-950405 |
| [37] |
S.R. Kallam, V.R. Eda, S. Sen, et al., Tetrahedron 73 (2017) 4285-4294. DOI:10.1016/j.tet.2017.05.080 |
| [38] |
D.R. Owen, C.M.N. Allerton, A.S. Anderson, et al., Science 374 (2021) 1586-1593. DOI:10.1126/science.abl4784 |
| [39] |
Y. Zhao, C. Fang, Q. Zhang, et al., Protein Cell 13 (2021) 689-693. |
| [40] |
E. Mahase, BMJ 375 (2021) n2713. DOI:10.1136/bmj.n2713 |
| [41] |
J.B. Chang, Bull. Natl. Nat. Sci. Found China 36 (2022) 630-634. |
| [42] |
Y. Furua, H. Egawa, Patent: WO2000010569A1, 2000.
|
| [43] |
T. Hara, N. Norimatsu, H. Kurushima, et al., Patent: WO2010087117A1, 2010.
|
| [44] |
S. Tsuzuki, K. Hayakawa, Y. Doi, et al., Infect. Dis. Ther. 11 (2022) 1075-1087. DOI:10.1007/s40121-022-00617-9 |
| [45] |
K. Shiraki, N. Sato, K. Sakai, et al., Pharmacol. Ther. 235 (2022) 108121. DOI:10.1016/j.pharmthera.2022.108121 |
| [46] |
H.A. Erdem, P.E. Korkma, D. Çağlayan, et al., Turk. J. Med. Sci. 51 (2021) 912-920. DOI:10.3906/sag-2008-33 |
| [47] |
J.B. Chang, X.H. Bao, Q. Wang, et al., Patent: CN101177442A, 2008.
|
| [48] |
J.B. Chang, Patent: EP2177527B1, 2013.
|
| [49] |
J.B. Chang, Patent: US8835615B2, 2014.
|
| [50] |
Q. Wang, W.D. Hu, S.Y. Wang, et al., Eur. J. Med. Chem. 46 (2011) 4178-4183. DOI:10.1016/j.ejmech.2011.06.020 |
| [51] |
J.B. Chang, Acc. Chem. Res. 55 (2022) 565-578. DOI:10.1021/acs.accounts.1c00697 |
| [52] |
J.L. Zhang, Y.H. Li, L.L. Wang, et al., Sig. Transduct. Target. Ther. 6 (2021) 414. DOI:10.1038/s41392-021-00835-6 |
| [53] |
Z.G. Ren, H. Luo, Z.J. Yu, et al., Adv. Sci. 7 (2020) e2001435. DOI:10.1002/advs.202001435 |
| [54] |
B. Yu, J.B. Chang, Sig. Transduct. Target. Ther. 5 (2020) 236. DOI:10.1038/s41392-020-00351-z |
| [55] |
W. Li, M. Xiao, J. Dalton, et al., Patent: US20200024270A1, 2020.
|
| [56] |
E.J. Rubin, L.R. Baden, C.C. Hardin, S. Morrissey, New Engl. J. Med. 387 (2022) e51. |
| [57] |
Anna Bratulic, https://firstwordpharma.com/story/5545887 (accessed April 11, 2022).
|
| [58] |
Veru, https://ir.verupharma.com/news-events/press-releases/detail/152/veruannounces-presentation-of-phase-3-study-of-sabizabulin (accessed August 9, 2022).
|
| [59] |
Y.Z. Tong, Patent: WO2012119559A1, 2012.
|
| [60] |
X.D. Hou, H.H. Yan, A. Wang, et al., BioRxiv (2022), doi: 10.1101/2022.06.29.498191.
|
| [61] |
F.A. Cadegiani, J. McCoy, C.G. Wambier, et al., Cureus 13 (2021) e13492. |
| [62] |
F.A. Cadegiani, R.A. Zimerman, D.N. Fonseca, et al., Cureus 13 (2021) e20691. |
| [63] |
O.T. Ranzani, L.S.L. Bastos, J.G.M. Gelli, et al., Lancet Respir. Med. 9 (2021) 407-418. DOI:10.1016/S2213-2600(20)30560-9 |
| [64] |
Y. Unoh, S. Uehara, K. Nakahara, et al., J. Med. Chem. 65 (2022) 6499-6512. DOI:10.1021/acs.jmedchem.2c00117 |
| [65] |
Shionogi, https://www.shionogi.com/global/en/news/2022/2/22-0225.html (accessed February 25, 2022).
|
| [66] |
Shionogi, https://www.shionogi.com/global/en/news/2022/03/e-20220316-2.html (accessed March 15, 2022).
|
| [67] | |
| [68] |
Shionogi, https://www.shionogi.com/global/en/news/2022/2/e-20220207.html (accessed February 7, 2022).
|
| [69] |
Z.T. Al-Salama, L.J. Scott, Drugs 78 (2018) 761-772. DOI:10.1007/s40265-018-0908-4 |
| [70] |
J.D. Rodgers, S. Shepard, Y.L. Li, et al., Patent: WO2009114512A1, 2009.
|
| [71] |
E.W. Ely, A.V. Ramanan, C.E. Kartman, et al., Lancet Respir. Med. 10 (2022) 327-336. DOI:10.1016/S2213-2600(22)00006-6 |
| [72] |
A.C. Kalil, T.F. Patterson, A.K. Mehta, et al., New Engl. J. Med. 384 (2021) 795-807. DOI:10.1056/NEJMoa2031994 |
| [73] |
M. Saber-Ayad, S. Hammoudeh, E. Abu-Gharbieh, et al., Pharmaceuticals 14 (2021) 680. DOI:10.3390/ph14070680 |
| [74] |
R. Rubin, JAMA 327 (2022) 2281. DOI:10.1001/jama.2022.9846 |
| [75] |
J.P. Sommadossi, A. Moussa, Patent: US20210009628A1, 2021.
|
| [76] |
Atea Pharmaceuticals, https://ir.ateapharma.com/news-releases/news-releasedetails/atea-pharmaceuticals-announces-first-patient-dosed-sunrise-3 (accessed November 29, 2022).
|
| [77] |
A. Shannon, V. Fattorini, B. Sama, et al., Nat. Commun. 13 (2022) 621. DOI:10.1038/s41467-022-28113-1 |
| [78] |
E. Berliba, M. Bogus, F. Vanhoutte, et al., Antimicrob. Agents Chemother. 63 (2019) e01201. |
| [79] |
Pardes Biosciences, https://ir.pardesbio.com/news-releases/news-releasedetails/pardes-biosciences-and-fs-development-corp-ii-announce-merger (accessed June 29, 2021).
|
| [80] |
L.D. Arnold, A. Jennings, W. Keung, Patent: GB2595975A, 2021.
|
| [81] |
Pardes Biosciences, https://ir.pardesbio.com/news-releases/news-releasedetails/pardes-biosciences-announces-fda-clearance-ind-application-pbi (accessed February 3, 2022).
|
| [82] |
Pardes Biosciences, https://www.globenewswire.com/news-release/2022/02/14/2384315/0/en/Pardes-Biosciences-Presents-Interim-Clinical-Data-fromOngoing-PBI-0451-Phase-1-Trial-Supporting-the-Potential-of-PBI-0451-as-aStand-Alone-Oral-Regimen-for-COVID-19-at-Conference-.html (accessed February 14, 2022).
|
| [83] |
Pardes Biosciences, https://ir.pardesbio.com/news-releases/news-releasedetails/pardes-biosciences-announces-commencement-phase-2-trial (accessed September 13, 2022).
|
| [84] |
WHO, https://covid19.who.int/(accessed June 7, 2023).
|
| [85] |
S. Khatiwada, A. Subedi, Curr. Nutr. Rep. 10 (2021) 125-136. DOI:10.1007/s13668-021-00354-4 |
| [86] |
L.M. Xian, Q.R. Li, T. Li, et al., Chin. Chem. Lett. 34 (2023) 107878. DOI:10.1016/j.cclet.2022.107878 |
| [87] |
X.R. Xiao, Z.F. Shao, L. Yu, Chin. Chem. Lett. 32 (2021) 2933-2938. DOI:10.1016/j.cclet.2021.03.047 |
| [88] |
Q.Y. Wang, P.Z. Li, T. Li, et al., Ind. Eng. Chem. Res. 60 (2021) 8659-8663. DOI:10.1021/acs.iecr.1c01437 |
| [89] |
W.Q. Ding, S. Wang, J.X. Gu, et al., Chin. Chem. Lett. 34 (2023) 108043. DOI:10.1016/j.cclet.2022.108043 |
 2024, Vol. 35
2024, Vol. 35 


 Zhigang Zeng is now an associate professor at Hubei University of Science and Technology and a senior research fellow at Hubei Huisheng Pharmaceutical Co., Ltd., China. His current research interest focuses on the drug research and development, organic synthesis methodologies including the related applications in green synthesis, (bio)catalysis;
Zhigang Zeng is now an associate professor at Hubei University of Science and Technology and a senior research fellow at Hubei Huisheng Pharmaceutical Co., Ltd., China. His current research interest focuses on the drug research and development, organic synthesis methodologies including the related applications in green synthesis, (bio)catalysis; Lei Yu received his B.Sc. from Nanjing University (2003). He received his Ph.D. from Zhejiang University under the direction of academician Xian Huang (2008). He conducted postdoctoral research at Yangzhou University (2008–2011) and Jiangsu Yangnong Chemical Group Company Limited/Nanjing University (2011–2013) respectively. He visited Prof. Mark Lautens' group at the University of Toronto (2013–2014) as a visiting scholar. He is now a professor at Yangzhou University. He is the head of the joint-laboratory of Shanghai Dingya Pharmaceutical Chemical Technology Co., Ltd. with Yangzhou University. He has published over 190 papers and the H-index is currently 43. He obtained over 40 authorized invention patents, including 4 times of assignment of the patent right and one time license for enterprise use; He created economic benefits with an annual output value exceeding 1 billion yuan (ca. $ 0.14 billion). His-current research interest is on element chemistry including the related applications on green synthesis, catalysis and novel materials development. For more information, please see the personal homepage at:
Lei Yu received his B.Sc. from Nanjing University (2003). He received his Ph.D. from Zhejiang University under the direction of academician Xian Huang (2008). He conducted postdoctoral research at Yangzhou University (2008–2011) and Jiangsu Yangnong Chemical Group Company Limited/Nanjing University (2011–2013) respectively. He visited Prof. Mark Lautens' group at the University of Toronto (2013–2014) as a visiting scholar. He is now a professor at Yangzhou University. He is the head of the joint-laboratory of Shanghai Dingya Pharmaceutical Chemical Technology Co., Ltd. with Yangzhou University. He has published over 190 papers and the H-index is currently 43. He obtained over 40 authorized invention patents, including 4 times of assignment of the patent right and one time license for enterprise use; He created economic benefits with an annual output value exceeding 1 billion yuan (ca. $ 0.14 billion). His-current research interest is on element chemistry including the related applications on green synthesis, catalysis and novel materials development. For more information, please see the personal homepage at: