b College of Chemistry, Nankai University, Tianjin 300071, China
Molecular recognition stands as a fundamental and pivotal topic in supramolecular chemistry, in which research of molecular recognition in aqueous environments is particularly significant [1–3]. Water, as the ubiquitous physiological solvent, is intimately involved in myriad biological processes that rely on molecular recognition [4]. Exploring molecular recognition in aqueous phase not only helps unravel the chemical essence of life's activities but also provides insights into the fundamental principles governing these processes [5,6]. The early exploration of aqueous-phase molecular recognition with synthetic receptors can be traced back to ions recognition by cryptands [7], and has since witnessed remarkable progress, closely intertwined with the studies of macrocyclic receptors and the deepening understanding of hydrophobic effect [8,9]. By harnessing the synergistic interplay between aqueous environments and molecular recognition, scientists have made remarkable strides in developing innovative supramolecular systems with superior functionalities and appealing applications. These progresses have influenced a variety of research areas, including molecular sensing and detection [10], ions recognition [11], water treatment [12], catalysis [13,14], smart soft materials [15], transmembrane transport [16], gene transfection [17], disease diagnosis and therapy [18,19], functional biomaterials [20,21], bioorthogonal tools [22,23], among others.
Among the numerous synthetic macrocycles developed during the past half century, cyclophanes emerge as an important class of artificial macrocyclic receptors, occupying a pivotal role in molecular recognition studies [24–27]. In a broad sense, cyclophanes can be defined as cyclic compounds including one or more aromatic moieties as an integral part of their structure. The aromatic moieties in cyclophanes not only facilitates chemical modifications to impart water solubility but also enables the formation of hydrophobic cavities for guest inclusion in aqueous environments. In 1955, Stetter and Roos reported the synthesis of cyclophanes CP1~CP3 (Fig. 1) and their ability to form stable 1:1 complexes with benzene and dioxane [28], which has been seen as first approach of synthetic receptor towards apolar molecules. However, X-ray crystallography revealed that the largest CP3 formed a clathrate structure, where benzene is accommodated between host molecules rather than within the intramolecular space [29]. Since the 1970s, research on the synthesis of new water-soluble cyclophanes and the investigation of their recognition properties have steadily increased. In 1980, Koga et al. reported a 1:1 complex between CP4 and 2, 7-naphthalenediol in diluted hydrochloric acid (Ka = 2.8 × 103 L/mol) and durene@CP4 complex in solid state [30], opening up new possibilities for water-phase guest recognition of cyclophane. Subsequently, Diederich et al. developed functionalized cyclophanes based on diphenylmethane units (CP5~CP8), and thoroughly investigated their guest-binding properties in water [24,31]. Over the following decades, the family of cyclophanes expanded rapidly, leading to the synthesis of novel water-soluble cyclophanes such as blue box [32], calixarenes [33], resorcinarenes [34], calixpyrroles [35], tetralactams [36], pillararenes [37], octopusarenes [38], biphenarenes [39], coronarenes [40], naphthotubes [41]. Water-soluble cyclophanes exhibit distinct recognition properties and diverse functions, making them highly appealing in host-guest chemistry.
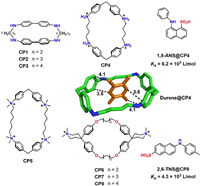
|
Download:
|
| Fig. 1. Some early reported water-soluble synthetic cyclophane (CCDC: 1100928 for Durene@CP4). | |
This review intends to comprehensively summarize cyclophane receptors tailored specifically for binding organic guests in aqueous environments. We aim to shed light on the significant impact of cavity modifications on the recognition properties of cyclophanes, categorizing them into two primary classes: those featuring an "exo-functionalized hydrophobic cavity" and those featuring an "endo-functionalized hydrophobic cavity". By exploring the distinct characteristics of different cyclophanes, we delve into typical strategies employed to enhance recognition affinity and/or selectivity. It is our fervent hope that this review will serve as a catalyst, inspiring the creation of novel synthetic receptors with captivating recognition properties and paving the way for real-world applications of cyclophanes.
2. How solvent effects of water affect interactions between polar/nonpolar groupsMolecular recognition in aqueous environments differs significantly from that in organic solvents, primarily due to the distinctive solvent effects of water [42]. Complex stability tends to decrease with higher solvent permittivity, driven by the electrostatic component of noncovalent interactions [5]. Solvation of binding partners, especially polar functional groups, further diminishes complex stability by offsetting free energy decrease gained from complex formation with the energy required to desolvation. As a result, complexes stable in nonpolar environments can weaken significantly in highly polar solvents with strong solvation [43]. Surprisingly, molecular recognition in water remains remarkably efficient despite its high permittivity and strong hydrogen bonds. Synthetic receptors have been demonstrated to function effectively in water, and biological recognition processes predominantly occur in aqueous media [4]. Some biological receptors, such as the biotin/avidin pairs, can even exhibit exceptional binding affinities reaching up to 1015 L/mol [44], which is rarely achieved in other solvents by synthetic receptors. The ultra-high affinity in biological recognition systems is attributed to the synergistic effect between the strong hydrophobic interactions within the binding pocket and the complementary recognition provided by multiple polar binding sites within the cavities. Hence, molecular recognition in water is intimately connected to the unique properties of water molecules and the overall water environment.
In this section, we will try to briefly introduce the impact of water on the binding interactions involving both nonpolar and polar groups. By examining these factors, we can gain valuable insights into the origin of recognition properties and facilitate a more insightful discussion on the performance of cyclophanes in water.
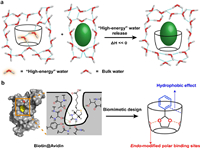
2.1. Hydrophobic effect driven nonpolar binding in waterThe hydrophobic effect (Fig. 2a) plays a crucial role in the binding processes of nonpolar molecules/groups in aqueous environments [9,45,46]. Although often driving molecular association/aggregation, hydrophobic effect not a kind of direct noncovalent interaction but rather a consequence of the energy difference between the solvation of water molecules and the intermolecular interactions within the associating species. Compared to polar and protic organic solvents, water exhibits the stronger cohesive intermolecular interactions (in the form of stronger hydrogen bonds) and the lower molecular polarizability [42]. Consequently, when water molecules cannot establish strong interactions with a solute, they tend to associate with other water molecules, promoting the association/aggregation of the nonpolar solutes. This effect can be driven by entropy, enthalpy, or a combination of both, depending on the shape, size, and polarity of the solutes involved. Supramolecular chemists' understanding about the nature of hydrophobic effect is constantly evolving and deepening, and it has been extensively discussed in some excellent reviews [9,47,48]. Building upon this knowledge, we would like to highlight several important aspects regarding the significance of the hydrophobic effect in host-guest recognition:
|
Download:
|
| Fig. 2. (a) The schematic illustration of hydrophobic effect based on "high-energy" water : upon complexing with a guest, the "high-energy" waters inside the hydrophobic cavity will be released to the bulk, forming more hydrogen bonds and thus providing enthalpy contribution. The "high-energy" water refers the water molecules inside the hydrophobic cavity, which could not form enough hydrogen bonds compare with bulk water. (b) Biomimetic design of cyclophane. Reproduced with permission [54]. Copyright 2018, American Chemical Society. | |
(1) Enhancing the hydrophobic effect is crucial for high and even super-high affinity binding in aqueous environments. This is particularly evident in the study of cucurbituril systems, where the release of "high-energy" water has been identified as a significant source of the extraordinary high affinity [45]. This has also been confirmed in the research of cyclophanes such as blue box [49] and deep cavitands [50].
(2) Houk et al. have conducted comprehensive studies on biological recognition receptors such as proteins and enzymes, comparing them with artificial macrocyclic receptors [51]. They pointed out that increasing the buried hydrophobic area between the host and guest can effectively enhance the hydrophobic effect and improve binding affinity.
(3) The hydrophobic effect is influenced by various factors related to the hydrophobic cavity, such as its shape, size, rigidity, and the presence of polarity-modifying groups. For instance, a concaved or closed hydrophobic cavity effectively hinders the formation of hydrogen bonds with water molecules, resulting in a "drying" state within the cavity [52]. The size of the cavity plays a significant role in determining the size of water molecule clusters and the average number of hydrogen bonds formed. Flexible cavities tend to collapse hydrophobically, impeding guest binding. Moreover, the polar-functional groups within or on the rim(s) of the cavity reduces its hydrophobicity, favoring a "wetting" state.
These considerations will be addressed and explored in our upcoming presentations and discussions regarding the recognition properties of different cyclophanes.
2.2. Electrostatic/hydrogen bonding interactions between polar groups in waterIn contrast to the enhancement of interactions between nonpolar groups through the hydrophobic effect in water, the interactions between polar groups, such as electrostatic interactions and hydrogen bonds, are often significantly weakened in aqueous environments [53]. The reasons are evident, as water has high permittivity and serves as both a hydrogen bond donor and acceptor. Consequently, polar groups tend to be well hydrated, requiring the displacement of surrounding water molecules when a binding occurs. This would reduce overall free energy decrease of the binding process. Therefore, the recognition of polar molecules with good affinity and selectivity in an aqueous environment poses a significant challenge in supramolecular chemistry [54].
However, as mentioned earlier, biological systems, such as protein/ligand interactions [55], enzyme/substrate recognition [56], and DNA complementary pairing [57], exhibit remarkable affinity and specificity. In these systems, the interactions between polar groups, such as electrostatic interactions and hydrogen bonding, play indispensable roles. Understanding the mechanisms underlying molecular recognition in biological systems provides crucial insights and provide inspiration in synthetic receptor design (Fig. 2b).
Firstly, while the interactions between polar groups are significantly weakened in aqueous environment, the hydration of these groups is substantially suppressed within hydrophobic microenvironments. Consequently, the electrostatic or hydrogen bonding recognition within such hydrophobic microenvironments remains highly efficient. For example, the hydrophobic pockets in proteins often contain residues capable of forming various electrostatic or hydrogen bonding interactions [58,59], which are vital for the specificity of substrate recognition.
Secondly, the cooperative effects of multiple binding sites and diverse types of interactions can significantly enhance the recognition affinity and selectivity. For instance, the DNA double helix relies on multiple hydrogen bonds and synergistic π-π stacking between base pairs, thereby ensuring robust complementary recognition. Therefore, through the rational preorganization of multiple polar interactions, it is possible to achieve high-affinity and selective molecular recognition in aqueous environments.
These observations provide important inspiration for us how to exploit polar groups in the design of aqueous-phase recognition receptors. By rationally positioning hydrogen bonding or electrostatic interaction sites within hydrophobic cavities or harnessing the synergistic interactions between multiple polar groups, we can effectively exploit the recognition capabilities of polar groups and overcome the challenges associated with recognizing polar molecules (e.g., biomolecules) in aqueous environments.
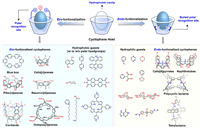
3. Exo- vs. endo-functionalization of cyclophane cavities to regulate the recognition propertiesAs abovementioned, both the hydrophobic cavity and polar group modifications on cyclophanes are crucial for recognition in the aqueous phase. Polar groups can not only enhance the water solubility of cyclophanes but also provide additional interactions to interact with guest molecules. To better understand the functionalization modes of cyclophanes, we roughly categorize cyclophanes into two major classes based on the spatial arrangement of decorated polar functional groups and their hydrophobic cavities (Fig. 3).
|
Download:
|
| Fig. 3. Exo- vs. endo-functionalized cavity to regulate the recognition properties of cyclophanes. | |
The first category is the cyclophanes which featuring an exo-functionalized hydrophobic cavity, where polar groups are modified at the edge of the hydrophobic cavity. Examples of exo-functionalized cyclophanes include blue box, calixarenes, pillararenes, and resorcinarenes, etc. These receptors are suitable for recognizing nonpolar guests or nonpolar molecules with polar headgroups.
The second category is the cyclophanes with an endo-functionalized cavity, where polar groups are modified inside the hydrophobic cavity and thus closely resembling the biological hydrophobic cavities. Examples of endo-functionalized cyclophanes include tetralactams, naphthotubes, polycyclic lactams, and calixpyrroles, etc. For these receptors, the hydrophobic effect is weakened by the polar groups inside the hydrophobic cavity. Nevertheless, the biomimetic endo-functionalized cavities render these receptors suitable for recognizing polar guests, such as carbohydrates, dioxane, and epoxy compounds. Additionally, the hydrogen bonds or/and salt bridges contribute extra interactions inside the hydrophobic cavity for improving the binding selectivity for the polar or amphiphilic guests.
It is worth noting that, for these both two categories, sometimes part of polar functional groups are located far from the hydrophobic cavity, and therefore they may only help to improve water-solubility and provide little contribution to the recognition of the cyclophanes.
4. Recognition properties of cyclophanes with exo-functionalized hydrophobic cavityIn this section, we will discuss the recognition properties of several representative types of water-soluble cyclophanes—especially the cases reported in recent years—which have no polar functional groups oriented inside the cavities.
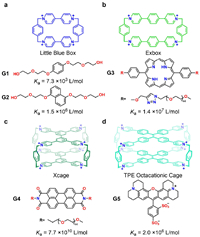
4.1. Blue box and derivativesCyclobis(paraquat-p-phenylene), also known as the blue box, is a tetracationic cyclophane that was first reported by Stoddart in 1988 [60] and has since become one of the most extensively investigated box-like receptors in supramolecular chemistry (Fig. 4) [32]. Its highly electron-deficient and hydrophobic cavity could include a series of electron-rich aromatic guests and phenyl glycopyranoside in aqueous phase [61,62], with binding constants ranging from 102 to 106 L/mol. For example, blue box could bind towards G1 (Ka = 7.3 × 103 L/mol, ΔH = −8.3 kcal/mol) and G2 (Ka = 1.5 × 106 L/mol, ΔH = −15.7 kcal/mol) in water [63]. The binding process mainly relies on enthalpy contribution. Blue box's excellent hosting ability, coupled with its unique redox-responsive property, makes it essential not only in supramolecular recognition but also in creating mechanically interlocked molecules and redox-controlled molecular machines.
|
Download:
|
| Fig. 4. The chemical formulas of (a) blue box, (b) Exbox, (c) Xcage and (d) TPECage and their corresponding guests. | |
Since 2013, a serials of new blue box analogues [64] with extended cavity sizes in terms of their lengths, widths, and depths were developed by Stoddart et al., such as Exbox [65], Excage [66] and Xcage [67]. Their enlarged positively charged cavities could accommodate polycyclic aromatic guests (i.e., pyrene, perylene, coronene, porphyrin and fullerenes) with excellent binding affinities in organic solvents, leading to various applications, including fullerene separation, semiconductive materials, drug delivery, catalysis, photo-up conversion and so on [64,67,68]. Exbox exhibited hosting ability towards G3 with Ka = 1.4 × 107 L/mol in water phase (Fig. 4b). Notably, in 2020, Stoddart et al. reported a rare case of ultrahigh-affinity recognition for a tricyclic octacationic receptor named Xcage (Fig. 4c) in aqueous solution. XCage exhibited picomolar affinities (up to 7.7 × 1010 L/mol, ΔG = −14.8 kcal/mol, ΔH = −14.1 kcal/mol) towards G3 and G4 in water, which was further applied to bioimaging [67,69]. The unique ultrahigh binding strength was ascribed to: (1) the large buried hydrophobic surface between the host-guest complexes ensures a strong hydrophobic effect; (2) the high rigidity of the conformations of both the host and the guests; (3) the good size and electrostatic match between the host and the guests. This example provides useful information for the design of ultrahigh-affinity cyclophanes.
In 2020, Cao et al. reported a new octacationic cage (TPECage) (Fig. 4d) containing two tetraphenylethene (TPE) units [70]. The TPE units gave rise to the significantly extended hydrophobic cavity with good conformational adaptivity, which not only allows to bind one large sized guest, but also bind two relatively smaller guests synergistically into the cavity. Besides, the incorporation of TPE units brought about good fluorescent emissive property (quantum yield: 30.95%) in water. The combination of adaptive recognition and fluorescent emission rendered TPECage to be very useful for sensing different analytes such as sulforhodamine 101 (G5), and chiral guests in aqueous solution [71].
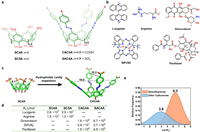
4.2. Calix[n]arenesCalix[n]arenes (C[n]As) are a class of cyclophanes composed of phenol units linked together by methylene bridges at their m-position. This affords them a concave bowl-like aromatic cavity, making them a vital class of cyclophanes. Given their facile accessibility and convenient chemical modification, several water-soluble derivatives of calix[n]arenes have been reported [33]. Sulfonatocalix[n]arenes (SCnAs) are the most extensively investigated calixarene hosts in aqueous phase, since the incorporation of multiple negatively charged sulfonate groups onto the upper rims of calix[n]arenes endows them significantly improved hosting ability. Typically, SCnAs showed good binding affinities towards positively charged organic guests. For example, SC4A (Figs. 5a-d) was found to bind lucigenin and arginine with binding constants of 2.8 × 107 L/mol and 1.5 × 103 L/mol, respectively [72,73]. Enlarging the cavity sizes (also the inner hydrophobic surfaces) by increasing the repeating units of SCnAs, i.e. from SC4A to SC5A, however, did not significantly enhance the guest binding affinities according to experimental results.
|
Download:
|
| Fig. 5. (a) The chemical formulas of sulfonatocalixarenes and azocalixarenes. (b) Chemical structures of guests for calixarenes. (c) Single crystal structures of SC4A and CAC4A (CCDC 652299, 736244), when the cavity is expanded by azobenzene groups, the hydrophobic surface and volume is significantly enlarged (Labeled distances on the graph are in Å). (d) Table of binding constants (L/mol) between selected calixarenes and guests in aqueous phase ("—" represent for not measured). (e) Statistics of binding constants (normalized distribution) of azocalixarenes and other calixarenes towards guests. | |
Moreover, calixarenes can be easily modified on the upper rim or lower rim. The azo-coupling reaction on calixarenes provides a powerful and facile approach for making azocalix[n]arenes with much deeper cavities. These azocalixarenes have exhibited outstanding recognition ability towards a wide range of hydrophobic guests owning to the enhanced hydrophobic effect of the expanded deep cavities. In recent years, a series of azocalix[4]arenes have been synthesized by Guo et al., and their recognition properties have been extensively investigated [74–76]. Notably, CAC4A and SAC4A can bind towards a series of charged drugs and dyes such as doxorubicin and SiPcN2 with an affinity of up to 108 L/mol in PBS buffer. Moreover, azocalixarenes also exhibited good binding affinities towards some neutral organic molecules such as paclitaxel with good affinities. For example, CAC4A can accommodate paclitaxel with a Ka value of 1.6 × 106 L/mol in PBS buffer.
From the single crystal structure of SC4A and CAC4A (Fig. 5c), the azo coupling expansion evidently causes a significantly enlarged hydrophobic pocket and inner hydrophobic surfaces, facilitating the improved encapsulation of larger-sized hydrophobic guests. Based on 81 host-guest titration data of azocalixarenes and over 900 titration data of other calixarenes in aqueous solution [77], the average Ka value of the inclusion complexes of azocalixarene reach 106.3 L/mol (Fig. 5e), which is significantly higher than the average Ka value of other calixarenes (103.8 L/mol). These findings emphasize the significant impact of expanding the hydrophobic cavity depth of cyclophanes as a highly effective approach to enhance binding affinities.
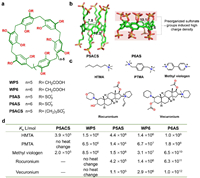
4.3. Pillar[n]arenesPillar[n]arenes (PnAs) are a type of pillar-shaped cyclophanes, synthesized by condensation of 1, 4-dimethoxybenzene with paraformaldehyde, which was firstly reported by Ogoshi et al. in 2008 [78]. PnAs manifest cylindrical shaped cavities with exo-modified polar groups. In 2010, Ogoshi group reported the first water-soluble pillar[5]arene [79]. Later, a plethora of water-soluble PnAs were synthesized and their recognition properties have been extensively investigated [80]. In recent years, increasing attentions have been paid to seeking applications of PnAs in biomedical materials and biotechnology, and their molecular recognition properties have played crucial roles. WP5 and WP6 with 10 or 12 carboxylate groups on the two rims of P5A and P6A, respectively, are two typical water-soluble PnAs that have been widely explored in guest recognition and related applications.
Notably, in 2020, Isaacs et al. developed [81] a new type of anionic modified pillararenes (P5AS and P6AS, Fig. 6) which exhibited significantly enhanced binding strength towards quaternary ammonium salts. As Fig. 6 illustrated, the authors compared the host-guest binding strengths of three different pillar[5]arene derivatives, WP5, P5AS and P5ACS. The three hosts possess the same pillar[5]arene backbone, and the only difference are the anionic functional groups. Remarkably, the sulfated pillar[5]arene, P5AS, exhibited much higher binding affinities than the other two hosts in most cases. The similar phenomenon is more pronounced in the comparison of pillar[6]arene derivatives. The binding strengths of the sulfated pillar[6]arene, P6AS, is generally higher than that of WP6 by 2 to 6 orders of magnitude, with the highest binding affinity reaching up to 1012 L/mol. The extraordinary high affinity of P6AS is attributed to the dense decoration of multiple negatively charged sulfonate groups on the upper and lower rims of the hydrophobic cavity. This strategic arrangement enables a synergistic interplay between hydrophobic interactions and multi-point electrostatic effects, leading to an exceptionally strong binding capacity towards quaternary ammonium salts.
|
Download:
|
| Fig. 6. (a) The chemical formulas of selected pillararene derivatives. (b) The single crystal structures of P5ACS and P6AS (CCDC: 1996179, 1996177). (c) The chemical formulas of some selected guests for the pillararene derivatives. (d) Table of binding constants (L/mol) between the pillararene derivatives and the guests in aqueous phase. | |
In addition to this example, Huang et al., Li et al., García-Río et al., and others have synthesized a few cationic [82–84] or neutral [85,86] pillararenes and investigated their recognition properties towards drugs or biomolecules. The good recognition properties of pillararenes renders them important components for the applications in biomaterials and nanotechnologies.
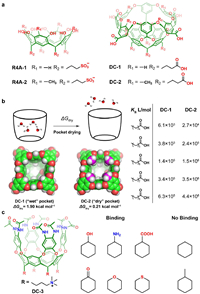
4.4. Resorcin[4]arenes and related cavitandsResorcin[4]arenes are macrocycles consisting of four electron-rich resorcinol units, first reported by Aoyama [87]. These macrocycles have a relatively shallow hydrophobic cavity in the cone conformation [88]. Introducing alkyl sulfonate groups onto the low rim of resorcin[4]arene represents a typical approach to bring about water solubility, as exemplified by R4A-1 and R4A-2. The two resorcin[4]arene derivatives are capable of binding towards cycloalkanol (Fig. 7a). R4A-2 exhibits higher binding affinities than R4A-1 since the methyl groups on the upper rim slightly expand the depth of the hydrophobic cavity [89] and thus lead to stronger hydrophobic effect. Therefore, increasing the depth of hydrophobic cavity may also be an efficient way to improve the hosting properties of resorcin[4]arene. To this end, a few extended resorcin[4]arenes known as cavitands were synthesized by Rebek, Diederich and Gibb et al. by rationally functionalizing at the upper rim, resulting in deep hydrophobic cavities [34,90,91]. The deep cavitands (DC) have been extensively investigated as excellent containers for linear organic molecules, and thus were also used to thoroughly investigate chemical reactivity in nanoconfined spaces by Rebek et al.
|
Download:
|
| Fig. 7. (a) The chemical formulas of selected resorcin[4]arenes and related deep cavitands. (b) Schematic illustration of spontaneous drying pocket in aqueous phase recognition. (c) The recognition of hydrophilic cyclic compounds by the water-soluble DC-3. | |
In 2020, Ashbaugh et al. conducted an inspirational study to discuss the hydrophobic effect—they also termed as "spontaneous drying" effect—of deep cavitands on the recognition property in water (Figs. 7a and b) [52]. The recognition behavior of two receptors, DC-1 and DC-2, were compared in the study. DC-1 has a "wet" cavity that can hold nearly four water molecules inside according to theoretical predictions. In contrast, the cavity of DC-2 with four additional methyl substitutes on the upper rim remains "dry" when dissolved in water. Although the two receptors possess similar cavity sizes, the binding affinities of DC-2 towards fatty acids increased 4 to 10 times compared to DC-1 because of the absence of water molecules inside the cavity. Molecular simulations revealed that the energy required to expel water outside the cavity (ΔGdry) was 1.90 kcal/mol for DC-1, whereas it was only 0.21 kcal/mol for DC-2. This phenomenon is similar to some nonpolar binding sites of proteins that remain "dry" in water, resulting in more enthalpy release in such a "dry" pocket. Therefore, this study indicates that by appropriately modifying the hydrophobic cavity with nonpolar groups, the cavity can be de-wetted, resulting in a more thermodynamically favorable binding process. This effect holds true even for cavities with very similar size.
Recently, Rebek and Yu et al. developed a series of new water-soluble resorcin[4]arene derived deep cavitands. For example, in 2021, they developed a cavitand (DC-3) containing eight amide groups on the upper rims of the hydrophobic cavity (Fig. 7c) [92]. The amide groups can provide additional hydrogen bonding sites in recognizing small polar molecules, such as cyclohexanol, cyclohexylamine, cyclohexanoic acid, cyclohexanone and so on, while cycloalkane without polar groups such as cyclohexane and methylcyclohexane could not be recognized by DC-3. Indeed, the similar strategy of functionalizing the deep cavitands using hydrogen bonding groups have been frequently exploited by Rebek and Yu et al. to modulate of the recognition properties of cavitands [93,94].
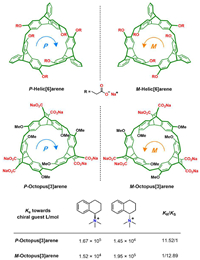
4.5. Helic[6]arenes and octopus[3]arenesIn recent years, Chen et al. have made notable contributions to the development of new cyclophane-based chiral receptors (Fig. 8). In 2016, they firstly reported the synthesis and chiral recognition property of a pair of enantiomeric trimeric triptycene macrocycles, namely, helic[6]arene [95]. Later, they functionalized helic[6]arene with six carboxylate groups to bring about water solubility. The chiral hydrophobic cavity of helic[6]arenes was explored to recognize quaternary phosphonium salts in water [96]. By encapsulating a cationic fluorescent dye in the chiral cavity of helic[6]arene, the resulting host-guest complexes could self-assemble into aggregates with circularly polarized luminescence [97].
|
Download:
|
| Fig. 8. The chemical formulas of helic[6]arenes and octopus[3]arenes, and the enantioselective recognition property of octopus[3]arenes towards a pair of chiral guests. | |
Indeed, the recognition of enantiomer of chiral guests with high selectivity remains a significant challenge for synthetic receptors. In 2022, Chen group reported a new class of chiral receptors named octopus[3]arenes [38]. Octopus[3]arenes are composed of three homochiral ethenoanthracene subunits. Notably, octopus[3]arenes have demonstrated high enantioselectivity (KS/KR) up to 12.89 in water phase among enantiomeric synthetic receptors. The binding affinities of these receptors was also impressive, reaching up to 106 L/mol. Their rigid hydrophobic cavities provide good recognition properties, which are expected to inspire the design of novel chiral receptors.
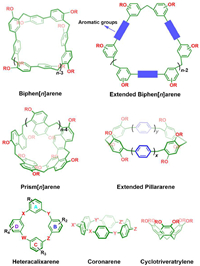
4.6. Other representative water-soluble cyclophanesAside from the abovementioned cases, there are still a large number of new water-soluble cyclophanes (Fig. 9) have been developed in recent decades, such as biphen[n]arenes [98,99], prism[n]arenes [100], extended pillar[n]arenes [101,102], heteracalixarenes [103], coronarenes [104], cyclotriveratrylene [105], as well as, cages [106,107] and capsules [108], among others. Due to the constraints of space, a comprehensive discussion of each case is not feasible within the given scope. These novel receptors have demonstrated appealing hosting properties towards a wide range of guests in water and hold promising prospects for further investigation and applications.
|
Download:
|
| Fig. 9. The chemical formulas of some other representative water-soluble cyclophanes developed in the last decade. | |
In this section, we introduce several cyclophanes that have been strategically modified with polar groups within their inner cavities, enabling them to selectively recognize polar organic molecules. These receptor molecules, featuring polar functional groups within the hydrophobic cavities, exhibit a biomimetic design, closely resembling the receptor pockets found in biological systems.
5.1. Calix[4]pyrrolesCalix[4]pyrroles are macrocycles containing four pyrrole units with methylene linkage. The four NH moieties provide hydrogen bonding sites inside the hydrophobic cavity, making them excellent receptors for recognizing anions [35]. However, the limited cavity depth renders calix[4]pyrrole a relatively poor host for organic guests.
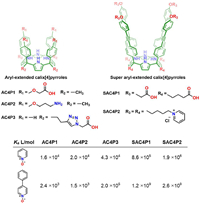
Flexible substitutes were firstly introduced either to the upper rim or the lower rim of calix[4]pyrrole by Pablo in 2009 to afford AC4P1, AC4P2 and AC4P3 (Fig. 10). These receptors manifested moderate to good recognition affinities (103~105 L/mol) towards pyridyl N-oxide and p-phenyl pyridyl N-oxide in water. In 2016, Pablo et al. developed "super aryl-extended" calix[4]pyrroles, SAC4P1 and SAC4P2 (Fig. 10) [109]. The deep hydrophobic cavity encircled by four diphenylethyne groups brought about much stronger hydrophobic effect upon guest inclusion. SAC4P1 and SAC4P2 were found to form ultrastable complex with p-phenyl pyridyl N-oxide in water as Ka reaches 109 L/mol [110]. The positive or negative charged functional chains on the two rims do not affect the binding constants significantly in this case, indicating the binding process is mainly driven by the hydrophobic effect and hydrogen bonding. Again, the case of super aryl-extended calix[4]pyrroles further demonstrated that extending the depth of hydrophobic cavity is a very effective strategy for improving the binding affinities of cyclophanes.
|
Download:
|
| Fig. 10. The chemical formulas of aryl-extended calix[4]pyrrole derivatives and their binding constants towards pyridyl N-oxide and p-phenyl pyridyl N-oxide. | |
In 2007, Smith et al. reported the synthesis of an anthracene-containing tetralactam macrocycle, TLacM [111], which can be conveniently functionalized with water soluble groups at the phenyl units. The amide linkages within the macrocycle provided hydrogen bond donors which pointed into the cavity. The near-infra emissive squaraine dyes were found to be the best matched guest molecules for TLacM [112]. This is because they not only have a planar aromatic backbone that can engage in favorable π-π interactions with the two anthracene walls but also possess two oxygen atoms as hydrogen bond acceptors to complementarily interact with the four NH groups of TLacM. The highly complementary recognition interactions together with the strong hydrophobic effect led to high binding constant (Ka) towards squaraine acid reached 1.0 × 109 L/mol in water [112].
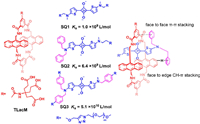
In 2018, Smith et al. proposed an effective strategy to further increase the binding affinity between tetralactams and squaraine dyes [113]. Instead of modifying the receptors, they introduced extra hydrophobic groups to the squaraine dyes to provide additional back-folding interactions (Fig. 11). Phenyl groups were added to the side chain of SQ1 to produce SQ2 and SQ3. It was found that, every single additional phenyl group could increase the binding constants by ca. 3~8 times. The maximum binding constant in water was Ka = 5.1 × 1010 L/mol. The ultrahigh affinities rendered the tetralactam/squaraine complexes to be very stable in biological mediums, making the complexes useful in bioimaging applications.
|
Download:
|
| Fig. 11. Recognition property of tetralactams towards squaraine dyes and scheme of back-folding interactions. | |
In 2022, Jiang et al. reported a new water-soluble tetralactam containing 2, 6-diethoxynaphthalene groups that can strongly bind to riboflavin in water (Ka = 1.2 × 107 L/mol) through hydrogen bonds and the hydrophobic effect [114]. The nanoconfined cavity was found to efficiently protect riboflavin from photodegradation under UV–vis irradiation, which is potentially useful in improving the performance of riboflavin as a drug and additive.
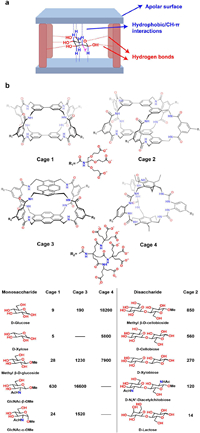
5.3. Polycyclic lactams and ureasRecognizing carbohydrates using synthetic receptors in water presents a significant challenge for supramolecular chemists due to the high hydrophilicity of carbohydrates and the weakened hydrogen bonds in water. To overcome this challenge, Davis et al. designed and synthesized a series of polycyclic lactams and ureas receptors for recognizing carbohydrates in water [53]. As illustrated in Fig. 12, these receptors feature a "temple" shaped cavity with hydrophobic "roof" and "floor" regions connected by several polar "pillars". The "roof" and "floor" provide hydrophobic/CH-π interactions towards the axial nonpolar hydrogen of carbohydrates, while the polar "pillars" offer hydrogen bonds with equatorial hydroxyl groups. This biomimetic design concept has been proved to be very effective in the recognition of carbohydrates in water.
|
Download:
|
| Fig. 12. (a) The design strategy of synthetic receptors for complementary recognition of carbohydrates. (b) The chemical formulas and binding constants towards several carbohydrates using different polycyclic lactams and ureas. | |
As an early version of polycyclic lactams, Cage 1 contained two biphenyl units and isophthalamide groups as linkers [115]. The polar "pillars" could offer a total of eight hydrogen bond donors directed toward the aromatic binding site. Cage 1 could form 1:1 complex with several monosaccharides in water and displayed some selectivity among the carbohydrates. Cage 2, an extended version of Cage 1, featured meta-terphenyl hydrophobic walls and ten hydrogen bond donors, were found to be a good receptor for disaccharide recognition [116]. They could recognize mono- or disaccharides in water with binding constants ranging from 5 L/mol to 850 L/mol. Obviously, the binding affinities of these receptors were not very satisfactory. In 2016, Davis further developed a new polycyclic lactam, Cage 3, which had two pyrenes as the "roof" and "floor" [117]. Cage 3 exhibited outstanding binding constant towards GlcNAc-β-OMe, reaching 16, 600 L/mol, which was a remarkable achievement for synthetic receptors targeting carbohydrates, benefiting from improved hydrophobic effect during recogntion.
Due to the vital role of d-glucose as a source of energy for living organisms, selective recognition of d-glucose is essential for synthetic receptors. The selective recognition of d-glucose was also realized by Davis et al. by synthesizing Cage 4, a polycyclic ureas receptors [118]. This bicyclic hexaurea exhibited good recognition affinity towards d-glucose with Ka = 18, 200 L/mol, which is comparable to the binding affinity of lectins or glucose transporters. Moreover, the selectivity over other related carbohydrates was also good, and thus hold great promise for future bio-related applications. Delicately controlling the property of the hydrophobic cavity and the hydrogen bonding sites is a powerful strategy in synthetic receptors design for selectively recognizing highly challenged hydrophilic guests in water.
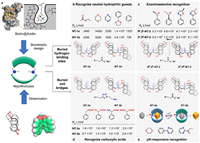
5.4. NaphthotubesIn 2004, Glass et al. reported a pair of water-soluble naphthalene-based fluorescent molecular macrocycles [119], which were obtained by dimerizing two V-shaped, rigidly connected bis-naphthalene units into a macrocycle. These receptors recognized lipid guests accompanied with quenched fluorescence. However, the amide binding sites inside the hydrophobic cavity were not utilized in the recognition of lipids in Glass's research. Inspired by natural receptors such as avidin, Professor Wei Jiang realized that these macrocycles, which have biomimetic hydrophobic cavities with endo-functionalized hydrogen bonding sites, might have great potentials in overcoming the challenging task of recognizing small hydrophilic molecules in water [41]. Therefore, Jiang et al. conducted a series of remarkable studies based on these macrocyclic molecules—which they named as "naphthotubes"—and their analogues with a specific focus on the biomimetic molecular recognition. In the past decade, they have made significant research progress in exploring the water-phase recognition properties of naphthotubes and expanding their applications in different areas (Fig. 13a).
|
Download:
|
| Fig. 13. (a) The biomimetic design of naphthotubes. Reproduced with permission [54]. Copyright, 2018, American Chemical Society. The summary of their recognition properties of different naphthotubes, including: (b) the recognition of neutral hydrophilic molecules, (c) the enantioselective recognition of chiral guests, (d) the recognition of carboxylic acids, and (e) the pH-responsive recognition of hydrophilic guests. Reproduced with permission [122]. Open access. | |
The endo-functional polar groups of naphthotubes are typically responsible for providing hydrogen bond binding sites or/and salty bridge sites, which are essential for the recognition of hydrophilic guests. For instance, NT-1a and NT-1b (Fig. 13b), which feature two amide protons oriented towards the hydrophobic cavity, were ideal receptors for binding with neutral hydrophilic guests [54]. These naphthotubes have demonstrated, by Jiang et al., to exhibit good binding constants (up to 1.4 × 106 L/mol) towards small organic guests including 1, 4-dioxane and 2-phenyl-1, 3-dioxolane in the aqueous phase. These results represent a remarkable achievement, since recognizing small hydrophilic organic guests such as 1, 4-dioxane was a longstanding difficult task in the supramolecular field.
It is noteworthy that, by exploiting chiral bis-naphthalene motifs, Jiang et al. also synthesized a pair of enantiopure naphthotubes, R2, S2-NT-2 and S2, R2-NT-2 (Fig. 13c), and conducted a comprehensive study on the enantioselective molecular recognition in water [120]. Impressive enantioselectivity values of up to 2.04 have been achieved for R and S, which represents a promising model for advancing our understanding of enantioselective recognition in nature.
Secondary amine groups are typically employed to provide salty bridge binding sites inside naphthotubes, which is widely exist in proteins but rarely used in synthetic receptors. Jiang et al. reported a pair of amine-functionalized naphthotubes, NT-3a and NT-3b (Fig. 13d), which was designed to recognize carboxylic acids in water [121]. The binding constants among guests were significantly affected by the structure of the carboxylic acids, especially their electrical property. An additional carboxylic group provided extra ionic interaction within the hydrophobic cavity, leading to the highest Ka value of up to 105 L/mol. When the carboxylic group was substituted with a hydroxyl or amine group, the binding constants were significantly reduced. This phenomenon underscored the crucial role that buried salt bridges play in molecular recognition.
Moreover, amine groups are pH-sensitive and thus can be used in pH-responsive recognition [122]. NT-4a and NT-4b (Fig. 13e) were further reported and their pKa values were determined to be 10.1 and 10.2, respectively. The endo-functionalized secondary amine group of NT-4a and NT-4b can be protonated and deprotonated by adjusting the pH value. Therefore, the naphthotubes showed drastically different guest-binding preferences at the two pH levels, thereby enabling pH-responsive recognition in water.
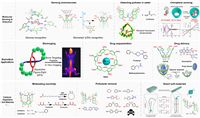
6. Applications of cyclophanes based on their recognition propertiesOver the past four decades, there has been extensive and in-depth research focused not only on expanding the structural diversity and understanding the recognition of cyclophanes but also on exploring the applications based on their water-based molecular recognition properties. These efforts have yielded remarkable achievements across various fields, including molecular sensing and detection, biomedical materials, catalysis, molecular separation, and smart soft materials, among others. However, due to limited space, we can only provide a brief overview of a small fraction of these applications, intending to showcase the tip of the iceberg of their vast application prospects (Fig. 14).
|
Download:
|
| Fig. 14. Applications of water-soluble cyclophanes in various fields. Reproduced with permission [125,126,130]. Copyright 2017, American Chemical Society; Copyright 2017, Wiley; Copyright 2023, Wiley. | |
Molecular recognition plays a pivotal role in the sensing and detection of trace amount of analytes. In this regard, cyclophanes have emerged as valuable tools for selectively sensing vital biomolecules and biomarkers in biofluids. A noteworthy example is the polycyclic lactam cages developed by Davis et al., which exhibit high selectivity for glucose sensing. This breakthrough holds great promise for monitoring diabetes and other glucose-related disease processes [118]. Another example came from Guo et al., they reported an efficient approach based on indicator displacement assay (IDA) method using a calixarene derivative to detect the cancer biomarker, lysophosphatidic acid, in serum, which has potential in diagnosis of ovarian cancer [123]. In addition to detecting biomolecules, monitoring the concentration of environmental pollutants is also an important application area. Jiang et al. have exploited their naphthotubes to detect hydrophilic organic pollutants in water [124]. In addition, they also reported the use of naphthotubes for chirality sensing of epoxides in water based on the induced circular dichroism signal [125].
The excellent water-phase molecular recognition properties of cyclophanes have also be widely exploited in the development of new biomedical materials. Firstly, by encapsulating organic dyes, cyclophanes can alter or improve the fluorescent properties of the dyes for bioimaging applications, as exemplified by Smith et al. They have done a lot of work on the application of fluorescent tetralactam/squaraine dyes pairs for bioimaging purposes [126]. The cavities of cyclophanes can also shield or sequester the detrimental effects of drug molecules or toxins. For example, pillar[6]MaxQ (P6AS) developed by Issacs et al. has been reported to be use as an in vivo sequestration agent for methamphetamine and fentanyl, etc. [127]. Moreover, by incorporating stimulus-responsive cleavable groups, cyclophanes can also be utilized as novel supramolecular drug delivery systems. An exemplary case is the hypoxia-responsive azocalixarenes developed by Guo et al. The deep-cavity azocalixarenes not only exhibited high affinity binding towards a wide range of drug molecules but also underwent selective cleavage of the azo bonds under hypoxic conditions, enabling controlled and precise drug release. As a result, the azocalixarenes have demonstrated outstanding performance in drug delivery and hold great promise for the treatment of hypoxia-related diseases [74].
The nanoconfinement of the cyclophane cavity can also impose significant effect on the chemical reactivity of encapsulated compounds, as Rebek et al. have revealed in their deep cavitands. For instance, they have showed that deep cavitands can improve the macrocyclization efficiency of long alkane chain substrates and alter the chemical reactivity of organic compounds in the water phase [13,128].
By incorporating cyclophane into polymeric materials, the recognition properties of cyclophane can be employed to extract and remove organic pollutants in water, as Jiang et al. demonstrated using their naphthotube-based crosslinked polymers [129].
Cyclophanes can also be used as the ring components in mechanically interlocked molecules to construct molecular machines and smart soft materials. Jiang et al. exploited their naphthotubes as the ring component to prepare a pseudopolyrotaxane which was capable of undergoing shear-induced formation of a transient hydrogel, and the mechanical property of the hydrogel can be further improved by force training [130].
As these examples demonstrated, owing to diverse structures and excellent molecular recognition properties, cyclophanes have enabled broad applications in various fields, depending on the specific targets of molecular recognition, such as biomolecules, drug compounds, fluorescent dyes, environmental pollutants, reaction substrates, polymer chains, and more. As researchers continue to advance the synthesis new cyclophanes and deepen our understanding about their recognition, their applications are poised to extend into even more research domains in the future.
7. Conclusion and perspectiveWe have provided a comprehensive overview of the recognition properties of selected cyclophanes and introduced their recognition properties in water. Given the structural diversity of these cyclophanes, there are numerous factors that influence their molecular recognition, including the geometry and size of the cavity, flexibility, type and position of functional groups, and charge distribution, among others. Consequently, a case-by-case analysis is often required to discuss these properties. Nevertheless, by summarizing all the examples, we can derive two particularly valuable insights about the molecular recognition of cyclophanes. Firstly, increasing the depth of hydrophobic cavity is a powerful strategy to enhance the binding affinities of cyclophane, since it can efficiently increase the buried hydrophobic surface area between host and guest molecules and bring about strong hydrophobic driven force. This strategy has been frequently utilized to achieve high binding affinity across different types of cyclophanes, including Xcage, azocalixarenes, deep cavitands, aryl-extended calix[4]pyrroles and so on. Secondly, the introduction of polar binding sites inside the hydrophobic cavities is a particularly useful strategy for improving the recognition selectivity and affinity towards hydrophilic organic guests in water, as exemplified by calix[4]pyrroles, tetralactams, polycyclic lactam cages and naphthotubes and so on. It is reasonable to anticipate that these two strategies will serve as important directions for further enhancing the molecular recognition properties of cyclophanes, and even other types of macrocyclic hosts.
From a forward-looking perspective, there is still ample room for exploration in the field of cyclophane chemistry:
(1) In terms of recognition properties, as mentioned earlier, there exists a significant disparity between the binding affinity and selectivity of cyclophanes compared to biological systems. This calls for supramolecular chemists to engage in more ingenious structural designs, aiming to achieve artificial receptors with exceptional recognition affinities and selectivity for challenging substrates, such as vital biomarkers and biomacromolecules, through relatively straightforward synthetic approaches. By doing so, we may eventually be able to provide powerful artificial recognition tools that can rival or even surpass biological recognition systems.
(2) From application standpoint, water-mediated molecular recognition plays a pivotal role, particularly in biological systems. However, the current focus of molecular recognition research remains predominantly limited to pure water solutions or pH-buffered solutions. Yet, many biologically relevant applications involve more complex physiological environments, such as blood, urine, or even inside living cells. In these environments, high salt concentrations and the presence of intricate biological interferents not only significantly diminish the effectiveness of molecular recognition but also amplify the challenges associated with characterizing and studying molecular recognition in such biofluids. Enhancing the resilience of cyclophanes (and other artificial receptors) against physiological interferences and improving the orthogonality of their recognition interactions in complex biomediums are crucial research endeavors for practical applications in biomedical materials. Furthermore, the development of novel and effective characterization techniques to investigate the process of molecular recognition in these intricate physiological media also represents a crucial avenue for future research endeavors.
Building upon this foundation, supramolecular chemists can further contemplate how to strategically couple the molecular recognition behavior of artificial receptors with in vivo cellular life processes, thereby enabling precise intervention in biological activities [131]. This endeavor holds significant implications for the development of clinically valuable materials for disease diagnosis and treatment, and it has the potential to greatly expand the influence of cyclophane chemistry, as well as the broader field of supramolecular chemistry.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 22271164, U20A20259), the Fundamental Research Funds for the Central Universities, and the NCC Fund (No. NCC2020FH04), which are gratefully acknowledged.
| [1] |
C.L. Schreiber, B.D. Smith, Nat. Rev. Chem. 3 (2019) 393-400. DOI:10.1038/s41570-019-0095-1 |
| [2] |
X. Wang, F. Jia, L.P. Yang, H. Zhou, W. Jiang, Chem. Soc. Rev. 49 (2020) 4176-4188. DOI:10.1039/D0CS00341G |
| [3] |
H.P. Ferguson Johns, E.E. Harrison, K.J. Stingley, M.L. Waters, Chem. Eur. J. 27 (2021) 6620-6644. DOI:10.1002/chem.202003759 |
| [4] |
E. Persch, O. Dumele, F. Diederich, Angew. Chem. Int. Ed. 54 (2015) 3290-3327. DOI:10.1002/anie.201408487 |
| [5] |
H.J. Schneider, Angew. Chem. Int. Ed. 48 (2009) 3924-3977. DOI:10.1002/anie.200802947 |
| [6] |
E.A. Meyer, R.K. Castellano, F. Diederich, Angew. Chem. Int. Ed. 42 (2003) 1210-1250. DOI:10.1002/anie.200390319 |
| [7] |
J.M. Lehn, Angew. Chem. Int. Ed. 27 (1988) 89-112. DOI:10.1002/anie.198800891 |
| [8] |
F. Diederich, J. Chem. Educ. 67 (1990) 813. DOI:10.1021/ed067p813 |
| [9] |
F. Biedermann, W.M. Nau, H.J. Schneider, Angew. Chem. Int. Ed. 53 (2014) 11158-11171. DOI:10.1002/anie.201310958 |
| [10] |
L. You, D. Zha, E.V. Anslyn, Chem. Rev. 115 (2015) 7840-7892. DOI:10.1021/cr5005524 |
| [11] |
Y. Li, X. Lou, C. Wang, et al., Chin. Chem. Lett. 34 (2023) 107877. DOI:10.1016/j.cclet.2022.107877 |
| [12] |
L. Tian, S. Zhou, J. Zhao, et al., J. Hazard. Mater. 441 (2023) 129873. DOI:10.1016/j.jhazmat.2022.129873 |
| [13] |
Q. Shi, M.P. Mower, D.G. Blackmond, J. Rebek Jr., Proc. Natl. Acad. Sci. U. S. A. 113 (2016) 9199-9203. DOI:10.1073/pnas.1610006113 |
| [14] |
K. Wang, X. Tian, J.H. Jordan, et al., Chin. Chem. Lett. 33 (2022) 89-96. DOI:10.1016/j.cclet.2021.06.026 |
| [15] |
H. Ke, L.P. Yang, M. Xie, Nat. Chem. 11 (2019) 470-477. DOI:10.1038/s41557-019-0235-8 |
| [16] |
Y.C. Pan, H.W. Tian, S. Peng, X.Y. Hu, D.S. Guo, Chin. Chem. Lett. 28 (2017) 787-792. DOI:10.1016/j.cclet.2016.12.027 |
| [17] |
K. Wang, M. Zuo, T. Zhang, H. Yue, X.Y. Hu, Chin. Chem. Lett. 34 (2023) 107848. DOI:10.1016/j.cclet.2022.107848 |
| [18] |
Y.C. Pan, X.Y. Hu, D.S. Guo, Angew. Chem. Int. Ed. 60 (2021) 2768-2794. DOI:10.1002/anie.201916380 |
| [19] |
J.J. Wu, F.Y. Chen, B.B. Han, et al., CCS Chem. 5 (2023) 885-901. DOI:10.31635/ccschem.022.202201859 |
| [20] |
B. Hazarika, V.P. Singh, Chin. Chem. Lett. (2023) 108220. |
| [21] |
W.C. Geng, Z. Zheng, D.S. Guo, VIEW (2020) 20200059. |
| [22] |
Y.L. Ma, S. Yan, X.J. Xu, H. Cao, R. Wang, Chin. Chem. Lett. (2023) 108645. |
| [23] |
Y.L. Ma, C. Sun, Z. Li, et al., CCS Chem. 4 (2022) 1977-1989. DOI:10.31635/ccschem.021.202101178 |
| [24] |
F. Diederich, Angew. Chem. Int. Ed. 27 (1988) 362-386. DOI:10.1002/anie.198803621 |
| [25] |
S. Sarkar, P. Ballester, M. Spektor, E.A. Kataev, Angew. Chem. Int. Ed. (2022) e202214705. |
| [26] |
H. Han, R. Fu, R. Wang, et al., J. Am. Chem. Soc. 144 (2022) 20351-20362. DOI:10.1021/jacs.2c08144 |
| [27] |
X.N. Han, Q.S. Zong, Y. Han, C.F. Chen, CCS Chem. 4 (2022) 318-330. DOI:10.31635/ccschem.021.202100870 |
| [28] |
H. Stetter, E.E. Roos, Chem. Ber. 88 (1955) 1390-1395. DOI:10.1002/cber.19550880909 |
| [29] |
R. Hilgenfeld, W. Saenger, Angew. Chem. Int. Ed. 21 (1982) 787-788. DOI:10.1002/anie.198207871 |
| [30] |
K. Odashima, A. Itai, Y. Iitaka, K. Koga, J. Am. Chem. Soc. 102 (1980) 2504-2505. DOI:10.1021/ja00527a083 |
| [31] |
F. Diederich, K. Dick, J. Am. Chem. Soc. 106 (1984) 8024-8036. DOI:10.1021/ja00338a005 |
| [32] |
X.Y. Chen, H. Chen, J.Fraser Stoddart, Angew. Chem. Int. Ed. 62 (2023) e202211387. DOI:10.1002/anie.202211387 |
| [33] |
D.S. Guo, Y. Liu, Acc. Chem. Res. 47 (2014) 1925-1934. DOI:10.1021/ar500009g |
| [34] |
S.M. Biros, J. Rebek Jr., Chem. Soc. Rev. 36 (2007) 93-104. DOI:10.1039/B508530F |
| [35] |
D.S. Kim, J.L. Sessler, Chem. Soc. Rev. 44 (2015) 532-546. DOI:10.1039/C4CS00157E |
| [36] |
J.M. Dempsey, Q.W. Zhang, A.G. Oliver, B.D. Smith, Org. Biomol. Chem. 16 (2018) 8976-8983. DOI:10.1039/C8OB02596G |
| [37] |
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [38] |
X.N. Han, P.F. Li, Y. Han, C.F. Chen, Angew. Chem. Int. Ed. 61 (2022) e202202527. DOI:10.1002/anie.202202527 |
| [39] |
J. Zhou, G. Yu, L. Shao, B. Hua, F. Huang, Chem. Commun. 51 (2015) 4188-4191. DOI:10.1039/C5CC00225G |
| [40] |
Q.H. Guo, L. Zhao, M.X. Wang, Angew. Chem. Int. Ed. 54 (2015) 8386-8389. DOI:10.1002/anie.201503179 |
| [41] |
L.P. Yang, X. Wang, H. Yao, W. Jiang, Acc. Chem. Res. 53 (2020) 198-208. DOI:10.1021/acs.accounts.9b00415 |
| [42] |
L. Escobar, P. Ballester, Chem. Rev. 121 (2021) 2445-2514. DOI:10.1021/acs.chemrev.0c00522 |
| [43] |
C.A. Hunter, Angew. Chem. Int. Ed. 43 (2004) 5310-5324. DOI:10.1002/anie.200301739 |
| [44] |
W. Liu, S.K. Samanta, B.D. Smith, L. Isaacs, Chem. Soc. Rev. 46 (2017) 2391-2403. DOI:10.1039/C7CS00011A |
| [45] |
F. Biedermann, V.D. Uzunova, O.A. Scherman, W.M. Nau, A. De Simone, J. Am. Chem. Soc. 134 (2012) 15318-15323. DOI:10.1021/ja303309e |
| [46] |
F. Biedermann, M. Vendruscolo, O.A. Scherman, A. De Simone, W.M. Nau, J. Am. Chem. Soc. 135 (2013) 14879-14888. DOI:10.1021/ja407951x |
| [47] |
Q. Sun, Chem. Phys. Lett. 672 (2017) 21-25. DOI:10.1016/j.cplett.2017.01.057 |
| [48] |
B. Kronberg, Curr. Opin. Colloid Interface Sci. 22 (2016) 14-22. DOI:10.1016/j.cocis.2016.02.001 |
| [49] |
S.T. Ryan, J. Del Barrio, I. Ghosh, et al., J. Am. Chem. Soc. 136 (2014) 9053-9060. DOI:10.1021/ja5032437 |
| [50] |
M. Petroselli, Y.Q. Chen, J.J. Rebek, Y. Yu, Green Synth. Catal. 2 (2021) 123-130. DOI:10.1016/j.gresc.2021.03.004 |
| [51] |
K.N. Houk, A.G. Leach, S.P. Kim, X. Zhang, Angew. Chem. Int. Ed. 42 (2003) 4872-4897. DOI:10.1002/anie.200200565 |
| [52] |
J.W. Barnett, M.R. Sullivan, J.A. Long, et al., Nat. Chem. 12 (2020) 589-594. DOI:10.1038/s41557-020-0458-8 |
| [53] |
J. Dong, A.P. Davis, Angew. Chem. Int. Ed. 60 (2021) 8035-8048. DOI:10.1002/anie.202012315 |
| [54] |
H. Yao, H. Ke, X. Zhang, et al., J. Am. Chem. Soc. 140 (2018) 13466-13477. DOI:10.1021/jacs.8b09157 |
| [55] |
D. Li, M. Wu, Signal Transduction Targeted Ther. 6 (2021) 291. DOI:10.1038/s41392-021-00687-0 |
| [56] |
T.C. Bruice, S.J. Benkovic, Biochemistry 39 (2000) 6267-6274. DOI:10.1021/bi0003689 |
| [57] |
H. Zhang, F. Li, B. Dever, et al., Angew. Chem. Int. Ed. 52 (2013) 10698-10705. DOI:10.1002/anie.201210022 |
| [58] |
C.D. Raposo, A.B. Canelas, M.T. Barros, Biomolecules 11 (2021) 188. DOI:10.3390/biom11020188 |
| [59] |
O.H. Laitinen, H.R. Nordlund, V.P. Hytonen, M.S. Kulomaa, Trends Biotechnol. 25 (2007) 269-277. DOI:10.1016/j.tibtech.2007.04.001 |
| [60] |
B. Odell, M.V. Reddington, A.M.Z. Slawin, et al., Angew. Chem. Int. Ed. 27 (1988) 1547-1550. DOI:10.1002/anie.198815471 |
| [61] |
S.A. Staley, B.D. Smith, Tetrahedron Lett. 37 (1996) 283-286. DOI:10.1016/0040-4039(95)02150-7 |
| [62] |
A.R. Bernardo, J.F. Stoddart, A.E. Kaifer, J. Am. Chem. Soc. 114 (2002) 10624-10631. |
| [63] |
M. Bria, G. Cooke, A. Cooper, et al., Tetrahedron Lett. 48 (2007) 301-304. DOI:10.1016/j.tetlet.2006.11.003 |
| [64] |
E.J. Dale, N.A. Vermeulen, M. Juricek, et al., Acc. Chem. Res. 49 (2016) 262-273. DOI:10.1021/acs.accounts.5b00495 |
| [65] |
J.C. Barnes, M. Juricek, N.L. Strutt, et al., J. Am. Chem. Soc. 135 (2013) 183-192. DOI:10.1021/ja307360n |
| [66] |
E.J. Dale, N.A. Vermeulen, A.A. Thomas, et al., J. Am. Chem. Soc. 136 (2014) 10669-10682. DOI:10.1021/ja5041557 |
| [67] |
W. Liu, S. Bobbala, C.L. Stern, et al., J. Am. Chem. Soc. 142 (2020) 3165-3173. DOI:10.1021/jacs.9b12982 |
| [68] |
Y. Shi, K. Cai, H. Xiao, et al., J. Am. Chem. Soc. 140 (2018) 13835-13842. DOI:10.1021/jacs.8b08555 |
| [69] |
W. Liu, C. Lin, J.A. Weber, et al., J. Am. Chem. Soc. 142 (2020) 8938-8945. DOI:10.1021/jacs.0c02311 |
| [70] |
H. Duan, Y. Li, Q. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 10101-10110. DOI:10.1002/anie.201912730 |
| [71] |
L. Cheng, K. Liu, Y. Duan, et al., CCS Chem. 3 (2021) 2749-2763. DOI:10.31635/ccschem.020.202000509 |
| [72] |
K. Wang, S.Y. Xing, X.G. Wang, H.X. Dou, Org. Biomol. Chem. 13 (2015) 5432-5443. DOI:10.1039/C5OB00053J |
| [73] |
N. Douteau-Guével, F. Perret, A.W. Coleman, J.P. Morel, N. Morel-Desrosiers, J. Chem. Soc., Perkin Trans. 2 (2002) 524-532. |
| [74] |
T.X. Zhang, Z.Z. Zhang, Y.X. Yue, et al., Adv. Mater. 32 (2020) 1908435. DOI:10.1002/adma.201908435 |
| [75] |
S. Li, R. Ma, X.Y. Hu, et al., Adv. Mater. 34 (2022) 2203765. DOI:10.1002/adma.202203765 |
| [76] |
Y.X. Yue, Z. Zhang, Z.H. Wang, et al., Small Struct. 3 (2022) 2200067. DOI:10.1002/sstr.202200067 |
| [77] |
Data source: https://suprabank.org/.
|
| [78] |
T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [79] |
T. Ogoshi, M. Hashizume, T.A. Yamagishi, Y. Nakamoto, Chem. Commun. 46 (2010) 3708-3710. DOI:10.1039/c0cc00348d |
| [80] |
X. Shu, K. Xu, D. Hou, C. Li, Isr. J. Chem. 58 (2018) 1230-1240. DOI:10.1002/ijch.201800115 |
| [81] |
W. Xue, P.Y. Zavalij, L. Isaacs, Angew. Chem. Int. Ed. 59 (2020) 13313-13319. DOI:10.1002/anie.202005902 |
| [82] |
Y. Ma, X. Ji, F. Xiang, et al., Chem. Commun. 47 (2011) 12340-12342. DOI:10.1039/c1cc15660h |
| [83] |
W. Chen, Y. Zhang, J. Li, et al., Chem. Commun. 49 (2013) 7956-7958. DOI:10.1039/c3cc44328k |
| [84] |
B. Gomez-Gonzalez, V. Francisco, R. Montecinos, L. Garcia-Rio, Org. Biomol. Chem. 15 (2017) 911-919. DOI:10.1039/C6OB02573K |
| [85] |
T. Ogoshi, R. Shiga, T.A. Yamagishi, J. Am. Chem. Soc. 134 (2012) 4577-4580. DOI:10.1021/ja300989n |
| [86] |
Y. Zhang, J. Chen, L. Chen, et al., Chin. Chem. Lett. 34 (2023) 107697. DOI:10.1016/j.cclet.2022.07.040 |
| [87] |
Y. Tanaka, Y. Aoyama, Bull. Chem. Soc. Jpn. 63 (1990) 3343-3344. DOI:10.1246/bcsj.63.3343 |
| [88] |
K. Wang, Q. Liu, L. Zhou, et al., Chin. Chem. Lett. (2023) 108559. |
| [89] |
K. Kobayashi, Y. Asakawa, Y. Kato, Y. Aoyama, J. Am. Chem. Soc. 114 (1992) 10307-10313. DOI:10.1021/ja00052a030 |
| [90] |
C. Gropp, B.L. Quigley, F. Diederich, J. Am. Chem. Soc. 140 (2018) 2705-2717. DOI:10.1021/jacs.7b12894 |
| [91] |
C.L. Gibb, B.C. Gibb, J. Am. Chem. Soc. 126 (2004) 11408-11409. DOI:10.1021/ja0475611 |
| [92] |
Y.H. Wan, Y.J. Zhu, J. Rebek Jr., Y. Yu, Molecules 26 (2021). DOI:10.3390/molecules26071922 |
| [93] |
Q. Shi, D. Masseroni, J. Rebek Jr., J. Am. Chem. Soc. 138 (2016) 10846-10848. DOI:10.1021/jacs.6b06950 |
| [94] |
J.M. Yang, Y. Yu, J. Rebek Jr., J. Am. Chem. Soc. 143 (2021) 2190-2193. DOI:10.1021/jacs.0c12302 |
| [95] |
G.W. Zhang, P.F. Li, Z. Meng, et al., Angew. Chem. Int. Ed. 55 (2016) 5304-5308. DOI:10.1002/anie.201600911 |
| [96] |
G.W. Zhang, Y. Han, Y. Han, Y. Wang, C.F. Chen, Chem. Commun. 53 (2017) 10433-10436. DOI:10.1039/C7CC05489K |
| [97] |
Y. Guo, Y. Han, C.F. Chen, Front. Chem. 7 (2019) 543. DOI:10.3389/fchem.2019.00543 |
| [98] |
Z.Y. Zhang, C. Li, Acc. Chem. Res. 55 (2022) 916-929. DOI:10.1021/acs.accounts.2c00043 |
| [99] |
X. Du, M. Ma, Y. Zhang, et al., Angew. Chem. Int. Ed. 62 (2023) e202301857. DOI:10.1002/anie.202301857 |
| [100] |
C. Zhai, L. Isaacs, Chem. Eur. J. 28 (2022) e202201743. DOI:10.1002/chem.202201743 |
| [101] |
J.R. Wu, C.Y. Wang, Y.C. Tao, et al., Eur. J. Org. Chem. 2018 (2018) 1321-1325. DOI:10.1002/ejoc.201800112 |
| [102] |
T. Chen, J. Wang, R. Tang, et al., Chin. Chem. Lett. 34 (2023) 108088. DOI:10.1016/j.cclet.2022.108088 |
| [103] |
M.X. Wang, Acc. Chem. Res. 45 (2012) 182-195. DOI:10.1021/ar200108c |
| [104] |
M.X. Wang, Sci. China: Chem. 61 (2018) 993-1003. |
| [105] |
D. Xia, Y. Li, K. Jie, B. Shi, Y. Yao, Org. Lett. 18 (2016) 2910-2913. DOI:10.1021/acs.orglett.6b01264 |
| [106] |
C.M. Hong, R.G. Bergman, K.N. Raymond, F.D. Toste, Acc. Chem. Res. 51 (2018) 2447-2455. DOI:10.1021/acs.accounts.8b00328 |
| [107] |
Y. Wu, C. Zhang, S. Fang, et al., Angew. Chem. Int. Ed. 61 (2022) e202209078. DOI:10.1002/anie.202209078 |
| [108] |
M. Yoshizawa, L. Catti, Acc. Chem. Res. 52 (2019) 2392-2404. DOI:10.1021/acs.accounts.9b00301 |
| [109] |
L. Escobar, G. Aragay, P. Ballester, Chem. Eur. J. 22 (2016) 13682-13689. DOI:10.1002/chem.201602987 |
| [110] |
L. Escobar, P. Ballester, Org. Chem. Front. 6 (2019) 1738-1748. DOI:10.1039/C9QO00171A |
| [111] |
J.J. Gassensmith, E. Arunkumar, L. Barr, et al., J. Am. Chem. Soc. 129 (2007) 15054-15059. DOI:10.1021/ja075567v |
| [112] |
E.M. Peck, W. Liu, G.T. Spence, et al., J. Am. Chem. Soc. 137 (2015) 8668-8671. DOI:10.1021/jacs.5b03573 |
| [113] |
W. Liu, A. Johnson, B.D. Smith, J. Am. Chem. Soc. 140 (2018) 3361-3370. DOI:10.1021/jacs.7b12991 |
| [114] |
H. Zhang, L.L. Wang, X.Y. Pang, L.P. Yang, W. Jiang, Chem. Commun. 57 (2021) 13724-13727. DOI:10.1039/D1CC05818E |
| [115] |
N.P. Barwell, M.P. Crump, A.P. Davis, Angew. Chem. Int. Ed. 48 (2009) 7673-7676. DOI:10.1002/anie.200903104 |
| [116] |
Y. Ferrand, M.P. Crump, A.P. Davis, Science 318 (2007) 619-622. DOI:10.1126/science.1148735 |
| [117] |
P. Rios, T.S. Carter, T.J. Mooibroek, et al., Angew. Chem. Int. Ed. 55 (2016) 3387-3392. DOI:10.1002/anie.201510611 |
| [118] |
R.A. Tromans, T.S. Carter, L. Chabanne, et al., Nat. Chem. 11 (2019) 52-56. DOI:10.1038/s41557-018-0155-z |
| [119] |
B.J. Shorthill, C.T. Avetta, T.E. Glass, J. Am. Chem. Soc. 126 (2004) 12732-12733. DOI:10.1021/ja047639d |
| [120] |
H. Chai, Z. Chen, S.H. Wang, et al., CCS Chem. 2 (2020) 440-452. DOI:10.31635/ccschem.020.202000160 |
| [121] |
X. Huang, X. Wang, M. Quan, et al., Angew. Chem. Int. Ed. 60 (2021) 1929-1935. DOI:10.1002/anie.202012467 |
| [122] |
X. Wang, M. Quan, H. Yao, et al., Nat. Commun. 13 (2022) 2291. DOI:10.1038/s41467-022-30012-4 |
| [123] |
Z. Zheng, W.C. Geng, J. Gao, Chem. Sci. 9 (2018) 2087-2091. DOI:10.1039/C7SC04989G |
| [124] |
L.M. Bai, H. Yao, L.P. Yang, W. Zhang, W. Jiang, Chin. Chem. Lett. 30 (2019) 881-884. DOI:10.1016/j.cclet.2018.11.033 |
| [125] |
L.L. Wang, Z. Chen, W.E. Liu, et al., J. Am. Chem. Soc. 139 (2017) 8436-8439. DOI:10.1021/jacs.7b05021 |
| [126] |
C. Zhai, C.L. Schreiber, S. Padilla-Coley, A.G. Oliver, B.D. Smith, Angew. Chem. Int. Ed. 59 (2020) 23740-23747. DOI:10.1002/anie.202009599 |
| [127] |
A.T. Brockett, W. Xue, D. King, et al., Chem 9 (2023) 881-900. DOI:10.1016/j.chempr.2022.11.019 |
| [128] |
S. Mosca, Y. Yu, J.V. Gavette, K.D. Zhang, J. Rebek Jr., J. Am. Chem. Soc. 137 (2015) 14582-14585. DOI:10.1021/jacs.5b10028 |
| [129] |
L.P. Yang, H. Ke, H. Yao, W. Jiang, Angew. Chem. Int. Ed. 60 (2021) 21404-21411. DOI:10.1002/anie.202106998 |
| [130] |
J.N. Jin, X.R. Yang, Y.F. Wang, et al., Angew. Chem. Int. Ed. 62 (2023) e202218313. DOI:10.1002/anie.202218313 |
| [131] |
C. Gao, Q. Wang, J. Li, et al., Sci. Adv. 8 (2022) eabn1805. DOI:10.1126/sciadv.abn1805 |
 2024, Vol. 35
2024, Vol. 35 

