α-Ketoamides and their derived peptidomimetics are key building blocks in natural products, promising medicinal warhead to target HIV, tumor, Alzheimer's disease, malaria and Parkinson's disease and proteasome inhibitors (Scheme 1a) [1-10]. Moreover, recently, α-ketoamides-containing peptidomimetics were disclosed as a potential oral treatment for Covid-2 SARS, which cause the coronavirus disease 2019 (Covid-19) pandemic [11, 12]. Further, they are versatile and valuable intermediates and synthons in a variety of functional group transformations and drugs' and natural products' total synthesis (e.g., Enalapril, Imidapril, Lisinopril) [13, 14] as well as bioluminescent probe [15-17] and oxidative responsive prodrugs [18, 19]. Despite the vast recent advances of synthetic methods for non-peptidomimetics α-ketoamides [2, 4, 7, 20-29], the classic multiple-step peptide synthesis using α-ketoacids or their derived amino acids remains the main synthetic approach for peptide containing α-ketoamide [26, 30-34]. In contrast to classic peptide synthesis, there were only few reports of late-stage functionalization approaches, which enable the precise modification of oligopeptides at the end of a synthetic sequence [35-45]. One of the late-stage ketoamide formation at peptide sequence is α-oxidation of α-amino acids or α-hydroxyl acids, which normally require stoichiometric metal oxidants with moderate regioselectivity [46-51]. Zhu and others developed multicomponent couplings of isocyanide and aldehydes with amino acids for α-ketoamides synthesis [52-56]. However, application of this elegant strategy to couple with oligopeptides remains unexplored. Thus, development of a site selective late-stage functionalization protocol for α-ketoamide moiety installation in peptidomemtics would be highly demanding.

|
Download:
|
| Scheme 1. Application and late-stage site selective functionalization of α-ketoamide peptidomimetics. | |
Site-specific chemical modification of peptides is becoming increasingly important in research and industry for monitoring cellular events or designing therapeutics, targeting ligands and molecular probes [57-60]. However the suitable chemical transformations for efficient biomolecule functionalization is limited by the stringent conditions and developing chemo- and site-selective transformations remains a prominent challenge [61-68]. Recently, great efforts have been devoted to developing site selective C—H functionalization [43, 69-77], multicomponent stapling reactions [39], cross coupling reactions [78, 79], deaminative conversion [80] and heteroatom (oxygen, nitrogen or sulfur) site nucleophilic substitution [81-85] of peptide sequence (Scheme 1b). Besides, decarboxylative diversification of peptides enables C−C bond cleavage and functionalization at C-terminal [86]. However, transformation of peptide sequence at N-terminal via C—C bond cleavage remains undeveloped.
Ring opening of cyclopropyl amines is one of powerful strategy towards ring construction (Scheme 2a). Werz, Waser and others reported Lewis acid catalytic ring-opening strategies for donor–acceptor type aminocyclopropanes [87-92]. Bower and coworkers disclosed the directed Rh catalytic C—C bond coupling of cyclopropyl amines [93-96]. Although radical ring opening of cyclopropyl amines for alkylation of DNA were disclosed to have relation with the genotoxin with cycolibactins and their derivatives [97, 98], only till recently the emergence of photoredox chemistry led to application of this radical pathway towards organic synthesis. Stephenson's and Zheng's group disclosed a strain-driven [3 + 2] cycloaddition of cyclopropylamines with olefins by visible-light photocatalysis [99-101]. Chen's group reported the selective C(sp3)−C(sp3) cleavage/alkynylation of cycloalkylamides under mild photoredox catalysis conditions [102]. Given the high rate of radical ring-opening of cyclopropyl amines [103], development of coupling process other than annulation remains unsolved and challenging owing to the lack of suitable catalytic systems to adjust coupling rates to this radical clock type process. Herein, we report a domino ring-opening/arylation of cyclopropyl amino amides and their derived peptides. The protocol under-goes a novel radical ring opening/cross-coupling pathway via Pd(I) catalysis, providing diverse α-ketoamide peptidomimetics and even more challenging peptide-natural product conjugates with broad functional group tolerance and excellent site-selectivity (Scheme 2b).
|
Download:
|
| Scheme 2. Typical ring-opening reaction of cyclopropyl amines. | |
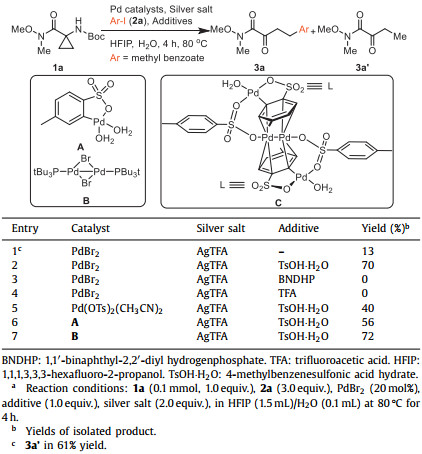
Initial investigation on the catalytic couplings of Weinreb amide (1a) with methyl 4-iodobenzoate (2a) under reported conditions [104-107] for cyclopropyl alcohol only afford ring-opening ketoamide 3a' (Table S1 in Supporting information). This could be attributed to the slower reaction rate of arylation over the ring opening. To enhance the efficiency of coupling process, AgTFA with 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as solvent were added to replace K2CO3, successfully providing 3a in 13% yield with 61% 3a' (entry 1, Table 1). Surprisingly, when 4-methylbenzenesulfonic acid hydrate (TsOH·H2O) was added, the reaction efficiency significantly increased, providing 3a in 70% yield without formation of 3a' (entry 2, Table 1). However, 1,1′-binaphthyl-2,2′-diyl hydrogenphosphate (BNDHP), trifluoroacetic acid (TFA) and other organic acids were ineffective additive (entries 3 and 4, Table 1, Table S3 in Supporting information), further confirming the essential role for sulfonic acid. According to previous reports, the possible role of TsOH·H2O in the Pd-catalyzed reactions can be categorized into two aspects: combination of Pd(II) with TsOH·H2O would afford either electrophilic Pd(OTs)2(CH3CN)2 [108-110] or complex A [111]. Pd(OTs)2(CH3CN)2 delivered lower yield (entry 5, Table 1). In contrast, complex A provided 3a in 56% yields (entry 6, Table 1), suggesting that complex A would be competent catalyst. As Bedford and coworkers disclosed that complex A would be spontaneously converted to unstable Pd(I) species C [111], investigation of possible involvement of Pd(I) species in this catalytic process was carried out.
|
|
Table 1 Optimization of reaction conditions.a |
To explore Pd(I) species in the catalytic cycle, the X-ray photoelectron spectroscopy (XPS) measurement of the reaction mixture using the PdBr2/TsOH·H2O system was carried out. The observed peak structures indicate the presence of two distinct oxidation states of Pd species (Fig. 1). These peaks can be attributed to Pd(I) (16.0 at%) and Pd(II) (84.0 at%) without apparent Pd(0) signals [112-114], which confirms the presence of a Pd(I) species during the reaction. Preparation of reported Pd(I) species in Bedford reference failed owing to its high instability and readily decomposition to palladium black. As complex C is extremely unstable, a stable dinuclear Pd(I) complex B [115-118], instead, was employed, providing a similar yield to standard conditions (entry 7, Table 1). Meanwhile, the kinetic experiments were carried out using three different Pd species, including: PdBr2, Pd(OTs)2(CH3CN)2 and complex B (Fig S2 in Supporting information). Comparing with PdBr2 and Pd(OTs)2(CH3CN)2, B did not exhibited induction period, which suggest the Pd(I) would be the competent catalysts. This result is in contrast to that for a previously reported DAF-Pd(I) species, which reduces the catalytic activity in allylic C—H acetoxylation of terminal alkenes and intramolecular aza-Wacker cyclization [119-121]. Thus, similar to our report on Pd(I)/Pd(II) catalytic cycle [114], we consider the ring-opening arylation processes would be a Pd(I)/Pd(II) catalysis.

|
Download:
|
| Fig. 1. (a) The X-ray photoelectron spectroscopy (XPS) data of the Pd. (b) The X-ray photoelectron spectroscopy (XPS) data of the Ag. | |
In the Pd(I) catalytic pathway, involvement of silver salts is uncommon. Owing to the halogenophilicity of silver [122, 123], Ag(I) was reported to abstract halogen during a reported Pd(I)-involved cross-coupling of enamines with α-bromocarbonyls by Loh [124]. In our case, we indeed detected Ag(I) as the only silver species in XPS [125], further confirming that Ag(I) acts as a halogen abstractor for aryl iodides instead of an oxidant [126, 127]. To our knowledge, this domino ring-opening arylation provide a distinct effective Pd(I) catalytic systems [113, 119, 128-139]. Further screening of catalyst, silver salts, sulfonic acids and other factors confirmed the best conditions for this domino process: 20 mol% PdBr2, 1.0 equiv. TsOH·H2O, 2.0 equiv. AgTFA, with HFIP/H2O as solvent at 80 ℃ for 4 h. Full details of the optimization study are provided in Supporting information.
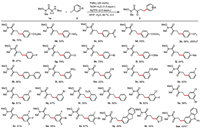
With the optimal conditions in hand, we investigated the scope of aryl iodides with 1a (Scheme 3). A wide range of aryl iodides bearing either electron-withdrawing or electron-donating substituents at ortho, meta and para sites proved to be viable substrates, affording the diverse δ-arylated α-ketoamides in good yields. Synthetically useful functional groups, including fluoro, chloro, bromo, ester, ether, aryl, trifluoromethyl, cyano, nitro, aldehyde, and ketone substituents, were well compatible with this protocol, illustrating the excellent site selectivity, chemoselectivity and potential for late-stage functionalization (3a−3i and 3k−3r). δ-Arylated α-ketoamide 3e were also successfully obtained with ample scope. 3m resulted in lower yield, which could be attribute to hydrolysis and decomposition of 3-iodobenzonitrile under the acidic condition. Similar to our previous Pd(I)/Pd(II) catalysis [114], the steric hinderance of ortho-substituted iodoarenes reduced the reaction efficiency of 3r. Additionally, multiple substituted iodobenzenes including medicinal important fluorine atom were also viable substrates, affording the desired product 3t-3y with 46%–67% yield. Furthermore, the robustness of the ring-opening arylation approach is demonstrated by, among others, the installation of the potentially bioactive thiophene motif (3z) and a carbazole-based fluorescent label (3aa). The potential of the ring-opening arylation strategy for fluorescent labelling is highlighted by the considerably turn-on of the fluorescent properties of the δ-arylated α-ketoamides products 3aa compared to the parent amino acids (Fig. S1 in Supporting information).
|
Download:
|
| Scheme 3. Arylation of weinreb amides: aryl iodide scope. Conditions: 1a (0.1 mmol, 1.0 equiv.), PdBr2 (20 mol%), TsOH·H2O (1.0 equiv.), Ar−I 2 (3.0 equiv.), AgTFA (2.0 equiv.), HFIP (1.5 mL)/H2O (0.1 mL), 80 ℃, under air, 4 h. a Yield when 1a (1.0 mmol). b Conditions: 1a (0.1 mmol, 1.0 equiv.), Pd(OAc)2 (15 mol%), TsOH·H2O (1.0 equiv.), Ar−I 2 (1.5 equiv.), AgTFA (2.0 equiv.), HFIP (1.0 mL), 70 ℃, under air, 7 h. | |
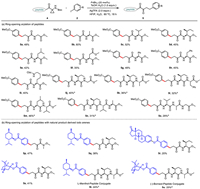
Encouraging by the good functional group compatibility, we evaluated potential of this coupling process for the envisioned bioorthogonal diversification of peptides 4 (Scheme 4a). The ring-opening arylation approach were viable to efficient syntheses of peptide-based derivatives bearing an α-ketoamide pharmacophoric unit at the N-terminal residue, which is inherently complementary drug candidates to the known peptidomimetics bearing α-ketoamide as C-terminus warheads [1-12]. Various dipeptides containing cyclopropyl amino acid residue at the N-terminus were well-tolerated (5a−5i). Dipeptides 4a−4e with alkyl substituents at the C-terminus reacted easily, providing the α-ketoamides in good yields (5a−5e, 42%−60%). When L-aspartic acid, L-phenyl glycine, L-phenylalanine or L-lysine were at the C-terminus, the arylated products 5f, 5g, 5h and 5i were obtained in 35%–48% yield, overriding the possibility of arylation at arenes and deprotection of Cbz group. Further optimization of reaction conditions enabled the facile access to late-stage functionalization of tripeptides (Table S6 in Supporting information). Diverse tripeptides and tetrapeptides were amenable substrates to afford desired α-ketoamides 5j−5o in 29%−46%.
|
Download:
|
| Scheme 4. Scope of peptides. Conditions: 4 (0.1 mmol, 1.0 equiv.), PdBr2 (20 mol%), TsOH·H2O (1.0 equiv.), Ar−I 2 (3.0 equiv.), AgTFA (2.0 equiv.), HFIP (2.0 mL)/H2O (0.2 mL), 80 ℃, under air, 18 h. a Conditions: 4 (0.1 mmol, 1.0 equiv.), PdBr2 (20 mol%), TsOH·H2O (1.0 equiv.), Ar−I 2 (3.0 equiv.), AgTFA (2.0 equiv.), HFIP (2.0 mL)/DMF (0.5 mL), 80 ℃, under air, 24 h. | |
Synthesizing covalent conjugates of a peptide and a complex molecule, e.g., natural products, remains challenging but important strategies in chemical biology and drug discovery [140-143]. These conjugates may have clinical value owing to their capacities to direct small-molecule toxins to certain tissues, widen therapeutic windows and tune pharmacokinetic and pharmacodynamics properties often beyond the capabilities of each component alone. To aim this goal, we applied the ring-opening protocol to the construction of peptide-natural product conjugates (Scheme 4b). The peptide-ketoamide-natural product hybrids were successfully synthesized via the ring-opening arylation strategy using aryl iodides derived from different naturally products. L-Menthol derived aryl iodide readily underwent the ring-opening coupling reactions with Weinreb amide and dipeptide to afford desired product 5p and 5q. Moreover, aryl iodides generated from (-)-borneol and estrone successfully provided the binding products 5r and 5s. Remarkably, tripeptides can successfully be linked with L-menthol and (-)-borneol derived aryl iodides under the standard conditions (5t, 5u).
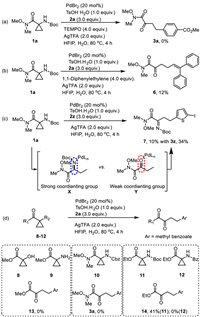
As we proposed that this Pd(I) catalytic domino process would undergo radical process, the further confirmation of radical routine over ionic ring-opening was carried out. Addition of TEMPO completely quenched the couplings (Scheme 5a), suggesting a radical pathway. Radical trap experiments with 1,1-diphenylethylene afford 6 in 12% (Scheme 5b). Notably, during the ring-opening arylation of 1a with 2z, a Minisci type arylation product 7 with Boc group was isolated with 10% yields (Scheme 5c), supporting the generation of X. It not only further supports the radical ring-opening pathway, but suggests that Boc group is involved during the entire ring-opening arylation process and might be cleaved after arylation. Indeed, substrates without Boc group (such as cyclopropanol 8 and cyclopropylamine 9) or with other protecting groups (e.g., Cbz, 10 and Bz, 12) did not afford any desired products. In contrast, ester 11 successfully afforded ring-opening arylation products (Scheme 5d). These results can be rationalized that in-situ generated Boc-protected imine group would be more prone to coordinate with Pd catalyst center for trapping transient radical generated from ring-opening than the weak coordinating carbonyl group in ketoamides.
|
Download:
|
| Scheme 5. Mechanism experiment. | |
Based on previous literatures [108-111, 128-131] and our results, a plausible mechanism has been proposed for this domino transformation (Scheme 6). Initially, PdBr2 reacts with TsOH·H2O to afford Pd(I) catalytic species C [108-110], which then readily undergoes oxidative addition with aryl iodides and anion-exchange with AgTFA to form Pd(II) species D. Subsequent coordination of D with 1a affords intermediate E, which undergoes an intramolecular oxidation to give Pd(I)-radical species F. Further combination of Pd(I) site with radical provides palladium species G, which produces the imine amide H through reductive elimination to regenerate the palladium catalytic species C. Deprotection of H with generated TFA gives the ketoamide 3a.

|
Download:
|
| Scheme 6. Proposed catalytic cycle. | |
In summary, we have developed an unprecedented palladium catalyzed ring-opening arylation strategy for late-stage functionalization of peptides. This domino position-selective diversification of peptides was accomplished under mild conditions with wide functional group compatibility. The robustness of this protocol allows the access to challenging peptides-natural product conjugates. The extensive mechanism investigation suggests that the ring-opening arylation undergoes a distinct radical ring-opening process through Pd(I) catalysis.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors gratefully acknowledge support from the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2017124).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.109250.
| [1] |
P. Chatterjee, W.M. Botello-Smith, H. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 17945-17952. DOI:10.1021/jacs.7b08938 |
| [2] |
C. De Risi, G.P. Pollini, V. Zanirato, Chem. Rev. 116 (2016) 3241-3305. DOI:10.1021/acs.chemrev.5b00443 |
| [3] |
A. Kling, K. Jantos, H. Mack, et al., J. Med. Chem. 60 (2017) 7123-7138. DOI:10.1021/acs.jmedchem.7b00731 |
| [4] |
D. Kumar, S.R. Vemula, G.R. Cook, ACS Catal. 6 (2016) 4920-4945. DOI:10.1021/acscatal.6b01116 |
| [5] |
N. Moskal, G.A. McQuibban, Cell Chem. Biol. 24 (2017) 1431-1433. DOI:10.1016/j.chembiol.2017.12.004 |
| [6] |
A. Peracchi, M. Veiga-da-Cunha, T. Kuhara, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) E3233-E3242. |
| [7] |
M. Robello, E. Barresi, E. Baglini, et al., J. Med. Chem. 64 (2021) 3508-3545. DOI:10.1021/acs.jmedchem.0c01808 |
| [8] |
M.L. Stein, H. Cui, P. Beck, et al., Angew. Chem. Int. Ed. 53 (2014) 1679-1683. DOI:10.1002/anie.201308984 |
| [9] |
F.G. Njoroge, K.X. Chen, N.-Y. Shih, et al., Acc. Chem. Res. 41 (2008) 50-59. DOI:10.1021/ar700109k |
| [10] |
B.I. Morinaka, E. Lakis, M. Verest, et al., Science 359 (2018) 779-782. DOI:10.1126/science.aao0157 |
| [11] |
L. Zhang, D. Lin, Y. Kusov, et al., J. Med. Chem. 63 (2020) 4562-4578. DOI:10.1021/acs.jmedchem.9b01828 |
| [12] |
L. Zhang, D. Lin, X. Sun, et al., Science 368 (2020) 409-412. DOI:10.1126/science.abb3405 |
| [13] |
C. Piemontesi, Q. Wang, J. Zhu, J. Am. Chem. Soc. 138 (2016) 11148-11151. DOI:10.1021/jacs.6b07846 |
| [14] |
J.S. Ham, B. Park, M. Son, et al., J. Am. Chem. Soc. 142 (2020) 13041-13050. DOI:10.1021/jacs.0c04278 |
| [15] |
X. Xie, F. Tang, G. Liu, et al., Anal. Chem. 90 (2018) 11629-11635. DOI:10.1021/acs.analchem.8b03207 |
| [16] |
K. Yoshioka, T. Komatsu, A. Nakada, et al., J. Am. Chem. Soc. 137 (2015) 12187-12190. DOI:10.1021/jacs.5b05884 |
| [17] |
N.A. Bindman, W.A. van der Donk, J. Am. Chem. Soc. 135 (2013) 10362-10371. DOI:10.1021/ja4010706 |
| [18] |
J. Fu, J. Han, T. Meng, et al., Chem. Commun. 55 (2019) 12904-12907. DOI:10.1039/C9CC05266F |
| [19] |
T. Meng, J. Han, P. Zhang, et al., Chem. Sci. 10 (2019) 7156-7162. DOI:10.1039/C9SC00910H |
| [20] |
J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Org. Lett. 22 (2020) 2206-2209. DOI:10.1021/acs.orglett.0c00387 |
| [21] |
A. de la Torre, D. Kaiser, N. Maulide, J. Am. Chem. Soc. 139 (2017) 6578-6581. DOI:10.1021/jacs.7b02983 |
| [22] |
S. Guo, J. Hua, Z. Dai, et al., ACS Sustain. Chem. Eng. 6 (2018) 7979-7988. DOI:10.1021/acssuschemeng.8b01339 |
| [23] |
L. Lu, X. Pei, Y. Mei, et al., Chem 4 (2018) 2861-2871. DOI:10.1016/j.chempr.2018.09.003 |
| [24] |
L. Lu, F. Qiu, H. Alhumade, et al., ACS Catal. 12 (2022) 9664-9669. DOI:10.1021/acscatal.2c02569 |
| [25] |
Y. Lv, P. Bao, H. Yue, et al., Green Chem. 21 (2019) 6051-6055. DOI:10.1039/C9GC03253C |
| [26] |
F. Penteado, E.F. Lopes, D. Alves, et al., Chem. Rev. 119 (2019) 7113-7278. DOI:10.1021/acs.chemrev.8b00782 |
| [27] |
Y. Tokuhiro, K. Yoshikawa, S. Murayama, et al., ACS Catal. 12 (2022) 5292-5304. DOI:10.1021/acscatal.2c00950 |
| [28] |
C. Zhang, N. Jiao, J. Am. Chem. Soc. 132 (2010) 28-29. DOI:10.1021/ja908911n |
| [29] |
F. Zhao, H.J. Ai, X.F. Wu, Angew. Chem. Int. Ed. 61 (2022) e202200062. DOI:10.1002/anie.202200062 |
| [30] |
C.L. Harrison, M. Krawiec, R.E. Forslund, et al., Tetrahedron 67 (2011) 41-47. DOI:10.1016/j.tet.2010.11.018 |
| [31] |
J. Ma, X. Cui, J. Xu, et al., J. Org. Chem. 87 (2022) 3661-3667. DOI:10.1021/acs.joc.1c02453 |
| [32] |
F. Rohrbacher, A. Zwicky, J.W. Bode, Helv. Chim. Acta 101 (2018) e1800039. DOI:10.1002/hlca.201800039 |
| [33] |
F. Thuaud, F. Rohrbacher, A. Zwicky, et al., Org. Lett. 18 (2016) 3670-3673. DOI:10.1021/acs.orglett.6b01692 |
| [34] |
F. Zhao, N. Meng, T. Sun, et al., Org. Chem. Front. 8 (2021) 6508-6514. DOI:10.1039/D1QO01351C |
| [35] |
X. Chen, F. Ye, X. Luo, et al., J. Am. Chem. Soc. 141 (2019) 18230-18237. DOI:10.1021/jacs.9b09127 |
| [36] |
J. Duan, Y.F. Du, X. Pang, et al., Chem. Sci. 10 (2019) 8706-8712. DOI:10.1039/C9SC03347E |
| [37] |
M. Imiolek, G. Karunanithy, W.L. Ng, et al., J. Am. Chem. Soc. 140 (2018) 1568-1571. DOI:10.1021/jacs.7b10230 |
| [38] |
T.J. Osberger, D.C. Rogness, J.T. Kohrt, et al., Nature 537 (2016) 214-219. DOI:10.1038/nature18941 |
| [39] |
L. Reguera, D.G. Rivera, Chem. Rev. 119 (2019) 9836-9860. DOI:10.1021/acs.chemrev.8b00744 |
| [40] |
C. Song, K. Liu, Z. Wang, et al., Chem. Sci. 10 (2019) 7982-7987. DOI:10.1039/C9SC02218J |
| [41] |
J. Tan, J. Wu, S. Liu, et al., Sci. Adv. 5 (2019) eaaw0323. DOI:10.1126/sciadv.aaw0323 |
| [42] |
J. Tang, Y. He, H. Chen, et al., Chem. Sci. 8 (2017) 4565-4570. DOI:10.1039/C6SC05530C |
| [43] |
W. Wang, M.M. Lorion, J. Shah, et al., Angew. Chem. Int. Ed. 57 (2018) 14700-14717. DOI:10.1002/anie.201806250 |
| [44] |
B.-B. Zhan, J. Fan, L. Jin, et al., ACS Catal. 9 (2019) 3298-3303. DOI:10.1021/acscatal.9b00544 |
| [45] |
X. Zhang, G. Lu, M. Sun, et al., Nat. Chem. 10 (2018) 540-548. DOI:10.1038/s41557-018-0006-y |
| [46] |
T. Li, X. Cui, Y. Cui, et al., ACS Catal. 10 (2020) 7950-7957. DOI:10.1021/acscatal.0c01895 |
| [47] |
S. Liu, Y.-M. Cui, F.-J. Nan, Org. Lett. 10 (2008) 3765-3768. DOI:10.1021/ol801419m |
| [48] |
S. Murahashi, A. Mitani, K. Kitao, Tetrahedron Lett. 41 (2000) 10245-10249. DOI:10.1016/S0040-4039(00)01823-2 |
| [49] |
D. Ranganathan, N.K. Vaish, K. Shah, J. Am. Chem. Soc. 116 (1994) 6545-6557. DOI:10.1021/ja00094a008 |
| [50] |
J.E. Semple, T.D. Owens, K. Nguyen, et al., Org. Lett. 2 (2000) 2769-2772. DOI:10.1021/ol0061485 |
| [51] |
G.G. Xu, F.A. Etzkorn, Org. Lett. 12 (2010) 696-699. DOI:10.1021/ol9027013 |
| [52] |
M. Bouma, G. Masson, J. Zhu, J. Org. Chem. 75 (2010) 2748-2751. DOI:10.1021/jo100302y |
| [53] |
J.W. Collet, C. Foley, A.Y. Shaw, et al., Org. Biomol. Chem. 15 (2017) 6132-6135. DOI:10.1039/C7OB00881C |
| [54] |
C. Foley, A. Shaw, C. Hulme, Org. Lett. 19 (2017) 2238-2241. DOI:10.1021/acs.orglett.7b00710 |
| [55] |
C. Foley, A. Shaw, C. Hulme, Org. Lett. 20 (2018) 1275-1278. DOI:10.1021/acs.orglett.7b03977 |
| [56] |
J.M. Grassot, G. Masson, J. Zhu, Angew. Chem. Int. Ed. 47 (2008) 947-950. DOI:10.1002/anie.200704840 |
| [57] |
K. Fosgerau, T. Hoffmann, Drug Discov. Today 20 (2015) 122-128. DOI:10.1016/j.drudis.2014.10.003 |
| [58] |
S.B. Gunnoo, A. Madder, Org. Biomol. Chem. 14 (2016) 8002-8013. DOI:10.1039/C6OB00808A |
| [59] |
Q.Y. Hu, F. Berti, R. Adamo, Chem. Soc. Rev. 45 (2016) 1691-1719. DOI:10.1039/C4CS00388H |
| [60] |
J.L. Lau, M.K. Dunn, Bioorg. Med. Chem. 26 (2018) 2700-2707. DOI:10.1016/j.bmc.2017.06.052 |
| [61] |
A. Boto, C.C. González, D. Hernández, et al., Org. Chem. Front. 8 (2021) 6720-6759. DOI:10.1039/D1QO00892G |
| [62] |
O. Boutureira, G.J. Bernardes, Chem. Rev. 115 (2015) 2174-2195. DOI:10.1021/cr500399p |
| [63] |
E.A. Hoyt, P.M.S.D. Cal, B.L. Oliveira, et al., Nat. Rev. Chem. 3 (2019) 147-171. DOI:10.1038/s41570-019-0079-1 |
| [64] |
O. Koniev, A. Wagner, Chem. Soc. Rev. 44 (2015) 5495-5551. DOI:10.1039/C5CS00048C |
| [65] |
C. Sornay, V. Vaur, A. Wagner, et al., Royal Soc. Open Sci. 9 (2022) 211563. DOI:10.1098/rsos.211563 |
| [66] |
C.D. Spicer, B.G. Davis, Nat. Commun. 5 (2014) 4740. DOI:10.1038/ncomms5740 |
| [67] |
T. Tamura, I. Hamachi, J. Am. Chem. Soc. 141 (2019) 2782-2799. DOI:10.1021/jacs.8b11747 |
| [68] |
S.J. Walsh, J.D. Bargh, F.M. Dannheim, et al., Chem. Soc. Rev. 50 (2021) 1305-1353. DOI:10.1039/D0CS00310G |
| [69] |
Z. Bai, Q. Chen, J. Gu, et al., ACS Catal. 11 (2021) 15125-15134. DOI:10.1021/acscatal.1c05030 |
| [70] |
X.X. Guo, D.W. Gu, Z. Wu, et al., Chem. Rev. 115 (2015) 1622-1651. DOI:10.1021/cr500410y |
| [71] |
X. Hou, N. Kaplaneris, B. Yuan, et al., Chem. Sci. 13 (2022) 3461-3467. DOI:10.1039/D1SC07267F |
| [72] |
L. Liu, X. Fan, B. Wang, et al., Angew. Chem. Int. Ed. 61 (2022) e202206177. DOI:10.1002/anie.202206177 |
| [73] |
X. Lu, S.J. He, W.M. Cheng, et al., Chin. Chem. Lett. 29 (2018) 1001-1008. DOI:10.1016/j.cclet.2018.05.011 |
| [74] |
D.G. Rivera, G.M. Ojeda-Carralero, L. Reguera, et al., Chem. Soc. Rev. 49 (2020) 2039-2059. DOI:10.1039/C9CS00366E |
| [75] |
S. Shabani, Y. Wu, H.G. Ryan, et al., Chem. Soc. Rev. 50 (2021) 9278-9343. DOI:10.1039/D0CS01441A |
| [76] |
H.R. Tong, B. Li, G. Li, et al., CCS Chem. 3 (2021) 1797-1820. DOI:10.31635/ccschem.020.202000426 |
| [77] |
Y. Weng, X. Xu, H. Chen, et al., Angew. Chem. Int. Ed. 61 (2022) e202206308. DOI:10.1002/anie.202206308 |
| [78] |
S. Li, D. Pissarnitski, T. Nowak, et al., ACS Catal. 12 (2022) 3201-3210. DOI:10.1021/acscatal.1c05502 |
| [79] |
T. Meng, L.A. Wells, T. Wang, et al., J. Am. Chem. Soc. 144 (2022) 12476-12487. DOI:10.1021/jacs.2c04627 |
| [80] |
J. Am. Chem. Soc. 144 (2022) 20135–20136.
|
| [81] |
E.M.D. Allouche, E. Grinhagena, J. Waser, Angew. Chem. Int. Ed. 61 (2022) e202112287. DOI:10.1002/anie.202112287 |
| [82] |
N. Declas, J.R.J. Maynard, L. Menin, et al., Chem. Sci. 13 (2022) 12808-12817. DOI:10.1039/D2SC04558C |
| [83] |
D. Karipal Padinjare Veedu, L.A. Connal, L.R. Malins, Angew. Chem. Int. Ed. 62 (2023) e202215470. DOI:10.1002/anie.202215470 |
| [84] |
O. Nwajiobi, A.K. Verma, M. Raj, J. Am. Chem. Soc. 144 (2022) 4633-4641. DOI:10.1021/jacs.2c00464 |
| [85] |
Z. Tan, S. Zhu, Y. Liu, et al., Angew. Chem. Int. Ed. 61 (2022) e202203374. DOI:10.1002/anie.202203374 |
| [86] |
L. Candish, K.D. Collins, G.C. Cook, et al., Chem. Rev. 122 (2022) 2907-2980. DOI:10.1021/acs.chemrev.1c00416 |
| [87] |
F. de Nanteuil, E. Serrano, D. Perrotta, et al., J. Am. Chem. Soc. 136 (2014) 6239-6242. DOI:10.1021/ja5024578 |
| [88] |
A. Kreft, A. Lucht, J. Grunenberg, et al., Angew. Chem. Int. Ed. 58 (2019) 1955-1959. DOI:10.1002/anie.201812880 |
| [89] |
H. Liu, Z. Fu, S. Gao, et al., Adv. Synth. Catal. 360 (2018) 3171-3175. DOI:10.1002/adsc.201800200 |
| [90] |
M. Petzold, P.G. Jones, D.B. Werz, Angew. Chem. Int. Ed. 58 (2019) 6225-6229. DOI:10.1002/anie.201814409 |
| [91] |
V. Pirenne, B. Muriel, J. Waser, Chem. Rev. 121 (2021) 227-263. DOI:10.1021/acs.chemrev.0c00109 |
| [92] |
O.O. Sokolova, J.F. Bower, Chem. Rev. 121 (2021) 80-109. DOI:10.1021/acs.chemrev.0c00166 |
| [93] |
A.G. Dalling, T. Yamauchi, N.G. McCreanor, et al., Angew. Chem. Int. Ed. 58 (2019) 221-225. DOI:10.1002/anie.201811460 |
| [94] |
G. Fumagalli, S. Stanton, J.F. Bower, Chem. Rev. 117 (2017) 9404-9432. DOI:10.1021/acs.chemrev.6b00599 |
| [95] |
O.O. Sokolova, J.F. Bower, Angew. Chem. Int. Ed. 61 (2022) e202205007. DOI:10.1002/anie.202205007 |
| [96] |
G.W. Wang, J.F. Bower, J. Am. Chem. Soc. 140 (2018) 2743-2747. DOI:10.1021/jacs.7b13087 |
| [97] |
A.R. Healy, H. Nikolayevskiy, J.R. Patel, et al., J. Am. Chem. Soc. 138 (2016) 15563-15570. DOI:10.1021/jacs.6b10354 |
| [98] |
Y. Jiang, A. Stornetta, P.W. Villalta, et al., J. Am. Chem. Soc. 141 (2019) 11489-11496. DOI:10.1021/jacs.9b02453 |
| [99] |
Y. Cai, J. Wang, Y. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 12259-12266. DOI:10.1021/jacs.7b06319 |
| [100] |
S. Maity, M. Zhu, R.S. Shinabery, et al., Angew. Chem. Int. Ed. 51 (2012) 222-226. DOI:10.1002/anie.201106162 |
| [101] |
D. Staveness, T.M. Sodano, K. Li, et al., Chem 5 (2019) 215-226. DOI:10.1016/j.chempr.2018.10.017 |
| [102] |
Z. Liu, S. Wu, Y. Chen, ACS Catal. 11 (2021) 10565-10573. DOI:10.1021/acscatal.1c02981 |
| [103] |
O.M. Musa, J.H. Horner, H. Shahin, et al., J. Am. Chem. Soc. 118 (1996) 3862-3868. DOI:10.1021/ja954268f |
| [104] |
K. Cheng, P.J. Walsh, Org. Lett. 15 (2013) 2298-2301. DOI:10.1021/ol4008876 |
| [105] |
N. Nithiy, A. Orellana, Org. Lett. 16 (2014) 5854-5857. DOI:10.1021/ol5027188 |
| [106] |
Q. Wang, R. Chen, J. Lou, et al., ACS Catal. 9 (2019) 11669-11675. DOI:10.1021/acscatal.9b04161 |
| [107] |
X. Zhou, S. Yu, L. Kong, et al., ACS Catal. 6 (2015) 647-651. |
| [108] |
R. Giri, J.K. Lam, J.Q. Yu, J. Am. Chem. Soc. 132 (2010) 686-693. DOI:10.1021/ja9077705 |
| [109] |
C.E. Houlden, C.D. Bailey, J.G. Ford, et al., J. Am. Chem. Soc. 130 (2008) 10066-10067. DOI:10.1021/ja803397y |
| [110] |
A.J. Reay, L.A. Hammarback, J.T.W. Bray, et al., ACS Catal. 7 (2017) 5174-5179. DOI:10.1021/acscatal.6b03121 |
| [111] |
R.B. Bedford, M.F. Haddow, C.J. Mitchell, et al., Angew. Chem. Int. Ed. 50 (2011) 5524-5527. DOI:10.1002/anie.201101606 |
| [112] |
H.M. Huang, P. Bellotti, P.M. Pfluger, et al., J. Am. Chem. Soc. 142 (2020) 10173-10183. DOI:10.1021/jacs.0c03239 |
| [113] |
G.Z. Wang, R. Shang, W.M. Cheng, et al., J. Am. Chem. Soc. 139 (2017) 18307-18312. DOI:10.1021/jacs.7b10009 |
| [114] |
Y. Cheng, S. Yu, Y. He, et al., Chem. Sci. 12 (2021) 3216-3225. DOI:10.1039/D0SC05409G |
| [115] |
C.C.C. Johansson Seechurn, T. Sperger, T.G. Scrase, et al., J. Am. Chem. Soc. 139 (2017) 5194-5200. DOI:10.1021/jacs.7b01110 |
| [116] |
G. Magnin, J. Clifton, F. Schoenebeck, Angew. Chem. Int. Ed. 58 (2019) 10179-10183. DOI:10.1002/anie.201903765 |
| [117] |
M. Mendel, I. Kalvet, D. Hupperich, et al., Angew. Chem. Int. Ed. 59 (2020) 2115-2119. DOI:10.1002/anie.201911465 |
| [118] |
G. Yin, I. Kalvet, F. Schoenebeck, Angew. Chem. Int. Ed. 54 (2015) 6809-6813. DOI:10.1002/anie.201501617 |
| [119] |
J.N. Jaworski, C.V. Kozack, S.J. Tereniak, et al., J. Am. Chem. Soc. 141 (2019) 10462-10474. DOI:10.1021/jacs.9b04699 |
| [120] |
J.N. Jaworski, S.D. McCann, I.A. Guzei, et al., Angew. Chem. Int. Ed. 56 (2017) 3605-3610. DOI:10.1002/anie.201700345 |
| [121] |
S.J. Tereniak, S.S. Stahl, J. Am. Chem. Soc. 139 (2017) 14533-14541. DOI:10.1021/jacs.7b07359 |
| [122] |
M. Naodovic, H. Yamamoto, Chem. Rev. 108 (2008) 3132-3148. DOI:10.1021/cr068413r |
| [123] |
J.M. Weibel, A. Blanc, P. Pale, Chem. Rev. 108 (2008) 3149-3173. DOI:10.1021/cr078365q |
| [124] |
R. Ding, Z.D. Huang, Z.L. Liu, et al., Chem. Commun. 52 (2016) 5617-5620. DOI:10.1039/C5CC10653B |
| [125] |
W. Huang, X. Kang, C. Xu, et al., Adv. Mater. 30 (2018) 1706962. DOI:10.1002/adma.201706962 |
| [126] |
Y. Yu, U.K. Tambar, Chem. Sci. 6 (2015) 2777-2781. DOI:10.1039/C5SC00505A |
| [127] |
A. Lobato, M.A. Salvado, J.M. Recio, Chem. Sci. 12 (2021) 13588-13592. DOI:10.1039/D1SC02152D |
| [128] |
M. Parasram, V. Gevorgyan, Chem. Soc. Rev. 46 (2017) 6227-6240. DOI:10.1039/C7CS00226B |
| [129] |
Q. Liu, X. Dong, J. Li, et al., ACS Catal. 5 (2015) 6111-6137. DOI:10.1021/acscatal.5b01469 |
| [130] |
N. Hazari, D.P. Hruszkewycz, Chem. Soc. Rev. 45 (2016) 2871-2899. DOI:10.1039/C5CS00537J |
| [131] |
P. Chuentragool, D. Kurandina, V. Gevorgyan, Angew. Chem. Int. Ed. 58 (2019) 11586-11598. DOI:10.1002/anie.201813523 |
| [132] |
M. Ratushnyy, N. Kvasovs, S. Sarkar, et al., Angew. Chem. Int. Ed. 59 (2020) 10316-10320. DOI:10.1002/anie.201915962 |
| [133] |
W.J. Zhou, G.M. Cao, G. Shen, et al., Angew. Chem. Int. Ed. 56 (2017) 15683-15687. DOI:10.1002/anie.201704513 |
| [134] |
K.P.S. Cheung, D. Kurandina, T. Yata, et al., J. Am. Chem. Soc. 142 (2020) 9932-9937. DOI:10.1021/jacs.0c03993 |
| [135] |
B. Zhao, R. Shang, G.Z. Wang, et al., ACS Catal. 10 (2019) 1334-1343. |
| [136] |
W.M. Cheng, R. Shang, Y. Fu, Nat. Commun. 9 (2018) 5215. DOI:10.1038/s41467-018-07694-w |
| [137] |
Z. Zhang, C.R. Rogers, E.A. Weiss, J. Am. Chem. Soc. 142 (2020) 495-501. DOI:10.1021/jacs.9b11278 |
| [138] |
C. Wang, G. Dong, J. Am. Chem. Soc. 140 (2018) 6057-6061. DOI:10.1021/jacs.8b03530 |
| [139] |
L. Song, L. Zhu, Z. Zhang, et al., Org. Lett. 20 (2018) 3776-3779. DOI:10.1021/acs.orglett.8b01363 |
| [140] |
R. Gianatassio, J.M. Lopchuk, J. Wang, et al., Science 351 (2016) 241-246. DOI:10.1126/science.aad6252 |
| [141] |
J. Ohata, S.C. Martin, Z.T. Ball, Angew. Chem. Int. Ed. 58 (2019) 6176-6199. DOI:10.1002/anie.201807536 |
| [142] |
S. Bloom, C. Liu, D.K. Kolmel, et al., Nat. Chem. 10 (2018) 205-211. DOI:10.1038/nchem.2888 |
| [143] |
D.T. Cohen, C. Zhang, C.M. Fadzen, et al., Nat. Chem. 11 (2019) 78-85. DOI:10.1038/s41557-018-0154-0 |
 2024, Vol. 35
2024, Vol. 35