b Key Laboratory of Biological Nanotechnology of National Health Commission, Changsha 410008, China
Cancer incidence and mortality rates are gradually increasing in the 21st Century, and it is predicted that the incidence of cancers will double by 2070 compared to 2020 [1,2]. Therefore, cancer is still a serious threat to human health. Recently, immunotherapy has revolutionized the field of cancer therapy due to its high efficiency and relatively low toxicity [3,4]. However, successful cancer immunotherapy ultimately requires the immune system of patients to recognize tumor antigens for immune activation. While some patients could spontaneously generate sufficient antigens to rouse a robust antitumor response, most patients do not. Fortunately, the cancer vaccine provides new hope for ensuring adequate levels of cytolytic effectors (T cells and antibodies) and offering secure, specific, and well-tolerated treatment compared to other immunotherapies [5].
Originally, vaccines were designed to prevent infectious diseases, but recently the concept of vaccines goes far beyond prevention [6,7]. Especially in the field of cancer immunotherapy, both prophylactic and therapeutic vaccines have been designed to treat different cancers with promising results. In the 1890s, Dr. William Coley, the pioneer of therapeutic vaccines for cancer, first treated the established malignant tumors with inactivated bacteria, which effectively activated the immune system of patients to cause tumor regression [8,9]. This laid the foundation for cancer immunotherapy research and cancer vaccines. After years of research and testing, several prophylactic and therapeutic cancer vaccines were approved, such as human papillomavirus (HPV) and hepatitis B virus (HBV) prophylactic vaccines to prevent viral cervical and liver cancer. In addition, U.S. Food and Drug Administration (U.S. FDA) also approved the first therapeutic cancer vaccine in 2010, Sipuleucel-T (ProvengeⓇ), which is an immune cell-based vaccine for hormone-refractory prostate cancer treatment [10]. In 2015, U.S. FDA and European Medicines Agency (EMA) further approved the talimogene laherparepvec (T-VEC) for melanoma treatment, which is an oncolytic virus-based vaccine [11].
Despite this inspiring foundation, the clinical translation of cancer vaccines from bench to bedside remains a challenge due to the high variability of tumor antigens and the poor immune response induced. Inspiringly, some studies have found that the immune response of vaccines may be related to the administration route. For instance, several clinical studies found that the immune response rate of the influenza vaccine by intradermal injection was higher than that of other administrations [12]. On the one hand, skin associates with the abundance of immune cells and unidirectional flow of capillary lymphatic vessels compared to muscle or subcutaneous tissue, which are critical for provoking immunity. Moreover, it can resolve the problem of gastrointestinal tract degradation and the metabolism of the vaccines. Therefore, transdermal administration is particularly attractive in the field of vaccination. Importantly, the advent of microneedles (MNs) further opens up a new possibility of safe, painless, and convenient vaccination with simple transportation, storage, and handling conditions, which is the core challenge in vaccine development.
Recently, the research on cancer vaccination through MNs has made great progress. MNs not only enhance the stability of cancer vaccines but also improve the bioavailability and efficiency of vaccines. The history of MNs-based cancer vaccines has been the subject of excellent reviews [13-15]. However, these reviews focused more on the advantages of MNs-based percutaneous administration over other administrations but ignored the crucial deficiencies of MNs, such as low vaccine loading. As a result, these vaccines may fail to trigger sufficiently strong and durable immune responses, suggesting that innovative strategies are required to optimize MNs-based vaccine therapy. This needs to take into account all the mechanisms of antitumor immunity to trigger a stronger response. Therefore, higher requirements are required for MNs-based cancer vaccines for better immunotherapy efficacy. With the rapid development of nanotechnology, the immune-engineering design of MNs-based cancer vaccines with different nanomaterials offers a new strategy for enhancing immune response, including an increase of antigen uptake by immune cells, recruitment of immune cells, enhancement of antigens presentation, and so on.
Herein, we presented a review of the current state of fundamental research on MNs-based cancer vaccines and categorized their immune-engineering design strategies, aiming to point out the direction of developing safer, more effective, and more stable MNs-based vaccines for cancer immunotherapy (Fig. 1). Specifically, we briefly outlined the advantages of using MNs in cancer vaccines, and systematically introduced the types of cancer vaccine according to the antigen, including in vitro and/or in-situ treated tumor cells or generated lysates, protein/peptide, and nucleic acid, etc. Then, nanoparticles (NPs), including polymeric, lipid-based, and self-assembled peptide/protein nanovaccines, etc. were reviewed as carriers to integrate into MNs-based cancer vaccines, which could further enhance the immune response of vaccines by increasing the uptake of antigens by immune cells and increasing the targeting of the vaccine to the lymph nodes (LNs), etc. Subsequently, we classified their designs for targeting delivery, including passive targeting to LNs and active targeting to immune cells through physicochemical properties of nanovaccines and ligand receptor-specific recognition, etc. Finally, the prospects and challenges of MNs-based cancer vaccines were also speculated.
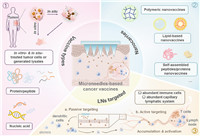
|
Download:
|
| Fig. 1. MNs-based vaccine delivery for cancer immunotherapy. | |
Conventionally, the majority of vaccines are administered intramuscularly or subcutaneously, with a few exceptions are administered orally, which means vaccines will pass the gastrointestinal tract and the systemic circulation in turn, and then capture and present by antigen-presenting cells (APCs, such as dendritic cells (DCs)) [16]. Subsequently, APCs travel to secondary LNs, where the interaction between APCs and T cells via major histocompatibility complex (MHC)-T cell receptor recognition and co-receptor engagement. In this process, the delivery of the vaccine to APCs is a critical event to stimulate the proliferation and differentiation of T and B cells in LNs with antitumor effects and long-lasting memory [17,18]. When the immune system is attacked by the same antigens again, it follows its original memory, inducing stronger humoral and cellular immunity to protect the individual from invasion [19]. However, vaccines are prone to degradation and clearance during gastrointestinal and systemic circulation before uptake by APCs, which is one of the main reasons for most vaccine failures. Therefore, researchers designed various delivery strategies with the help of different materials, but the therapeutic effect was unsatisfactory. Inspiringly, some studies have found that the administration route of vaccines may address this issue. Thus, alternatives have been investigated to improve the efficiency of vaccines, including oral mucosa [20], nasal [21], and intradermal [22] delivery. Among them, the skin is a highly immunologically active organ and is of great interest for vaccination because of its availability [23,24].
Skin is the largest organ in the body and consists of the epidermis, dermis, and subcutaneous tissue [25]. In the epidermis and dermis, there are several types of immune cells, such as Langerhans cells (LCs), DCs, and macrophages, which are the most specialized APCs [26]. What is more, there are rich unidirectional flow capillary lymphatic vessels in the skin, which can absorb the interstitial fluid in the dermis due to their endothelial cells being relatively loose compared to the blood vessels. Therefore, delivering vaccines through the skin has a unique innate physiological advantage, where vaccines can migrate to LNs by different strategies to effectively initiate an adaptive immune response, including active and passive delivery strategies [27,28]. Nevertheless, the structure of the stratum corneum resembles a “brick wall”, which is the main barrier to drug delivery [29,30]. To address this issue, several techniques have been developed, including ion introduction [31], ultrasonic introduction [32], electroporation [33], laser ablation [34], and MNs.
MNs, a promising percutaneous device dating back to 1971, are comprised of multiple needles arranged on a base and designed using a variety of materials including polymers, carbohydrates, metal, silicon, and so on, in which each needle with a height ranging from 25 µm to 2000 µm, 1 µm to 25 µm width, and an external diameter of not more than 30 µm [35]. According to the performance of MNs, MNs can be roughly divided into five categories, including solid MNs, coated MNs, hollow MNs, dissolving MNs, and hydrogel-forming MNs that form transient and tiny aqueous channels in the skin without touching the rich nerve endings and capillaries in the dermis by adjustable needle length, facilitating transdermal drug delivery in a minimally invasive and painless manner [36-38].
All in all, MNs delivery of cancer vaccines has the following advantages: firstly, MNs are expected to promote the immune response of vaccines due to the innate immune advantage of skin; secondly, MNs can effectively enhance the stability of cancer vaccines at room temperature by solidifying the vaccine, thus avoiding cold chain and effectively facilitating vaccination programs in developing countries or remote areas; thirdly, MNs allow precisely the cancer vaccines delivery, resulting in a high local concentration of vaccine, which helps to save vaccine doses, thereby reducing costs and toxicity; last but not least, MNs-based cancer vaccination can overcome the disadvantages of syringes to achieve a painless vaccination, reduce the risk of infection spread, as well as anxiety, and improve patient compliance [39,40].
3. Types of cancer vaccinesCancer vaccines generally employ tumor antigens combined with adjuvants for exogenous administration to activate the APCs. The uptake of co-delivered antigens and adjuvants by APCs through systemic circulation and optimal APCs maturation are critical events for activating adaptive immunity. However, the conventional administration route of vaccination is painful and invasive for vaccine receivers, and the benefits are unsatisfactory due to first-pass metabolism. Alternatively, MNs are developed to offer painless, tolerable, and efficient intradermal delivery approaches for cancer vaccination, because the height of MNs can be adjusted that facilitate accurate delivery of vaccines into the skin to escape from systemic circulation. MNs provide a common platform for various types of cancer vaccine delivery during the last decade, including nucleic acid vaccines (DNA, RNA), protein/peptide vaccines, whole tumor cells or lysates vaccines, and in-situ vaccines. In this section, we provided an overview of MNs-based cancer vaccines according to different cancer vaccine antigens (Table S1 in Supporting information).
3.1. DNA vaccinesDNA vaccines usually derive from bacterial plasmids and travel to the nucleus to express antigens and manipulate the immune response offering advantages including product stability, absence of pathogenic infection, and easy preparation and modification to match mutations in the antigenic spectrum [41]. However, the clinical performance of naked DNA cancer vaccines is poor due to multiple biological barriers, low independent transfection efficiency, low immunogenicity, and degradation by DNase and lysozymes [42]. The following approaches can improve the above problems: (1) rational optimization of encoded antigens: designing hybrid plasmids encoding homo- and/or hetero-antigens, using neoantigen DNA vaccines with tumor specificity; (2) optimization of vaccine formulations: combining gene vectors and adjuvants. For example, Duong et al. [43] designed a smart DNA vaccine delivery system, in which negatively charged poly(I:C) and positively charged ultra-pH-responsive oligo sulfamethazine conjugated poly(β-amino ester urethane) (OSM-(PEG-PAEU)) were assembled with layer by layer on the 9 × 9 MNs array (600 µm needle), then the pOVA-loaded cationic polymer DA3 was coated on the surface of the outermost anionic complex (Fig. S1A in Supporting information). In the high pH environment of the skin after MNs insertion, OSM-(PEG-PAEU) converted into anionic copolymers, releasing DNA vaccine and poly(I:C) based on electrostatic repulsion. Due to the electrostatic interaction of poly(I:C) and DNA vaccine, they eventually were uptake by immune cells through negative-charged cell membranes and then the antigens were expressed (Figs. S1B and C in Supporting information). MNs-based smart DNA vaccine system significantly promoted the production of anti-OVA IgG1 and interferon γ (IFN-γ) positive tumor-infiltrating CD8+ T cells in vivo (Figs. S1D and E in Supporting information) and inhibited melanoma compared to subcutaneous injection. Similarly, the group loaded pOVA and poly(I:C) into the cationic polymer conjugate DA3 via ionic interactions to form nanocomplexes that were incorporated into the MNs for tumor eradication (Fig. S1F in Supporting information) [44]. Enzymatic degradation of pOVA and poly(I:C) was protected by the cationic polymer conjugate DA3, which promoted uptake by immune cells and enhanced the entrance of pOVA into the nucleus to encode antigens based on the proton sponge effect.
3.2. RNA vaccinesRNA vaccines also provide a platform for cancer therapy, in particular, mRNA is translated in the cytoplasm to encode tumor antigens for cancer immunotherapy, which reduce insertion mutation risk compared to DNA vaccines [45]. In addition, RNA is transcribed in vitro to increase yield and enable rapid, scalable production, as well as RNA acts as an adjuvant by providing co-stimulatory signals [46]. However, RNA is poorly stable and rapidly degraded by extracellular RNase in vivo, and mRNA is prone to translation errors. Accordingly, efficient, nontoxic vectors and multiple structural modification strategies are available to improve stability and delivery efficiency. Furthermore, mRNA synthesized by RNA sequence engineering becomes more easily translated. Nevertheless, subcutaneous delivery of naked mRNA has been shown to yield high protein translation efficiency [47]. Koh et al. [48] attempted to load naked mRNA in dissolving MNs for activating immunity against tumors. The results showed that the luciferase mRNA (mLuc) in the MNs could be preserved in solid form for at least two weeks under ambient conditions and MNs-derived mLuc from Day 5 to Day 15 produced comparable levels of luciferase expression to Day 0 by subcutaneous injection. In tumor model treatment, the OVA mRNA-loaded MNs also induced effective serum anti-OVA antibodies and delayed tumor progression. In summary, MNs offer an attractive delivery platform for mRNA-based vaccines.
3.3. Protein/peptide vaccinesBesides nucleic acid cancer vaccines, MNs-delivered protein or peptide vaccines also serve an essential role in cancer prevention and treatment. In contrast to nucleic acid vaccines, protein/peptide cancer vaccines are directly processed at the APCs to activate immunity, offering low production costs, low oncogenicity, and little allergenic response [49]. Unfortunately, naked antigenic protein and peptide are generally less immunogenic and result in immune tolerance due to immunosuppression of the tumor and limited T-cell recruitment. Beyond nanocarriers and adjuvants, several approaches were proposed to address the challenges, including the selection of optimal antigenic targets, optimization of peptide composition, length, and sequence to improve binding affinity and bioavailability, preparation of multiple epitope vaccines, protein/peptide binding and modification. For instance, Kim et al. [50] coupled a cytotoxic T-cell epitope peptide (SIINFEKL) to hyaluronate (HA) in a biodegradable HA MNs patch, which effectively delivered antigens to the skin immune system (Fig. S2A in Supporting information). HA promoted antigen internalization into APCs through HA receptor-mediated endocytosis and acted as a model damage molecule by engaging Toll-like receptors to activate immune cells. Importantly, the FITC-labeled HA-SIINFEKL fluorescence signal was still observed for 24 h, probably because of the steric bulk and the non-fouling effect of HA that protected HA-SIINFEKL from peptidase degradation, which prolonged interaction between HA-SIINFEKL and APCs (Fig. S2B in Supporting information). In vivo antitumor results showed that MNs-based HA-SIINFEKL significantly inhibited B16 melanoma growth and enhanced CD8+ T cell infiltration compared to MNs-based SIINFEKL. Similarly, Zeng and colleagues assembled cationic nanoarginine (R9) domain-modified Trp2 peptide with adjuvant CpG in layers to form 128 immunopolyelectrolyte multilayers on the surface of levopolylactic acid MNs to induce a strong memory immune response [51].
3.4. Whole tumor cells or lysates vaccinesRecently, strategies for the in vitro preparation of tumor antigens for vaccination using whole tumor cells or lysates have been progressively investigated to enhance antitumor immune responses. Generally, cancer vaccines containing a single antigen are likely to fail to produce an effective antitumor immune response due to tumor heterogeneity and the non-expression of immunogenic antigens to obtain immune escape [52,53]. Compared to narrowly defined tumor antigens, whole tumor cells or lysates provide a wide range of tumor-associated antigens (TAAs) sources to offer patient-individualized antigens and prevent the emergence of immunoresistant tumor variants [54]. Ye et al. [55] reported on the both prophylactic and therapeutic effects of MNs patch delivery of whole tumor cell lysates vaccines on tumors (Fig. S3A in Supporting information). They co-encapsulated melanin-containing B16F10 whole tumor lysates and the adjuvant granulocyte-macrophage colony-stimulating factor (GM-CSF) in MNs. The 15 × 15 MNs array with a height of 800 µm and conical construction, providing sufficient strength for insertion into the skin. MNs promoted the uptake and presentation of antigens by DCs, which facilitated immune response via abundant lymphatic vessels in the dermis. The results showed that the MNs-delivered vaccine significantly facilitated the release of cytokines (IFN-γ, tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6)) and infiltration of CD8+ T cells, inhibited the development of distant tumors, and prolonged the survival period. Notably, self-antigens and signaling molecules in whole tumor cell lysates can suppress immune responses, and live tumor cells are poorly immunogenic and secrete or contain cytokines to depress DCs and T cell function [56]. To overcome these challenges, various strategies including cyclic freeze-thaw, ultraviolet (UV) irradiation, thermal therapy, hyperthermia, and hypochlorite oxidation have been proposed. Yang et al. [57] used a NIR laser to irradiate B16 cells in vitro to generate sufficient TAAs and co-loaded it with GM-CSF and Tat-beclin 1 into dissolving MNs to enhance antigen cross-presentation and T cells activity to induce a robust antitumor immune response (Fig. S3B in Supporting information).
3.5. In-situ vaccine generationIn recent years, the concept of in-situ cancer vaccines has been proposed for cancer immunotherapy. It aims to activate antitumor immunity by triggering the immunogenic cell death (ICD) of tumor cells in the body through chemotherapy, photothermal therapy, photodynamic therapy, radiotherapy, and sonodynamic therapy, and so on [58,59]. ICD of tumor cells induces the release of TAAs, danger-associated molecular patterns (DAMPs), and a range of pro-inflammatory cytokines to promote the uptake, presentation, and maturation of DCs, as well as regulate the tumor immunosuppressive microenvironment to further enhance antitumor effects [60,61]. Compared to other cancer vaccines, this conversion of the patient’s tumor into a vaccine ensures the specificity of the antigen and avoids the tedious process of in vitro tumor antigen identification and the need for in vivo antigen recognition [62]. Similar to whole tumor cells or lysates vaccines, in-situ vaccine generation also leads to the release of immunosuppressive cytokines, and the effective recognition of TAAs released through ICD is also an important parameter for successful immune activation. Therefore, several strategies have been proposed to optimize in-situ vaccines, such as combining immune adjuvants and cytokines to reprogram the tumor immunosuppressive microenvironment, and nanoparticles being used to capture released TAAs to facilitate their DCs delivery. Our group developed a photothermal nanovaccine to enhance the immunotherapy of melanoma by an array of 10 × 10 dissolving MNs platform with a 100% skin insertion ratio (Fig. S4A in Supporting information) [63]. The photothermal nanovaccine was constructed of polyserotonin (PST) core adsorbed β-catenin silencing DNAzyme and tannic acid (TA)/Mn2+ coordination-based metal-organic-framework (MOF) shell. The results revealed that the nanovaccine triggered the tumor cells’ ICD through a photothermal effect, then the released TAAs were captured and presented to DCs by the bio-adhesive nature of the PST, as well as the DAMPs were released to enhance the tumor immunogenicity (Figs. S4B–E in Supporting information). Furthermore, the DNAzyme was activated by Mn2+ to silence the β-catenin that regulated the secretion of C—C motif chemokine ligand 4 (CCL4) to promote DCs infiltration and activation. The system of MNs-based photothermal nanovaccine achieved safe and effective antitumor efficacy of primary and distant tumors without extra therapeutic agents.
4. MNs-based cancer vaccines delivered by nanocarriersA series of complex facts about the host, the tumor, and the environment also directly affect tumor-specific immunity. Tumor antigens alone induce less strong and sustained T cells response, and the design of vaccines often requires consideration of multiple mechanisms of immune homing, inhibition, and escape. Therefore, cancer vaccines often need to combine tumor antigens with different components, including adjuvants and chemokines, and so on, to achieve step-by-step regulation for provoking robust immunity. Although multiple cancer vaccine components are simply mixed for co-delivery, the naked nucleic acid and protein/peptide are susceptible to enzymatic degradation and elimination, as well as the adjuvant dissociates from the antigen and is rapidly degraded when the vaccine enters the body. Seriously, free adjuvant triggers the autoimmune response against the host [64].
Nanoparticles offer an excellent co-delivery platform. With the development of nanotechnology, NPs with different compositions, sizes, shapes, and surface properties are manufactured as a common vehicle for multiple components in cancer vaccines to enhance the immunogenicity of antigens and achieve the desired immune response [65,66]. NPs provide several advantages for cancer vaccines with the protection of vaccines from degradation, sustained release of depot effect, and targeting effect to facilitate absorption [67,68]. NPs also possess inherent immunostimulatory properties that are comparable to those of conventional vaccine adjuvants and provide both antigens and adjuvants to the same APCs to enhance antigen immunogenicity [69,70].
Considering the advantages of nanocarriers and the important role of skin in immunization, dermal delivery of nanocarrier-based cancer vaccines is an excellent administration route for cancer immunotherapy. However, breaking through the stratum corneum barrier is a priority, and MNs, a recent trend in transdermal drug delivery, have effectively overcome this challenge. Currently, several materials were deployed as nanocarriers for cancer vaccines, which were loaded in MNs for percutaneous immunization (Fig. S5 in Supporting information).
4.1. Polymeric nanovaccinesThe materials for preparing polymer NPs are divided into synthetic polymers, including poly(lactic-co-glycolic acid) (PLGA), poly(glycolic acid) (PGA), poly(lactic acid) (PLA), polyethylenimine (PEI), etc. and natural polymers (e.g., chitosan, alginate, dextran, and hyaluronic acid, etc.) [71,72].
PLGA is one of the most widely used synthetic polymers, approved by the FDA for clinical applications due to biocompatibility and low toxicity, and is extensively applied for the delivery of drugs, nucleic acids, and vaccines based on controlled release property [73]. Zaric et al. [74] conducted a classic study, in which they encapsulated the model antigen OVA in PLGA NPs and delivered by MNs. The results revealed that MNs-based OVA-NPs were mainly retained at the vaccination site with depot effect and presented by DCs in the skin, activating an effective CD8+ T cell response, as well as promotion of IFN-γ. Furthermore, compared to OVA-MNs, OVA-NPs-MNs maintained the stability of OVA that still induced a similar level of specific CD8+ T immune responses to the initial ones after 10 weeks.
PEI is a cationic polymer that is widely used for nucleic acid delivery as it forms complexes with nucleic acids through electrostatic interactions and possesses a proton sponge effect that significantly enhances transfection [75]. PEI is also highly cytotoxic, causing cell death due to a high positive surface charge. Nevertheless, toxicity can be reduced by using linear and low molecular weight PEI or charge shielding [76]. Hu et al. [77] proposed a novel DNA cancer vaccine delivery system based on MNs, in which DNA was loaded in PEI1800 with low toxicity to fabricate complexes with mannose and cell-penetrating peptide modified on the surface (CPP-PEI1800-Man/DNA nanocomplexes).
Chitosan is a naturally cationic polysaccharide polymer with biocompatible and biodegradable properties and exhibits a strong affinity for nucleic acid and cell surface. Antigens and adjuvants were loaded into chitosan by physical encapsulation, chemisorption, and electrostatic interactions to obtain natural polymer-based nanovaccines that enhanced uptake and protected antigens from degradation [72]. Li et al. [78] designed rapidly dissolving MNs loaded with positively charged chitosan NPs encapsulating the antigen OVA and the adjuvant CpG by electrostatic interactions (Fig. S6A in Supporting information). The NPs zeta potential was 25.8 mV, following incubation with DCs for 2 h, the uptake efficiency of NPs by DCs reached 93.37% through the interaction of NPs with the cell membrane, while the free OVA was only 38.7% (Fig. S6B in Supporting information).
4.2. Lipid-based nanovaccinesLipid-based NPs have been widely used for vaccine delivery with biocompatibility and inherent adjuvant property, where the positive charge of the outer lipid layer promotes adhesion to negatively charged cell membrane through ionic interaction, leading to sustained release of antigen [79]. Additionally, liposomes can mimic cell membrane morphology to deliver hydrophobic and hydrophilic active substances [80]. Therefore, due to their physicochemical properties, low cytotoxicity, and structural flexibility, liposomes are more suitable than other carriers for the delivery of vaccines, and in particular have been extensively investigated in recent years for the delivery of mRNA vaccines [81]. Zhao et al. [82] prepared OVA-PD-Lipos by wrapping OVA and the adjuvant platycodin (PD) in liposomes for immunization by HA-based dissolving MNs with a height of 356 µm and reliable skin insertion capability (Fig. S7 in Supporting information). Liposomes with a surface charge of 45.7 mV enhanced the uptake of OVA by mouse bone marrow DCs and remarkably reduced the toxicity and hemolysis of PD by reducing the interaction of platycodin with cholesterol in the cell membrane. The results showed that MNs-based OVA-PD-Lipos elicited remarkable OVA-specific IgG, IgG1, and IgG2b levels in mice.
4.3. Self-assembled peptides/proteins nanovaccinesBased on complex molecular interactions, such as electrostatic interactions, hydrophobic interactions, aromatic interactions, or hydrogen bonding, peptides or proteins self-assemble into thermodynamically stable supramolecular nanostructure and facilitate the stability of antigens to improve immunogenicity [83]. For example, Cole et al. [84] designed a dissolving MNs delivery platform based on RALA-encapsulated pPSCA (encoded Prostate Stem Cell Antigen) for the prevention and treatment of prostate cancer (Fig. S8A in Supporting information). RALA is a cationic peptide delivery sequence consisting of a novel 30 amino acid that electrostatically encapsulates DNA encoding the prostate stem cell antigen to form RALA/pPSCA cationic NPs. RALA/pPSCA cationic NPs protected pPSCA from serum nuclease degradation and significantly enhanced cell transfection. Similarly, RALA acted as a pDNA carrier, forming nanocomplexes that were loaded into MNs to trigger an immune response against TC-1 cervical cancer cells in vivo (Fig. S8B in Supporting information) [85].
5. Targeting delivery strategies for MNs-based cancer vaccinesThe LNs distributed throughout the body with high-density APCs, B cells, and T cells, which are the most important lymphoid organs for vaccine-induced adaptive immunity [86]. Hence, cancer vaccines targeted delivery to LNs is an attractive cancer immunotherapy strategy. The skin, as the perfect site for immunization, is rich in capillary lymphatic vessels, which provide a bridge between the skin and LNs, as well as offer the basis for targeting cancer vaccines to LNs through percutaneous delivery [87]. Among the targeting strategies, passive lymphatic drainage, receptor-promoted cytokinesis, vesicular transfer mechanisms, and uptake by APCs are effective approaches for cancer vaccines to enter the capillary lymphatic vessels, especially for nanovaccines [28]. Additionally, higher interstitial oncotic pressure in the skin offers advantages for targeting LNs [88]. Based on these facts, MNs offer great advantages for the direct exposure of cancer vaccines to capillary lymphatic vessels in the skin for targeted LNs delivery.
5.1. Passive targeting-LNs accumulationPassive targeted accumulation of LNs relies on the physicochemical properties of the nanovaccine. Size is an important parameter in the migration of nanovaccines into the capillary lymphatics through loose gaps in the endothelium of capillary lymphatic vessels [87,89]. It is generally accepted that small-sized particles (<10 nm) are absorbed through the capillaries into the blood after dermal administration, 10–100 nm particles enter the capillary lymphatic vessels via lymphatic drainage, while the efficiency of particles of >100 nm is significantly reduced by obstruction of the extracellular matrix in the tissues through lymphatic drainage, and larger particles (>200 nm) do not move freely directly into the lymphatic capillaries but are instead migrated to the LNs by APCs through lymphatic transport [90-94]. Therefore, designing the size of the cancer nanovaccine is important for the passive targeting of LNs accumulation. For instance, Kim et al. [95] co-loaded hydrophilic antigen OVA, hydrophobic adjuvant TLR7/8 agonist (R848), and Poloxamer F127 in MNs for cancer vaccination (Fig. S9A in Supporting information). After insertion of the pyramid-shaped MNs, the released cargoes were self-assembled in-situ in the skin, forming nanomicelles with the size of 30 nm to 40 nm. The results demonstrated that R848-loaded nanomicelles promoted the uptake of OVA-FITC by RAW264.7 cells. Importantly, the fluorescent probe signal in nanomicelles was observed from Langerhans cells (CD207+), medullary macrophages (F4/80+), DCs (DEC205+), and subcapsular macrophages (CD169+) in LNs, indicating nanomicelles particle size was suitable for efficiently transporting R848 and antigens to the LNs via lymphatic drainage. Furthermore, MNs-based nanomicelles delivery induced a low level of IL-6 in serum compared to subcutaneous injection, due to the NPs-mediated delivery of R848 to LNs targeted accumulation that minimized systemic exposure. In vivo results demonstrated an increase in antibody and IFN-γ levels were elicited, resulting in a significant antitumor effect.
Moreover, the passive targeted accumulation of LNs can also be enhanced by adjusting the shape, surface charge, rigidity, and surface hydrophobicity of nanovaccines’ physical and chemical properties [96]. Normally, the positive surface charge is a disadvantage for NPs, making them more susceptible to removal during systemic circulation. However, in the case of MNs delivering safe doses of nanovaccines, the positive surface charge is a favorable factor, which enhanced the escape capacity of endosomes and the activation of skin-resident immune cell in particular [97]. Furthermore, Leak and colleagues used cationic ferritin to demonstrate the presence of anionic sites at endothelial cell junctions in capillary lymphatic vessels [98]. Given these, Wu et al. [99] prepared nanovaccines with positive (OVA-SAT) and negative (OVA-SCT) surface charges based on transfersomes, respectively, and incorporated them into MNs for skin immunization (Figs. S9B and C in Supporting information). Transferosomes were elastic and deformable vesicles that facilitated the diffusion and migration of antigens in the skin. Although the higher internalization efficiency of negatively charged nanovaccines by DCs due to HA-CD44 interactions, positively charged nanovaccines exhibited greater endo/lysosome escape that promoted OVA presentation. In vivo tracking study showed that cationic nanovaccine OVA-SAT was better enriched in LNs than anionic nanovaccine OVA-SCT for 120 h after percutaneous immunization by MNs (Fig. S9D in Supporting information).
5.2. Active targeting-LNs homingAPCs residing in the skin are responsible for the uptake, processing, and presentation of vaccines to the LNs to activate the immune response. Therefore, designing cancer vaccines to target APCs actively and exploiting homing effect would be an ideal strategy. Especially, highly expressed receptors on the surface of DCs, including CD40, CD44, CD11c, DC-SIGN, and DEC205, offer the opportunity for active target delivery of cancer vaccines to LNs [100,101]. For example, Zhou et al. [102] devised a strategy for targeting tumor-draining lymph nodes (tdLNs) and DCs with transfersomes based on nanovaccines complexed MNs with pyramid morphology and 84.4% insertion ratio (Fig. S10A in Supporting information). Apart from the fact that the transfersomes enhanced lymphatic uptake via the skin route, they also modified the transfersomes with HA-GMS-αCD40 to endow the transfersomes with DCs active targeting ability via αCD40-CD40 and HA-CD44. OVA or TAA, αPD1, and poly(I:C) were co-encapsulated in functionalized transfersomes for vaccination via dissolving MNs. The results revealed that the highest fluorescence signal of functionalized transfersomes-based nanovaccines in tdLNs was observed for 96 h after MNs-based immunization, achieving a significant anti-melanoma effect. Furthermore, mannose receptors expressed by DCs will also facilitate the targeted delivery of cancer vaccines to DCs [103-105]. Xu et al. [106] prepared a polymeric nanocomplex of paclitaxel (PTX)-encapsulated sulfobutylether-β-cyclodextrin (SBE)/mannosylated N,N,N trimethyl chitosan (mTMC)/DNA, which provided pTrp-2 cancer vaccine targeting DCs through mannose and produced high antitumor activity in mice using MNs-assisted transcutaneous immunization (Fig. S10B in Supporting information).
Besides active targeting of DCs, endothelial cells of the capillary lymphatic vessels express vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1) and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE1), which are necessary to help immune cells enter the capillary lymphatic vessels. Therefore, modification of cancer vaccines by suitable ligands to target endothelial cells is a potential way to achieve effective delivery of cancer vaccines to LNs [90].
Recently, biomimetic cancer nanovaccines based on various cell membrane modifications have also emerged as a research hotspot. NPs are encapsulated by cell membranes that inherit the functions and components (e.g., proteins and carbohydrates) of the original cells (cancer cells, red blood cells, neutrophils, platelets, macrophages, and bacteria, etc.), and the components of the cell membrane are recognized as antigens that offer the high potential of cancer vaccines delivery [107-109]. In particular, cancer cell membrane-modified nanovaccines possess homologous recognition of tumor cells and active delivery of TAAs from the cell membrane surface to DCs that enhance uptake to activate subsequent antitumor immune responses [110,111]. Park et al. [112] encapsulated the imiquimod (R837)-loaded nanomicelles (F127-R837 NPs) into HCT116 cancer cell membrane to prepare F127-R837@M NPs for skin cancer vaccination by dissolving MNs. The results showed that F127-R837@M NPs were efficiently absorbed by RAW264.7 cells, and F127-R837@M NPs delivered by dissolving MNs significantly suppressed tumors compared to F127-R837 NPs.
Albumin hitchhiking is another interesting and attractive strategy for the active homing delivery of cancer vaccines to LNs. Albumin is the most abundant protein in the blood and is also widely present in interstitial fluid, where it is mainly responsible for the transport of hydrophobic molecules, such as hormones, long-chain fatty acids, and drugs [113]. Due to the high concentration of albumin in the blood, the albumin hitchhiking prevents the nanovaccines from crossing the capillaries and entering the circulation in the skin, while most of them enter the lymphatic system. Given these facts, An et al. [114] utilized dissolving MNs to deliver an amphiphilic vaccine consisting of OVA and CpG, which effectively delivered vaccine to drained LNs for inducing the immune response through the transport and uptake mechanism of albumin hitchhiking (Fig. S11A in Supporting information). The results suggested the increase in fluorescence intensity and size of inguinal and axillary LNs isolated from mice based on MNs delivery, compared to intradermal injection. In addition, MNs-based modified CpG delivery showed higher DCs accumulation in LNs than unmodified CpG and was similar to intradermal injection (Fig. S11B in Supporting information).
6. Conclusions and future perspectivesAmong various cancer immunotherapies, the therapeutic vaccine becomes the research hotspot recently because it could offer specific, safe, and tolerable treatment. However, its therapeutic efficacy is still suboptimal, which limits clinical translation. Recent studies show that percutaneous delivery of vaccines offers a novel strategy for vaccine redevelopment. In particular, MNs-based cancer vaccine has become a new research hotspot due to various unique advantages. However, low drug packaging of MNs limits the immune response rate, so higher requirements are needed for such an administration route. To this end, we have a categorical discussion of existing immune-engineering design strategies for MNs-based cancer vaccines, which have important guiding significance for the development of safer, more potent, and more durable MNs-based cancer vaccines. Meanwhile, there is a burgeoning of research activities to investigate the various parameters of MNs-based cancer vaccine delivery to optimize immune responses, including MNs type, material, structure, shape, size, etc. These parameters are important in piercing skin with adequate mechanical strength, establishing the effective dose, enhancing the stability of the vaccine, achieving controlled release, triggering an effective immune response, and improving patient compliance. Besides, MNs delivery strategy is conducive to combining vaccines with synergetic therapy, such as phototherapy, chemotherapy, and immune checkpoint inhibition therapy, which is superior to monotherapy that combats tumor immune escape established by downregulation of MHC complexes presentation, Treg cells immunosuppression and upregulation of co-repressed signaling pathways to facilitate clinical translation.
Despite these advantages of MNs for cancer vaccine delivery, only a few have entered clinical trials and there are still several issues that require attention. Firstly, MNs delivery systems are not suitable for all types of cancer vaccines, and sometimes it depends on the type of tumor. For instance, MNs-based in-situ vaccines combined with phototherapy are more suitable for superficial tumors and their metastasis, but not for deep primary tumors. Therefore, how to apply these effective strategies universally is still one of the current research directions. Secondly, although MNs have a minimally invasive nature, repeated administration reminds us to pay attention to their safety, such as allergies and infection. Therefore, the selection of MNs biomaterials and the incorporation of nanotechnology to prolong the release of tumor antigens to enhance the efficacy and persistence of cancer vaccines, achieving a shift from multiple to single vaccination remains a focus of future attention. Besides, MNs-based cancer vaccine researches are still at cell and animal levels, and there needs more work to figure out the racial differences between animals and human, which is critical for clinical translation. Especially, the skin elasticity that affects the insertion of MNs and the density of capillary lymphatic vessels depend on the physiological state of the patients at various ages, and therefore the patients have to be taken into account to optimize the MNs-based cancer vaccines. In addition, aseptic scale-up production, batch-to-batch reproducibility, cost-effectiveness, and strict government supervision are also crucial for the successful clinical translation of MNs-based cancer vaccines.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 82073799), the Natural Science Foundation of Hunan Province in China (No. 2021JJ20084), and the Science and Technology Innovation Program of Hunan Province (No. 2021RC3020).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108706.
| [1] |
I. Soerjomataram, F. Bray, Nat. Rev. Clin. Oncol. 18 (2021) 663-672. DOI:10.1038/s41571-021-00514-z |
| [2] |
N. Wang, J. Chen, W. Chen, et al., BMC Health Serv. Res. 22 (2022) 1247. DOI:10.1186/s12913-022-08610-1 |
| [3] |
L. Milling, Y. Zhang, D.J. Irvine, Adv. Drug Deliv. Rev. 114 (2017) 79-101. DOI:10.1016/j.addr.2017.05.011 |
| [4] |
A. Huang, W. Zhou, Chin. J. Cancer Res. 35 (2023) 19-43. DOI:10.21147/j.issn.1000-9604.2023.01.04 |
| [5] |
Z. Wang, Q. Chen, H. Zhu, et al., Chin. Chem. Lett. 32 (2021) 1888-1892. DOI:10.1016/j.cclet.2021.01.036 |
| [6] |
Y. Igarashi, T. Sasada, J. Immunol. Res. 2020 (2020) 5825401. |
| [7] |
R.S. Riley, C.H. June, R. Langer, M.J. Mitchell, Nat. Rev. Drug Discov. 18 (2019) 175-196. DOI:10.1038/s41573-018-0006-z |
| [8] |
Z. Feng, Y. Wang, H. Xu, et al., Acta Pharm. Sin. B 13 (2023) 1014-1027. DOI:10.1016/j.apsb.2022.09.015 |
| [9] |
L. Shen, T. Zhou, Y. Fan, et al., Chin. Chem. Lett. 31 (2020) 1709-1716. DOI:10.1016/j.cclet.2020.02.007 |
| [10] |
C.S. Higano, E.J. Small, P. Schellhammer, et al., Nat. Rev. Drug Discov. 9 (2010) 513-514. DOI:10.1038/nrd3220 |
| [11] |
S.Z. Shalhout, D.M. Miller, K.S. Emerick, H.L. Kaufman, Nat. Rev. Clin. Oncol. 20 (2023) 160-177. DOI:10.1038/s41571-022-00719-w |
| [12] |
Y. Levin, E. Kochba, I. Hung, R. Kenney, Hum. Vaccin. Immunother. 11 (2015) 991-997. DOI:10.1080/21645515.2015.1010871 |
| [13] |
A.F. Moreira, C.F. Rodrigues, T.A. Jacinto, et al., Pharmacol. Res. 148 (2019) 104438. DOI:10.1016/j.phrs.2019.104438 |
| [14] |
H. Amani, M.A. Shahbazi, C. D’Amico, et al., J. Control. Release 330 (2021) 185-217. DOI:10.1016/j.jconrel.2020.12.019 |
| [15] |
D. Li, D. Hu, H. Xu, et al., Biomaterials 264 (2021) 120410. DOI:10.1016/j.biomaterials.2020.120410 |
| [16] |
S. Feng, G. Song, L. Liu, et al., J. Dermatol. 49 (2022) 1310-1319. DOI:10.1111/1346-8138.16582 |
| [17] |
R.M. Steinman, J. Banchereau, Nature 449 (2007) 419-426. DOI:10.1038/nature06175 |
| [18] |
Y. Hu, L. Lin, Z. Guo, et al., Chin. Chem. Lett. 32 (2021) 1770-1774. DOI:10.1016/j.cclet.2020.12.055 |
| [19] |
B.L. Bartlett, A.J. Pellicane, S.K. Tyring, Dermatol. Ther. 22 (2009) 104-109. DOI:10.1111/j.1529-8019.2009.01223.x |
| [20] |
R.L. Creighton, K.A. Woodrow, Adv. Healthc. Mater. 8 (2019) e1801180. DOI:10.1002/adhm.201801180 |
| [21] |
Y.L. Mato, Int. J. Pharm. 572 (2019) 118813. DOI:10.1016/j.ijpharm.2019.118813 |
| [22] |
I.J. Choi, A. Kang, M.H. Ahn, et al., J. Control. Release 286 (2018) 460-466. DOI:10.1016/j.jconrel.2018.08.017 |
| [23] |
B.G. Weniger, G.M. Glenn, Vaccine 31 (2013) 3389-3391. DOI:10.1016/j.vaccine.2013.05.048 |
| [24] |
E. Criscuolo, V. Caputo, R.A. Diotti, et al., J. Immunol. Res. 2019 (2019) 8303648. |
| [25] |
P. Makvandi, M. Kirkby, A.R.J. Hutton, et al., Nanomicro Lett. 13 (2021) 93. |
| [26] |
K. Kabashima, T. Honda, F. Ginhoux, G. Egawa, Nat. Rev. Immunol. 19 (2019) 19-30. DOI:10.1038/s41577-018-0084-5 |
| [27] |
S. Liao, P.Y. von der Weid, Semin. Cell Dev. Biol. 38 (2015) 83-89. DOI:10.1016/j.semcdb.2014.11.012 |
| [28] |
A.D. Permana, F. Nainu, K. Moffatt, E. Larrañeta, R.F. Donnelly, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 13 (2021) e1690. DOI:10.1002/wnan.1690 |
| [29] |
G.K. Menon, G.W. Cleary, M.E. Lane, Int. J. Pharm. 435 (2012) 3-9. DOI:10.1016/j.ijpharm.2012.06.005 |
| [30] |
X. Zhou, Y. Hao, L. Yuan, et al., Chin. Chem. Lett. 29 (2018) 1713-1724. DOI:10.1016/j.cclet.2018.10.037 |
| [31] |
Y. Wang, L. Zeng, W. Song, J. Liu, Drug Deliv. Transl. Res. 12 (2022) 15-26. DOI:10.1007/s13346-021-00898-6 |
| [32] |
B.E. Polat, D. Hart, R. Langer, D. Blankschtein, J. Control. Release 152 (2011) 330-348. DOI:10.1016/j.jconrel.2011.01.006 |
| [33] |
K. Ita, Pharmaceutics 8 (2016) 9. DOI:10.3390/pharmaceutics8010009 |
| [34] |
S. Del Río-Sancho, D. Pan Delgado, G.F. de la Fuente, et al., Eur. J. Pharm. Biopharm. 156 (2020) 165-175. DOI:10.1016/j.ejpb.2020.08.027 |
| [35] |
Y. Liu, T. Huang, Z. Qian, W. Chen, Chin. Chem. Lett. 34 (2023) 108103. DOI:10.1016/j.cclet.2022.108103 |
| [36] |
G. Ma, C. Wu, J. Control. Release 251 (2017) 11-23. DOI:10.1016/j.jconrel.2017.02.011 |
| [37] |
Y. Zeng, H. Zhou, J. Ding, W. Zhou, Theranostics 11 (2021) 8270-8282. DOI:10.7150/thno.60758 |
| [38] |
Y. Zhang, Y. Xu, H. Kong, et al., Exploration 3 (2023) 20210170. DOI:10.1002/EXP.20210170 |
| [39] |
S.C. Balmert, C.D. Carey, G.D. Falo, et al., J. Control. Release 317 (2020) 336-346. DOI:10.1016/j.jconrel.2019.11.023 |
| [40] |
B. Bediz, E. Korkmaz, R. Khilwani, et al., Pharm. Res. 31 (2014) 117-135. DOI:10.1007/s11095-013-1137-x |
| [41] |
J. Rice, C.H. Ottensmeier, F.K. Stevenson, Nat. Rev. Cancer 8 (2008) 108-120. DOI:10.1038/nrc2326 |
| [42] |
A. Lopes, G. Vandermeulen, V. Préat, J. Exp. Clin. Cancer Res. 38 (2019) 146. DOI:10.1186/s13046-019-1154-7 |
| [43] |
H.T.T. Duong, Y. Yin, T. Thambi, et al., Biomaterials 185 (2018) 13-24. DOI:10.1016/j.biomaterials.2018.09.008 |
| [44] |
H.T.T. Duong, Y. Yin, T. Thambi, et al., J. Mater. Chem. B 8 (2020) 1171-1181. DOI:10.1039/c9tb02175b |
| [45] |
A. Gupta, J.L. Andresen, R.S. Manan, R. Langer, Adv. Drug Deliv. Rev. 178 (2021) 113834. DOI:10.1016/j.addr.2021.113834 |
| [46] |
M.A. McNamara, S.K. Nair, E.K. Holl, J. Immunol. Res. 2015 (2015) 794528. |
| [47] |
K.K.L. Phua, K.W. Leong, S.K. Nair, J. Control. Release 166 (2013) 227-233. DOI:10.1016/j.jconrel.2012.12.029 |
| [48] |
K.J. Koh, Y. Liu, S.H. Lim, et al., Sci. Rep. 8 (2018) 11842. DOI:10.1038/s41598-018-30290-3 |
| [49] |
W. Liu, H. Tang, L. Li, et al., Cell Prolif. 54 (2021) e13025. DOI:10.1111/cpr.13025 |
| [50] |
H. Kim, K.Y. Seong, J.H. Lee, et al., ACS Biomater. Sci. Eng. 5 (2019) 5150-5158. DOI:10.1021/acsbiomaterials.9b00961 |
| [51] |
Q. Zeng, J.M. Gammon, L.H. Tostanoski, Y.C. Chiu, C.M. Jewell, ACS Biomater. Sci. Eng. 3 (2017) 195-205. DOI:10.1021/acsbiomaterials.6b00414 |
| [52] |
I. Dagogo-Jack, A.T. Shaw, Nat. Rev. Clin. Oncol. 15 (2018) 81-94. DOI:10.1038/nrclinonc.2017.166 |
| [53] |
S.H. van der Burg, R. Arens, F. Ossendorp, T. van Hall, C.J. Melief, Nat. Rev. Cancer 16 (2016) 219-233. DOI:10.1038/nrc.2016.16 |
| [54] |
C.L. Chiang, G. Coukos, L.E. Kandalaft, Vaccines 3 (2015) 344-372. DOI:10.3390/vaccines3020344 |
| [55] |
Y. Ye, C. Wang, X. Zhang, et al., Sci. Immunol. 2 (2017) eaan5692. DOI:10.1126/sciimmunol.aan5692 |
| [56] |
A. Harari, M. Graciotti, M. Bassani-Sternberg, L.E. Kandalaft, Nat. Rev. Drug Discov. 19 (2020) 635-652. DOI:10.1038/s41573-020-0074-8 |
| [57] |
D. Yang, M. Chen, Y. Sun, et al., J. Control. Release 357 (2023) 641-654. DOI:10.1001/jamaophthalmol.2023.1821 |
| [58] |
X. Xiong, J. Zhao, J. Pan, et al., Nano Lett. 21 (2021) 8418-8425. DOI:10.1021/acs.nanolett.1c03004 |
| [59] |
X. Wang, C. Li, Y. Wang, et al., Acta Pharm. Sin. B 12 (2022) 4098-4121. DOI:10.3390/electronics11244098 |
| [60] |
L. Li, S. Wang, W. Zhou, Cancers 15 (2022) 26. DOI:10.3390/cancers15010026 |
| [61] |
L. Fu, X. Ma, Y. Liu, Z. Xu, Z. Sun, Chin. Chem. Lett. 33 (2022) 1718-1728. DOI:10.1016/j.cclet.2021.10.074 |
| [62] |
Y. Bo, H. Wang, Adv. Mater. (2023) e2210452. DOI:10.1002/adma.202210452 |
| [63] |
J. Zhu, R. Chang, B. Wei, et al., Research 2022 (2022) 9816272. |
| [64] |
Z.B. Wang, J. Xu, Vaccines 8 (2020) 128. DOI:10.3390/vaccines8010128 |
| [65] |
C.T. Perciani, L.Y. Liu, L. Wood, S.A. MacParland, ACS Nano 15 (2021) 7-20. DOI:10.1021/acsnano.0c08913 |
| [66] |
H. Dai, Q. Fan, C. Wang, Exploration 2 (2022) 20210157. DOI:10.1002/EXP.20210157 |
| [67] |
C.H. Kapadia, S. Tian, J.L. Perry, et al., J. Control. Release 269 (2018) 393-404. DOI:10.1016/j.jconrel.2017.11.020 |
| [68] |
J. Liu, L. Miao, J. Sui, Y. Hao, G. Huang, Asian J. Pharm. Sci. 15 (2020) 576-590. DOI:10.1016/j.ajps.2019.10.006 |
| [69] |
F. Lebre, C.H. Hearnden, E.C. Lavelle, Adv. Mater. 28 (2016) 5525-5541. DOI:10.1002/adma.201505395 |
| [70] |
X. Liu, C. Zheng, Y. Kong, H. Wang, L. Wang, Chin. Chem. Lett. 33 (2022) 328-333. DOI:10.1016/j.cclet.2021.07.025 |
| [71] |
A. George, P.A. Shah, P.S. Shrivastav, Int. J. Pharm. 561 (2019) 244-264. DOI:10.1016/j.ijpharm.2019.03.011 |
| [72] |
E.A. Grego, A.C. Siddoway, M. Uz, et al., Curr. Top. Microbiol. Immunol. 433 (2021) 29-76. |
| [73] |
B.S. Ou, O.M. Saouaf, J. Baillet, E.A. Appel, Adv. Drug Deliv. Rev. 187 (2022) 114401. DOI:10.1016/j.addr.2022.114401 |
| [74] |
M. Zaric, O. Lyubomska, O. Touzelet, et al., ACS Nano 7 (2013) 2042-2055. DOI:10.1021/nn304235j |
| [75] |
D. Wibowo, S.H.T. Jorritsma, Z.J. Gonzaga, et al., Biomaterials 268 (2021) 120597. DOI:10.1016/j.biomaterials.2020.120597 |
| [76] |
S. Iyer, R. Yadav, S. Agarwal, S. Tripathi, R. Agarwal, Clin. Microbiol. Rev. 35 (2022) e0012321. DOI:10.1128/CMR.00123-21 |
| [77] |
Y. Hu, B. Xu, J. Xu, et al., Polym. Chem. 6 (2015) 373-379. DOI:10.1039/C4PY01394H |
| [78] |
Z. Li, Y. He, L. Deng, Z.R. Zhang, Y. Lin, J. Mater. Chem. B 8 (2020) 216-225. DOI:10.1039/c9tb02061f |
| [79] |
M. Henriksen-Lacey, A. Devitt, Y. Perrie, J. Control. Release 154 (2011) 131-137. DOI:10.1016/j.jconrel.2011.05.019 |
| [80] |
L. Rahnfeld, P. Luciani, Pharmaceutics 12 (2020) 567. DOI:10.3390/pharmaceutics12060567 |
| [81] |
K. Thapa Magar, G.F. Boafo, X. Li, Z. Chen, W. He, Chin. Chem. Lett. 33 (2022) 587-596. DOI:10.1016/j.cclet.2021.08.020 |
| [82] |
J.H. Zhao, Q.B. Zhang, B. Liu, et al., Int. J. Nanomed. 12 (2017) 4763-4772. DOI:10.2147/IJN.S132456 |
| [83] |
J. Lee, D. Kim, J. Byun, et al., Adv. Drug Deliv. Rev. 186 (2022) 114325. DOI:10.1016/j.addr.2022.114325 |
| [84] |
G. Cole, A.A. Ali, E. McErlean, et al., Acta Biomater. 96 (2019) 480-490. DOI:10.1016/j.actbio.2019.07.003 |
| [85] |
G. Cole, A.A. Ali, C.M. McCrudden, et al., Eur. J. Pharm. Biopharm. 127 (2018) 288-297. DOI:10.1016/j.ejpb.2018.02.029 |
| [86] |
T. Cai, H. Liu, S. Zhang, J. Hu, L. Zhang, J. Nanobiotechnol. 19 (2021) 389. DOI:10.1142/9789811218309_bmatter |
| [87] |
M. Chen, G. Quan, Y. Sun, et al., J. Control. Release 325 (2020) 163-175. DOI:10.1016/j.jconrel.2020.06.039 |
| [88] |
Y. Ding, Z. Li, A. Jaklenec, Q. Hu, Adv. Drug Deliv. Rev. 179 (2021) 113914. DOI:10.1016/j.addr.2021.113914 |
| [89] |
Y.N. Zhang, J. Lazarovits, W. Poon, et al., Nano Lett. 19 (2019) 7226-7235. DOI:10.1021/acs.nanolett.9b02834 |
| [90] |
A.H. Sabri, Y. Kim, M. Marlow, et al., Adv. Drug Deliv. Rev. 153 (2020) 195-215. DOI:10.1016/j.addr.2019.10.004 |
| [91] |
G.M. Ryan, L.M. Kaminskas, C.J. Porter, J. Control. Release 193 (2014) 241-256. DOI:10.1016/j.jconrel.2014.04.051 |
| [92] |
M.P. Manspeaker, S.N. Thomas, Adv. Drug Deliv. Rev. 160 (2020) 19-35. DOI:10.1016/j.addr.2020.10.004 |
| [93] |
M. Abdallah, O.O. Müllertz, I.K. Styles, et al., J. Control. Release 327 (2020) 117-128. DOI:10.1016/j.jconrel.2020.07.046 |
| [94] |
Y. Sui, J. Li, J. Qu, et al., Asian J. Pharm. Sci. 17 (2022) 583-595. DOI:10.1016/j.ajps.2022.05.004 |
| [95] |
N.W. Kim, S.Y. Kim, J.E. Lee, et al., ACS Nano 12 (2018) 9702-9713. DOI:10.1021/acsnano.8b04146 |
| [96] |
X. Ke, G.P. Howard, H. Tang, et al., Adv. Drug Deliv. Rev. 151-152 (2019) 72-93. DOI:10.1016/j.addr.2019.09.005 |
| [97] |
Y.C. Tseng, Z. Xu, K. Guley, H. Yuan, L. Huang, Biomaterials 35 (2014) 4688-4698. DOI:10.1016/j.biomaterials.2014.02.030 |
| [98] |
L.V. Leak, Microvasc. Res. 31 (1986) 18-30. DOI:10.1016/0026-2862(86)90003-8 |
| [99] |
X. Wu, Y. Li, X. Chen, et al., J. Mater. Chem. B 7 (2019) 4854-4866. DOI:10.1039/c9tb00448c |
| [100] |
H. Kim, T.S. Griffith, J. Panyam, J. Pharmacol. Exp. Ther. 370 (2019) 715-724. DOI:10.1124/jpet.118.254953 |
| [101] |
R.E. Serda, Int. J. Nanomed. 8 (2013) 1683-1696. |
| [102] |
Z. Zhou, J. Pang, X. Wu, et al., Nano Res. 13 (2020) 1509-1518. DOI:10.1007/s12274-020-2737-5 |
| [103] |
N.L. Trevaskis, L.M. Kaminskas, C.J. Porter, Nat. Rev. Drug Discov. 14 (2015) 781-803. DOI:10.1038/nrd4608 |
| [104] |
R. Yang, J. Xu, L. Xu, et al., ACS Nano 12 (2018) 5121-5129. DOI:10.1021/acsnano.7b09041 |
| [105] |
Y. Song, C.M. Dong, Chin. Chem. Lett. 33 (2022) 4084-4088. DOI:10.1016/j.cclet.2022.01.051 |
| [106] |
J. Xu, B. Xu, J. Tao, et al., Small 13 (2017) 1700666. DOI:10.1002/smll.201700666 |
| [107] |
R. Liu, C. Luo, Z. Pang, et al., Chin. Chem. Lett. 34 (2023) 107518. DOI:10.1016/j.cclet.2022.05.032 |
| [108] |
Z. Meng, Y. Zhang, X. Zhou, J. Ji, Z. Liu, Adv. Drug Deliv. Rev. 182 (2022) 114107. DOI:10.1016/j.addr.2021.114107 |
| [109] |
H. Liu, Z. Miao, Z. Zha, Chin. Chem. Lett. 33 (2022) 1673-1680. DOI:10.1016/j.cclet.2021.10.057 |
| [110] |
Y. Guo, Z. Wang, X. Shi, M. Shen, Exploration 2 (2022) 20210171. DOI:10.1002/EXP.20210171 |
| [111] |
M. Li, M. Qin, G. Song, et al., Asian J. Pharm. Sci. 16 (2021) 97-109. DOI:10.1016/j.ajps.2020.06.006 |
| [112] |
W. Park, K.Y. Seong, H.H. Han, S.Y. Yang, S.K. Hahn, RSC Adv. 11 (2021) 10393-10399. DOI:10.1039/d1ra00747e |
| [113] |
P. Famta, S. Shah, N. Jain, et al., J. Control. Release 353 (2023) 166-185. DOI:10.1016/j.jconrel.2022.11.034 |
| [114] |
M. An, H. Liu, Small 13 (2017) 1700164. DOI:10.1002/smll.201700164 |
 2024, Vol. 35
2024, Vol. 35 

