b Institute of Molecular Materials and Devices, Fudan University, Shanghai 200433, China
COVID-19 is an infectious disease caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2). Its high transmission rate and the high proportion of asymptomatic infection have posed a significant threat to human health and life [1–5]. In the absence of specific therapeutic drugs, it is crucial to strengthen clinical screening and quarantine with early accurate technology to reduce the risk of disease transmission. The SARS-CoV-2 diagnostic techniques are classified into three categories currently: Molecular techniques for detecting viral RNA sequences, antigens or host antibodies techniques for detecting the presence of the virus, and Imaging techniques for detecting changes in the lung [6–13]. Among the current developing approaches, reverse transcription-polymerase chain reaction (RT-PCR) has always been considered the gold standard for detecting SARS-CoV-2. RT-PCR techniques have several advantages, such as high sensitivity and specificity at the early stages of infection. However, this method requires well-trained operators, expensive equipment, time-consuming steps, and standard laboratory conditions; these factors severely limit rapid and large-scale testing in under-equipped hospitals [14,15]. The immunological detection method is an auxiliary method for the rapid diagnosis of SARS-CoV-2 as it can test results efficiently and operate conveniently. Nevertheless, human antibodies are formed 2 to 3 weeks after virus infection, false-positive results may occur in patients previously infected with SARS-CoV-2 [16–21]. The abovementioned aspects make it unsuitable for detecting suspected cases promptly and quickly in controlling epidemic situations. Therefore, a specific and convenient method for diagnosing SARS-CoV-2 is urgently needed to protect public safety [22–25]. Thus, the real-time detection strategy by point-of-care testing (POCT) platforms is an excellent supplement for early diagnosis. POCT platforms implement at the sample collection site and use portable analytical instruments with matching reagents that meet the detection needs of fever clinics, community hospitals, customs, and airports.
Here, we summarize recent POCT achievements of SARS-CoV-2 and introduce the nucleic acid detection technology platform, immunological detection platform, and nanomaterial-based biosensors detection platforms. At the same time, the application scopes, the advantages, and the limitations of each detection method are clarified. In addition, we put forward prospects for developing a more convenient, efficient, and sensitive POCT technology; we hope to provide a reference for epidemic prevention and clinical diagnosis for future work.
2. Overview of rapid diagnosis by POCT platforms 2.1. Advantages of POCT diagnosisPOCT is a real-time strategy representing an accurate, efficient sample collection testing site. Numerous definitions of POCT diagnosis indicate a similar thing, "sample-in answer-out", which obtain test results quickly so that the patient can be diagnosed at the earliest. The diagnostic platform plays a vital role in the early implementation of isolation, contact tracing, and control measures that limit the spread followed. Traditional diagnostic platforms PCR-based detections are time-consuming and with significant demand for intensive equipment.
POCT diagnosis is low cost, portable, repeatable, and conducive to large-scale screening with real-time testing [26]. It is also suitable for clinical laboratories and nonlaboratory personnel under simple training following a few operation steps. The infected person could be found immediately instead of sending the sample to the central laboratory for a long turnaround time [27]. In addition, it benefits low-income areas or communities that cannot provide POCT devices and extended testing [28–31]. The global pandemic has exposed apparent discrepancies in POCT techniques, and the significance of the development for preventing pandemics at their initial stage is highlighted [32–35]. In this review, we summarize related studies concerning POCT platforms.
2.2. The common techniques for POCTThe number of kits developed using the RT-PCR method to test the SARS-CoV-2 nucleic acid has a considerable scale. RT-PCR is a nucleic acid amplification technology wherein the RNA is translated into its complementary deoxyribonucleic acids (cDNA), and the specific region of the cDNA is amplified by PCR repeatedly multiplied for detection [36,37]. Although the RT-PCR method is considered the gold standard with high sensitivity for SARS-CoV-2 detection, it is usually inconvenient to operate, depends on well-trained laboratory personnel, and has a long turnaround time [38,39]. The sample amplification period has a barrier in the form of the necessity for specialized heat cycling equipment, which is highly costly and complicated. Challenging to realize and equip in places where resources are scarce [40]. These limitations in the diagnostic platforms prompted researchers to develop strategies that simplify the process and correspond to sensitivity and specificity. Combined with the current epidemic prevention needs, Isothermal amplification is the most promising alternative method of RT-PCR, which amplifies nucleic acids at a constant temperature without thermal cycling progress and can test SARS-CoV-2 as similar as using RT-PCR at the same level [41].
At present, kinds of isothermal amplification technologies that can be implemented in the POCT diagnostic platform, such as loop-mediated isothermal amplification (LAMP) [42–47], recombinase polymerase amplification (RPA) [48–52], rolling circle amplification (RCA) [53–57], and nicking enzyme-assisted reaction (NEAR) [58–61] (Fig. 1). Moreover, some electrochemical and visual sensors have been developed leveraging advances in nanotechnology with improved sensitivity, specificity and cost-effectivity.

|
Download:
|
| Fig. 1. Principle of nucleic acid amplification in point-of-care testing of SARS-CoV-2. Schematic diagram of RT-PCR (A), LAMP-PCR (B), RPA-PCR (C), NEAR-PCR (D), RCA-PCR (E), and CRISPR (F). Copied with permission [37,48]. Copyright 2022, Wiley. | |
RT-LAMP is the most widely used isothermal amplification technique for SARS-CoV-2 nucleic acid detection without a thermal cycler. At the initial stage of RT-LAMP, the RNA genome of SARS-CoV-2 is reverse-transcribed into cDNA, then designed primers that can bind to six different regions are used to enhance the sensitivity of mixed LAMP [42]. Notably, oligos containing a DNA strand can be regarded as primers for reverse transcriptase. This auto-cycling program promotes various double-stranded looped DNA structures and test results caused by the fluorescent response, pH sensitivity, and turbidity. The detection time of SARS-CoV-2 by RT-LAMP is shorter than the traditional RT-PCR.
RT-LAMP is carried out at a single temperature between 60 and 65 ℃ and generates up to 109 copies of DNA within 1 h. LAMP-based detection technologies have been used in the research of the POCT platform for SARS-CoV-2 detection [43]. Several studies have evaluated some experimental parameters for RT-LAMP, such as limit of detection (LOD), target genes, and primer sequences. Anders et al. [44] designed a two-color RT-LAMP assay protocol using a specific primer set for the nucleocapsid (N) gene to test SARS-CoV-2 viral RNA. Compared to the RT-PCR technology, which also used a sensitive primer set, the RT-LAMP with high specificity and sensitivity is more reliable. Lu et al. [45] designed a mismatch-tolerant amplification technique called visual RT-LAMP assay based on its N gene. The LOD per 25 µL reaction is up to 118.6 copies of SARS-CoV-2 RNA, and the procedure is completed within 30 min. Despite many benefits, LAMP techniques have several limitations in optimizing primer design and reaction conditions. Researchers have made efforts to optimize RT-LAMP for developing sensitive detection of SARS-CoV-2. Gonzalez et al. [46] used a three-dimensional (3D)-printed incubation chamber to detect and amplify genetic sequences that encode the protein. This straightforward method detects and amplifies SARS-CoV-2 nucleic acids from 62 to 2 × 105 DNA copies. These results of RT-LAMP have demonstrated that it achieved an efficient POCT technology based on nucleic acid amplification detection of SARS-CoV-2. At the same time, the amplified program needs external equipment to provide a constant temperature, which is unconducive to keep the equipment portable and miniature. Recently, Abbott Diagnostics made a POCT approach using nasal swabs for sample collection [47]. The colorimetric LAMP to detect viral RNA is a speedier diagnostic method for detecting SARS-CoV-2 in their molecular lysate samples. El-Tholoth et al. [49] enhanced the sensitivity of a modified two-step LAMP protocol; with this protocol, a success rate of 100% can be achieved at 7–10 copies of viral RNA per reaction, while the PCR method provides a similar success rate at 700 viral RNA copies needed. This nested structure can achieve higher sensitivity and better tolerance to inhibitors. However, there are still some shortcomings at this stage. The amplification products need to be purified during electrophoresis detection, and when the template concentration is low, non-specific signals will be generated, affecting the experimental results. Therefore, developing isothermal amplification methods at room temperature in the future is more promising.
3.2. Recombinase polymerase amplification (RPA)RPA is another nucleic acid isothermal amplification method used for SARS-CoV-2 detection. The reaction temperature is lower than LAMP, and the whole reaction can be realized at room temperature without external heating elements. The process of RPA mainly depends on three enzymes: recombinase that can bind single-stranded nucleic acid, single-stranded DNA binding protein (SSB), and strand replacement DNA polymerase. DNA synthesis is started through the chain replacement reaction of DNA polymerase, and the target region on the template is amplified exponentially [48]. Amplified products are detected by gel electrophoresis, tag-specific antibodies, fluorescent signals, and a quencher on primers.
The substituted DNA single-strand binds to SSB to prevent further substitution of stable open double-stranded structure [48]. It can also be combined with an information probe to use side flow test paper for the detection or add dye for visual detection and reading, which will be more suitable for POCT detection. The single-tube RT-RPA method is 100% consistent with RT-PCR. RPA process can amplify target DNA fragments without heating parts, and the reaction speed is faster than LAMP.
Behrmann [50] used a suitable set of RT-RPA primer and exonuclease probe internally quench (Exo-IQ) sequences targeting the SARS-CoV-2 N gene by computational and manual design. The RT-RPA assay was determined to be 7.74 copies per reaction and no cross-reactivity to other screened coronaviruses. Compared to RT-PCR, this developed RT-RPA assay produced 100% diagnostic sensitivity and specificity. It has excellent application prospects in virus detection as another obvious advantage: designing primers and probes is relatively simple. Its fluorescence detection reaction system only needs one pair of primers and one probe [51].
Wahed et al. [52] used some nucleocapsid gene RNA molecules to establish an RT-RPA assay for detecting MERS-CoV, and it only took 3 to 7 min to detect a minimum of 10 copies of RNA molecules. This method has a fast response and has been used to amplify different targets from kinds of organisms and virus samples. Although RPA technology is considered promising to replace PCR nucleic acid detection, there are still some shortcomings at this stage. For example, when the template concentration is low, non-specific signals will be generated, which will affect the experimental results.
3.3. Rolling circle amplification (RCA)RCA as an isothermal amplification method has been used widely for nucleic acid testing. The assay amplifies DNA or RNA primers by using DNA or RNA polymerases and annealing to a circular DNA template. The RCA amplicon is a concatemer complementary to the circular template and contains multiple sequence repeats [53]. RCA could produce amplicons ~10-fold and perform by simple water bath or heating block less than 90 min with minimal reagents. RCA has the advantages of isothermal reaction, high amplification efficiency, and easy combination with other technologies, such as an electrochemical biosensor, which enables quantitative results obtained rapidly either using POCT or conducted in the laboratory. With the development of RCA technology, researchers have developed RCA detection technology for miRNA, pathogens, proteins, and other substances [54].
In addition, RCA is also realized in the construction of DNA nanostructures, the preparation of drug carriers, and biochemical analysis. Chaibun [55] reported a new assay that is detectable based on RCA to detect SARS-CoV-2 with redox-active labels. A total of 106 samples, including 41 SARS-CoV-2 and 9 samples positive for other respiratory viruses, were tested by this assay, which reported a concordance 100% result with RT-PCR. This one-step sandwich hybridization assay can test as low as 1 copy/µL of N and spike (S) genes within 2 h. This approach allows testing kits to be allocated to outbreak areas broadly, as well as remote regions where laboratory facilities are insufficient, which particularly; is beneficial for some developing countries [56,57].
3.4. Nicking enzyme amplification reaction (NEAR)NEAR is driven by reverse transcriptase, nicking enzymes, and isothermal amplification DNA polymerase [58]. The DNA template hybridizes with the primer, the following template replaces the extension product, and the complementary strand of the replacement product is extended to form a nicking enzyme recognition site [59]. The recognition site cuts a specific short sequence of one strand of the double-stranded DNA to create a gap. The isothermally amplified DNA polymerase extends the nucleotides from the 3′ end of the primer to continue synthesizing the short sequence. Finally, the amplified double-strand of NEAR is obtained. A template of target DNA is continuously amplified through cycles of cleavage and extension, and molecular beacons are designed to generate fluorescent signals for quantification.
A new assay based on the NEAR technology has been widely used for commercial POCT detection of SARS-CoV-2 directional amplification of the RdRp designed by Abbott’s ID and completed within 5-13 min. Nguyen et al. [60] demonstrated that the ID NOW assay provides a reliable alternative to laboratory-based RT-PCR methods and is performed in the emergency department using dry swabs. This assay is a user-friendly molecular biology test based on NEAR; compared with the RT-PCR study, the assay yielded a sensitivity of 98.0% and a specificity of 97.5% and can achieve exponential rate amplification [61]. The advantages of this method are speed and sensitivity, but the disadvantage is that the design of short sequences has a high possibility of false positives.
3.5. Clustered regularly interspaced short palindromic repeats (CRISPR)CRISPR is a powerful gene-editing technology that can be used to trim, cut, replace, or add to an organism’s DNA sequence. Activated Cas enzymes, which can precisely cleave target RNA and nonspecifically cleave RNA or DNA in the surrounding environment, can be used to detect nucleic acids [62]. The quantitative properties of isothermal amplification methods can be achieved by nonspecific amplification of non-target sequences or primer-dimers.
Moreover, studies have shown that CRISPR and its related proteins, mainly Cas12a and Cas13, can be used to detect specific nucleic acids in samples. They bind to RNA or DNA targets, respectively, specified by guide RNA sequences that can bind to fluorophore quencher DNA probes to generate signal amplification [63–69]. CRISPR-based assays have higher specificity and sensitivity compared with RT-PCR and metagenomic next-generation sequencing (mNGS) methods.
Specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) via reverse transcribes the viral RNA target into cDNA, and then isothermal amplification technology amplifies it. Common SHERLOCK involves two reaction steps, individual liquid sample treatment and open tube operation. It only requires purified nucleic acid samples and can be completed quickly and accurately detect SARS-CoV-2 with high sensitivity [70].
Zhang et al. [71] Tested the synthetic SARS-CoV-2 RNA fragment and found that at a deficient virus RNA level (10–100 copy)/µL), this technique can still accurately detect the SARS-CoV-2 sequence. The test results can be intuitively displayed on the test paper, and the whole process does not require complex equipment and high-end technicians to confirm whether the patient has SARS-CoV-2 infection on site quickly. Hou et al. used the SHERLOCK method targeting orf1ab to obtain near single-copy sensitivity and reasonable specificity [72]. In 52 clinical samples of patients, it is verified that it has 100% clinical sensitivity, and the turnover time is only 40 min, which proves its diagnostic potential. And one-pot SHERLOCK test developed that combines LAMP with a CRISPR-mediated detection step; it converted the classic two-step SHERLOCK into a single-step reaction without sample extraction. The detection limit of 100 copies with commercial lateral flow test strips or fluorescent readings has been validated with clinical samples for POCT [73].
In a way named DNA endonuclease-targeted CRISPR trans reporter (DETECTOR). The first stage of this method is isothermal amplification after viral RNA is transformed into DNA [74,75]. The cas12 enzyme is then triggered by using specific target sequences in the amplified DNA, which cuts the single-stranded DNA reporter gene to release a fluorescent signal. Ding et al. [76] proposed the All-In-One Dual CRISPR-Cas12 (AIOD-CRISPR) assay. Its sensitivity can reach 4.6 copies per microliter. When combined with an isothermal amplification technology, the CRISPR-based method can produce fast readings and the sensitivity is better than RT-PCR. there is still a lack of appropriate real-time detection equipment [77–79].
Guo et al. [80] established a viral nucleic acid detection platform that integrates sample processing, recombinase-assisted amplification, and CRISPR detection, with a detection limit of 1 × 104 copies per milliliter. To make the platform more suitable for immediate detection, a Portable blue cassette with color LEDs for visualization. Of course, CRISPR can also use side flow test paper to detect signals, but the sensitivity will be reduced. CRISPR/Cas strategy based on RNA combined with isothermal amplification can improve the reliability, which is very suitable for family detection and shows a broad prospect for developing next-generation molecular diagnosis technology and applying POCT detection [81,82].
3.6. Expectations on nucleic acid detection POCT platformsAmong different nucleic acid detection technology platforms, nucleic acid extraction is essential to achieving accurate pathogen detection [83]. Conventional nucleic acid extraction is divided into two methods: manual extraction and instrument automated extraction. However, the extracted products are added manually to the detection system in the actual detection process. When processing samples containing highly infectious viruses, the infection risk of experimental operators will be increased. An integrated automated nucleic acid detection platform came into being to avoid the problems caused by separate nucleic acid extraction from samples. This closed automated system integrates sample extraction, nucleic acid detection, and result output. It can avoid errors and problems caused by excessive manual operation and decrease cross-contamination risk during sample detection.
The SARS-CoV-2 detection technology based on an integrated automation platform integrates sample extraction, nucleic acid detection, and result in output into one system, achieving the fully automatic detection of pathogen nucleic acid in a "sample in-result out" method. The platform does not require professional experimental operators and a strict laboratory environment. The instrument is portable and has high detection efficiency and good repeatability, so it is especially suitable for rapid on-site screening of pathogens [84]. However, the integrated automation platform is still in the development stage, the price of instruments and consumables is high, Therefore, there is ample space for development before clinical promotion.
4. POCT platforms based on other technologies 4.1. The immunological detection platformsSARS-CoV-2 immunological detection mainly includes antigen detection and antibody detection. Antibody-based tests take blood, plasma, or serum samples for testing [85]. Antigen-based assays can detect the presence of viral antigens and diagnose an active infection. Immunoassays can help clinicians identify different stages of a patient’s viral infection and are an effective complement to SARS-CoV-2 nucleic acid detection. According to different detection techniques, immunological detection classified enzyme-linked immunosorbent assay (ELISA) [86–88], immunofluorescence chromatography (lateral flow immunoassay, LFIA) [89,90], and chemiluminescence immunoassay (CLIA) [91–93].
ELISA is always used on the antibody test, exploited specific binding of antibody and subsequent enzyme reaction, which generates a colorimetric readout. While traditional ELISAs are laboratory-based and expensive, LFIA can overcome some limitations. To date, LFIA is the most successful paper-based POCT diagnostic device depending on the natural wicking property of paper. The foundational principle of LFIA is exploiting the specific binding between antigens and antibodies. It has the advantages of fast detection, a cost-effective and straightforward analytical technique suitable for POCT diagnostics (Fig. 2). LFIA obtained results readable by the naked eye within minutes. However, they suffer from quantification limitations and solid-phase effects. In theory, the SARS-CoV-2 antigen can be detected once an individual is infected [94,95]. When the virus enters the human body, a window period during the immune response produces antibodies and immune response, and the detection pathway tends to induce "false negative" results [96]. Thus, the sensitivity of LFIAs is often limited. Table 1 presents various POCT diagnosis technologies and evaluates them based on the testing types, samples, detection targets, and limits. These methods are also summarized for sensitivity, specificity, and clinical validation.

|
Download:
|
| Fig. 2. Rapid antigen and rapid antibody tests. Analytical workflow of rapid antigen/antibody test for the detection of SARS-CoV-2 viral antigens through lateral flow immunoassay. Copied with permission [85]. Copyright 2022, Springer. | |
|
|
Table 1 Comparison of the POCT diagnosis technologies based on different platforms for the SARS-CoV-2 detection. |
Electrochemical detection can be realized using biosensors that measure electrical signals from antigen-antibody binding. It has significant advantages over other methods, such as analysis cost, quantitative detection, high sensitivity and selectivity, and the potential for portability [97]. Developing new high-performance biosensors has become a hotspot in scientific research to prevent and detect diseases by combining optical, electrochemical, mechanical, magnetic, and thermal transport modes [98,99]. Most biosensors can use antibodies, proteins, enzymes, aptamers, cells, and nucleic acids as receptor probes [100]. The rapid development of emerging technologies with the advent of lab-on-a-chip (LOC) [101], lab-on-disk (LOAD) [102], microfluidic paper-based assays (µPADs) [103], lateral flow assays (LFA) [104], micro-PCR [105], Promoted the improvement trend of in vitro diagnosis from centralized laboratory to POCT detection [106].
Chen et al. constructed the platform for anchoring N gene targeted aptamer the novel label-free method based SPR aptasensor using thiol-modified niobium carbide MXene quantum dots has been utilized to detect SARS-CoV-2 [107]. Types of electrochemical biosensors have been used for the diagnosis of viral infectious diseases, such as metal nanoparticles (NPs), graphene oxide (GO) [108], graphene [109], quantum dots (QDs) [110], and Nanoreactors [111]. They can be functionalized with antibodies/nucleic acids and suited for high-sensitivity SARS-CoV-2 analysis [112]. Samper et al. report a novel low-cost electrochemical capillary-flow device to quantify IgG antibodies targeting SARS-CoV-2 down to 5 ng/mL in low volume (10 µL) with a quantitative electrochemical readout less than 20 min [113]. Biosensor-based target analysis is considered a possible option to ease the burden of PCR-based techniques, which has proven to be a superior platform [114–116].
Currently, advances in electrochemical methods combined with wireless media for SARS-CoV-2 detection are mainly by large-scale graphene electrodes [117,118]. At the same time, a series of quantitative detection methods based on electrochemistry, fluorescence, absorbance, and surface plasmon resonance has been proposed on POCT devices for quantitative readout [119]. Singh et al. successfully detected SARS-CoV-2 virus antigen in saliva samples using a ready-made portable blood glucose meter [120].
4.2.2. Field-effect transistor (FET) biosensorsAnother biosensor that does not require sample pretreatment is the FET biosensor, with high sensitivity, high specificity, and no labeling. This device can be integrated with microelectronic systems for POCT detection [121]. The signal transduction capabilities of conventional FET biosensors depend on their channel materials; the intrinsic electronic properties and biorecognition process of channel materials determine their performance. Graphene is a potential substrate for biosensing applications due to its excellent electrical conductivity, large surface area, and good handling properties [122,123]. More and more studies have focused on detecting DNA probe-based graphene field-effect transistor (GFET) biosensors (Fig. 3). Loan et al. fabricated a GFET sensing device with probe DNA immobilized on ultrathin graphene flakes grown using chemical vapor deposition (CVD) [124]. They use a residue-free gold transfer graphene film instead of a polymethyl methacrylate (PMMA)-mediated transfer process, with a detection limit as low as 1 pmol/L obtained, which is five times higher than previous results. Kang et al. [125] realized a GFET biosensor modified near the graphene surface ultra-sensitive SARS-CoV-2 antibody detection with a LOD down to 10–16 g/mL level.

|
Download:
|
| Fig. 3. (A) Triple-probe TDF dimer GFET sensor for SARS-CoV-2 RNA testing. Copied with permission [129]. Copyright 2022, American Chemical Society. (B) A multi-antibodies transistor assay is developed for sensitive and highly precise antigen pool testing. Copied with permission [127]. Copyright 2021, American Chemical Society. (C) SARS-CoV-2 nucleic acid testing. Workflows for SARS-CoV-2 nucleic acid testing by GFETs. Copied with permission [121]. Copyright 2022, Nature. (D) Nucleic acid assay by using a graphene field-effect transistor with Y-shaped DNA dual probes (Y-dual probes) Copied with permission [122]. Copyright 2021, American Chemical Society. | |
GFET biosensors using DNA probes as identification elements can achieve high-sensitivity detection of trace substances due to their fast response speed, efficient signal transduction, label-free detection, convenient operation, and easy integration [126]. Dai et al. developed a multi-antibody transistor assay for testing sensitive and highly accurate antigen repertoire. Using multiple antibodies to capture the SARS-CoV-2 spike S1 protein with different conformations, it binds to the antigen that is as low as 0.34 fmol/L, and the detection limit in artificial saliva reaches 3.5 × 10−17 g/mL, 4–5 orders of magnitude lower than existing transistor sensors. Their work addresses a long-standing problem with antigen pool testing, making it an invaluable tool for future accurate diagnosis and universal screening [127]. Guo [128] developed a portable bifunctional electrical detector based on GFET biosensors with ultra-low limits of detection of ~0.1 and ~1 fg/mL in phosphate buffer saline, respectively, through either nucleic acid hybridization or antigen-antibody protein interaction for SARS-CoV-2. The diagnostic results exhibit rapid detection speed and high throughput within 10 min for nucleic acid detection; their assay provides excellent agreement with RT-PCR accurately.
Due to the competitive sensing performance with different antigen testing routes, Wei et al. [129] precisely developed a multi-antibody FET sensor for antigen pool testing. A portable integrated platform is demonstrated to distinguish SARS-CoV-2 positive cases among 10-in-1 pooled samples with 1 min and ~100% same with PCR results. The pool testing is valuable to identify infected individuals for diagnosing SARS-CoV-2 from the population as their lower cost per test and diagnosing capacity improved, as well as for testing other infectious viruses such as Ebola and Zika by replacing multi-antibodies. Although FET is an ideal POCT candidate for detecting of SARS-CoV-2, Most biosensors are in the laboratory stage with poor stability in the external environment, so the realization of large-scale commercialization remains a huge challenge.
4.2.3. Colorimetric biosensorsColorimetry is a straightforward and visually appreciable detection method without complex equipment [130]. Colorimetric biosensors assays based on gold nanoparticles (AuNPs) are commonly used to diagnose various human diseases that exploit the color change occurring through antigen-antibody interactions in colloidal suspensions [131–133]. In particular, AuNPs are most favored for colorimetric analysis because of their unique optical properties, ease of synthesis, simplicity, and utility of their surface functionality (Fig. 4). Several portable devices showed promising results in detecting the viral genome. The advantage of portable devices is that they can be used to detect SARS-CoV-2 both in the hospital and outside the hospital quickly. In recent studies, a smartphone app was used for quantitative colorimetric analysis of color changes on the paper surface with cytokines.

|
Download:
|
| Fig. 4. Schematic overview of the electrochemical capillary-flow immunoassay. (A) Exploded view. (B) Electrochemical immunoassay and detection mechanism. Copied with permission [113]. Copyright 2021, American Chemical Society. (C, D) Schematic diagram of the synthesis of Nb2C-SH QDs and construction of the Nb2C-SH QD-based SPR aptasensor for detecting N-gene of SARS-CoV-2. Copied with permission [107]. Copyright 2021, Springer. (E) An Overview of the proposed point-of-care, aptamer-based COVID-19 assay. Copied with permission [120]. Copyright 2020, Elsevier. | |
Pan et al. [134] developed a colorimetric bioassay based on AuNPs that diagnosed positive cases within 10 min from the isolated RNA. They adopted the biosamples modifying an all-inclusive targeting strategy by capping two regions of the SARS-CoV-2 N gene. Colorimetric biosensors applications on smartphone interfaces, microfluidic channels, and bioassay cartridges are promising solutions for further data interpretation and communication [135]. It is an easy-to-use technology that finds more POCT diagnostic platforms for COVID-19 control and future pandemics.
Nanotechnology for colorimetric biosensors realized miniaturization of test devices and improved sensitivity without special facilities and qualified personnel. Such applications can be detected and sequenced for SARS-CoV-2 mutating pathogens, reducing false-negative rates, thereby effectively collecting epidemiological data for future crucial drug and vaccine application strategies [136].
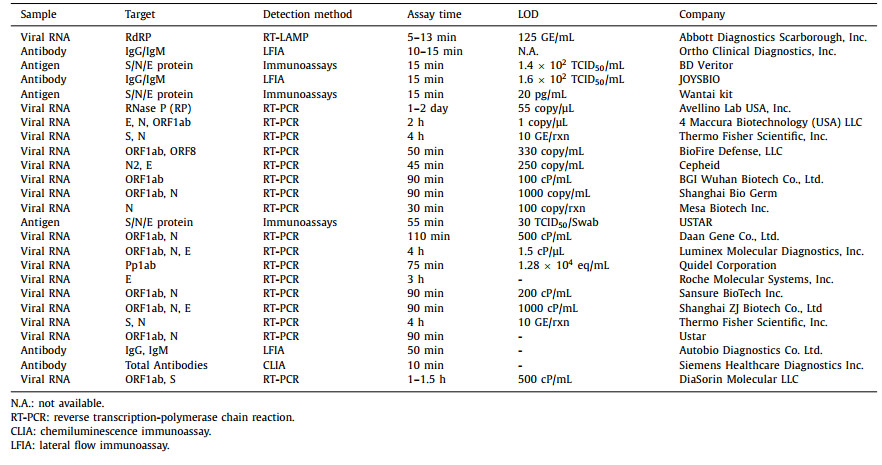
5. Commercially market-oriented SARS-CoV-2 assaysSeveral biological companies have developed commercialized nucleic acid POCT detection reagents and supporting instruments. Table 2 summarizes a series of commercial kits from different countries and regions, including antigen testing, antibody testing, and nucleic acids testing, involving target analyses, detection time, clinical specimens, sensitivity, specificity, and manufacturers. As the POCT technology rapidly developed with the SARS-CoV-2 detection, this table contents require constant updating at any time [137–145]. More or less, comparing and selecting the most suitable test from different commercial kits providing for SARS-CoV-2 detection is essential.
|
|
Table 2 Commercial point-of-care testing assays from different countries and regions. |
Currently, most of the POCT methods for SARS-CoV-2 detection are in the conceptual research stage, and there is still a distance from commercial application. These methods approved for the market needs to be used on a unique supporting instrument that realizes the integration of extraction, amplification, and detection, significantly promoting the development of POCT detection. And the POCT diagnosis kits urgently need encouraging further in-depth studies and large-scale clinical validations with an excellent commercial potential platform.
It is worth noting that an increasing number of commercial tests for POCT based on RT-PCR assays are developing. In response to the epidemic of asymptomatic infection with virus mutations, the POCT diagnostic platform is trying to move in the following directions: (1) Optimizing the currently used portable microanalysis equipment to maintain efficient detection while reducing costs. (2) Developing multiplexing multiple targets, which can detect various targets at the same time to improve detection accuracy and sensitivity. (3) Combining artificial intelligence technology with smartphone terminals enhances user-friendliness and facilitates user detection methods.
The above suggestions are listed from the customer and market demand perspective, which is a great challenge. Some significant obstacles exist in transforming from an academic research laboratory environment to commercialization. Not only are the apparent gaps at the technical level, but more research results also need the help of enterprises before they can be transformed into market-oriented competitive products. Judging from the current research, more advanced and feasible technologies or tools for epidemic prevention and control and quick-reading detection will appear in the future.
6. Conclusions and outlooksNowadays, from a life sciences perspective, SARS-CoV-2 may not be the last pandemic we will experience. Knowing how to detect viruses technologically and face the next pandemic confidently is essential, a lesson we learned from our sufferings from the contemporary COVID-19 pandemic. For future available POCT systems, a more portable, high-throughput diagnostic platform is urgently needed. The development of epidemic information technology positively affects vaccination statistics and treatment for clinical factors.
Open and transparent exchanges of disease progression prevention, control experience, and feasible technology conducive to coping with future epidemics (Fig. 5). Undoubtedly, information technology for virus detection is also critical, while establishing a global information data management platform is an obstacle. In theory, statistical databases can help build a worldwide query-able public health data portal to share social health records.

|
Download:
|
| Fig. 5. Expecting on development the epidemic information technology for precise public health surveillance. | |
That means a POCT diagnostic platform with multiple detection methods complementing each other can cover different stages of the virus on a large scale, such as screening of suspected cases, initial infection, disease treatment, and later stages of cure. However, from the perspective of researchers and the direction of POCT diagnostic technology, there are some thorny scientific challenges in current testing technology research. For example, a general approach to receptors is lacking in meeting the need to identify a broad range of SARS-CoV-2 variants, and current receptor approaches vary. At the same time, the improvement of sensitivity in overcoming the problem of false-negative detection is indispensable. For the optimization of sampling and the visual reading of detection results, there is much room for improvement and optimization, making it more convenient for people to monitor the epidemic prevention and control situation on a large scale.
Current POCT detection research has the advantages of simple operation, short detection time, and no need for a professional laboratory. It makes up for traditional detection technology’s shortcomings that rely on professional equipment and long detection cycles. Although significant progress has been made in biosensors and nucleic acid amplification technologies, the need for epidemic prevention and control has driven intense research and development. The future ideal POCT product needs to have the characteristics of portable, high-precision, and high-throughput detection; it needs to be miniaturized, automated, and visualized. This review summarized recent POCT platforms to provide a reference for epidemic prevention and clinical management.
Declaration of competing interestThe authors of the manuscript entitled "Recent progress on rapid diagnosis of COVID-19 by point-of-care testing platforms" declare that the authors have no competing interests.
AcknowledgmentsThis work was supported by the National Key R&D Program of China (No. 2021YFC2301100), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB30000000), the National Natural Science Foundation of China (No. 61890940), the Chongqing Bayu Scholar Program (No. DP2020036), Program of Shanghai Academic Research Leaders (No. 23XD1420200) and Fudan University.
| [1] |
H. Chen, J. Guo, C. Wang, et al., Lancet 395 (2020) 809-815. DOI:10.1016/S0140-6736(20)30360-3 |
| [2] |
W.J. Guan, Z.Y. Ni, Y. Hu, et al., N. Engl. J. Med. 382 (2020) 1708-1720. DOI:10.1056/NEJMoa2002032 |
| [3] |
P. Elliott, O. Eales, B. Bodinier, et al., Nat. Commun. 13 (2022) 4500. DOI:10.1038/s41467-022-32121-6 |
| [4] |
M. Yuce, E. Filiztekin, K.G. Ozkaya, Biosens. Bioelectron. 172 (2020) 112752. |
| [5] |
R. Weissleder, H. Lee, J. Ko, M.J. Pittet, Sci. Transl. Med. 12 (2020) 546. |
| [6] |
R. Li, S. Pei, B. Chen, et al., Science 368 (2020) 489-493. DOI:10.1126/science.abb3221 |
| [7] |
M.J. Mina, K.G. Andersen, Science 371 (2021) 126-127. DOI:10.1126/science.abe9187 |
| [8] |
X. Yuan, C. Yang, Q. He, et al., ACS Infect. Dis. 6 (2020) 1998-2016. DOI:10.1021/acsinfecdis.0c00365 |
| [9] |
C.Y. Lee, I. Degani, J. Cheong, et al., Biosens. Bioelectron. 178 (2021) 113049. DOI:10.1016/j.bios.2021.113049 |
| [10] |
H. Tegally, E. Wilkinson, M. Giovanetti, et al., Nature 592 (2021) 438-443. DOI:10.1038/s41586-021-03402-9 |
| [11] |
M. Azhar, R. Phutela, M. Kumar, et al., Biosens. Bioelectron. 183 (2021) 113207. DOI:10.1016/j.bios.2021.113207 |
| [12] |
G. Xun, S.T. Lane, V.A. Petrov, B.E. Pepa, H. Zhao, Nat. Commun. 12 (2021) 2905. DOI:10.1038/s41467-021-23185-x |
| [13] |
L. Dortet, C. Emeraud, C. Vauloup-Fellous, et al., Emerg. Microbes Infect. 9 (2020) 2212-2221. DOI:10.1080/22221751.2020.1826892 |
| [14] |
V.M. Corman, O. Landt, M. Kaiser, et al., Eurosurveillance 25 (2020) 3. |
| [15] |
I. Smyrlaki, M. Ekman, A. Lentini, et al., Nat. Commun. 11 (2020) 4812. DOI:10.1038/s41467-020-18611-5 |
| [16] |
S. Pfefferle, S. Reucher, D. Nrz, et al., Eurosurveillance 25 (2020) 9. DOI:10.2807/1560-7917.ES.2020.25.9.2000152 |
| [17] |
R. Lu, X. Zhao, J. Li, et al., Lancet 395 (2020) 565-574. DOI:10.1016/S0140-6736(20)30251-8 |
| [18] |
J.F. Chan, S. Yuan, K.H. Kok, et al., Lancet 395 (2020) 514-523. DOI:10.1016/S0140-6736(20)30154-9 |
| [19] |
J. Yu, N. Ding, H. Chen, et al., Acad. Radiol. 27 (2020) 614-617. DOI:10.1016/j.acra.2020.03.025 |
| [20] |
C. Wang, P.W. Horby, F.G. Hayden, G.F. Gao, Lancet 395 (2020) 470-473. DOI:10.1016/S0140-6736(20)30185-9 |
| [21] |
Y.R. Guo, Q.D. Cao, Z.S. Hong, et al., Mil. Med. Res. 7 (2020) 11. |
| [22] |
S. McDonald, D.M. Courtney, A.E. Clark, et al., Acad. Emerg. Med. 27 (2020) 764-766. DOI:10.1111/acem.14039 |
| [23] |
S. Young, S.N. Taylor, C.L. Cammarata, et al., J. Clin. Microbiol. 59 (2020) e02338. |
| [24] |
P. Zhou, X.L. Yang, X.G. Wang, et al., Nature 579 (2020) 270-273. DOI:10.1038/s41586-020-2012-7 |
| [25] |
V. Redecke, K. Tawaratsumida, E.T. Larragoite, et al., Sci. Rep. 11 (2021) 24507. DOI:10.1038/s41598-021-04298-1 |
| [26] |
S. Roger, C. Lefeuvre, A. Pivert, et al., J. Med. Virol. 94 (2022) 1723-1727. DOI:10.1002/jmv.27505 |
| [27] |
Y. Pan, D. Zhang, P. Yang, et al., Lancet Infect. Dis. 20 (2020) 411-412. DOI:10.1016/S1473-3099(20)30113-4 |
| [28] |
A.V. Ritchie, N. Goel, C. Tie, et al., J. Clin. Microbiol. 59 (2020) e01262. |
| [29] |
M. Pieri, E. Nicolai, M. Nuccetelli, et al., Arch. Virol. 167 (2022) 1285-1291. DOI:10.1007/s00705-022-05422-w |
| [30] |
P.C. Fragkou, V. Papaevangelou, A. Antoniadou, et al., In Vivo 34 (2020) 3039-3045. DOI:10.21873/invivo.12138 |
| [31] |
M.M. Gibani, C. Toumazou, M. Sohbati, et al., Lancet Microbe 1 (2020) e300-e307. DOI:10.1016/S2666-5247(20)30121-X |
| [32] |
Y. Huang, S. Chen, Z. Yang, et al., Am. J. Respir. Crit. Care. Med. 201 (2020) 1435-1438. DOI:10.1164/rccm.202003-0572LE |
| [33] |
G. Bianco, M. Boattini, A.M. Barbui, et al., J. Clin. Virol. 139 (2021) 104838. DOI:10.1016/j.jcv.2021.104838 |
| [34] |
M. Zandi, M. Fani, Biosens. Bioelectron. 200 (2022) 113924. DOI:10.1016/j.bios.2021.113924 |
| [35] |
L. Miscio, A. Olivieri, F. Labonia, et al., J. Transl. Med. 18 (2020) 488. DOI:10.1186/s12967-020-02651-y |
| [36] |
Y. Kiyasu, M. Owaku, Y. Akashi, et al., J. Infect. Chemother. 28 (2022) 543-547. DOI:10.1016/j.jiac.2021.12.027 |
| [37] |
Q. Ye, D. Lu, T. Zhang, J. Mao, S. Shang, J. Med. Virol. 94 (2022) 1866-1875. DOI:10.1002/jmv.27617 |
| [38] |
N. Odiwuor, J. Xiong, F. Ogolla, et al., Anal. Chim. Acta 1200 (2022) 339590. DOI:10.1016/j.aca.2022.339590 |
| [39] |
M. Ishikane, H. Unoki-Kubota, A. Moriya, et al., J. Infect. Chemother. 28 (2022) 729-734. DOI:10.1016/j.jiac.2022.02.004 |
| [40] |
L. Dong, J. Zhou, C. Niu, et al., Talanta 224 (2021) 121726. DOI:10.1016/j.talanta.2020.121726 |
| [41] |
I. Torres, J. Qualai, E. Albert, et al., J. Med. Virol. 93 (2021) 5233-5235. DOI:10.1002/jmv.27039 |
| [42] |
H.Q. Nguyen, H.K. Bui, V.M. Phan, et al., Biosens. Bioelectron. 195 (2021) 113655. |
| [43] |
W.E. Huang, B. Lim, C.C. Hsu, Microb. Biotechnol. 13 (2020) 950-961. DOI:10.1111/1751-7915.13586 |
| [44] |
V.L. Dao Thi, K. Herbst, K. Boerner, et al., Sci. Transl. Med. 12 (2020) eabc7075. DOI:10.1126/scitranslmed.abc7075 |
| [45] |
R. Lu, X. Wu, Z. Wan, Y. Li, et al., Int. J. Mol. Sci. 21 (2020) 2826. DOI:10.3390/ijms21082826 |
| [46] |
E. González-González, I.M. Lara-Mayorga, I.P. Rodríguez-Sánchez, et al., Anal. Methods 13 (2021) 169-178. DOI:10.1039/D0AY01658F |
| [47] |
J.A. Plante, Y. Liu, J. Liu, et al., Nature 592 (2021) 116-121. DOI:10.1038/s41586-020-2895-3 |
| [48] |
A.A. El Wahed, P. Patel, M. Maier, et al., Anal. Chem. 93 (2021) 2627-2634. DOI:10.1021/acs.analchem.0c04779 |
| [49] |
M. El-Tholoth, H.H. Bau, J. Song, Anal. Chem. 38 (2021) 13063-13071. |
| [50] |
O. Behrmann, I. Bachmann, M. Spiegel, et al., Clin. Chem. 66 (2020) 1047-1054. DOI:10.1093/clinchem/hvaa116 |
| [51] |
Z.A. Crannell, B. Rohrman, R.R. Kortum, et al., Anal. Chem. 86 (2014) 5615-5619. DOI:10.1021/ac5011298 |
| [52] |
A.A.E. Wahed, P. Patel, D. Heidenreich, et al., PLoS Curr. 5 (2013) 24459611. |
| [53] |
L.A. Heger, N. Elsen, M. Rieder, et al., BMC Infect. Dis. 22 (2022) 486. DOI:10.1186/s12879-022-07447-7 |
| [54] |
J. Yin, Z. Zou, Z. Hu, et al., Lab Chip 20 (2020) 979-986. DOI:10.1039/C9LC01143A |
| [55] |
T. Chaibun, J. Puenpa, T. Ngamdee, et al., Nat. Commun. 12 (2021) 802. DOI:10.1038/s41467-021-21121-7 |
| [56] |
X. Xu, L. Wang, D. Zhu, Y. Wang, W. Jiang, Talanta 186 (2018) 293-298. DOI:10.1016/j.talanta.2018.04.047 |
| [57] |
A. Mane, S. Jain, A. Jain, et al., Sci. Rep. 12 (2022) 7355. DOI:10.1038/s41598-022-11284-8 |
| [58] |
R.T. Aruleba, T.A. Adekiya, N. Ayawei, et al., Bioeng.-Basel 9 (2022) 4. |
| [59] |
J.V. Ness, L.K.V. Ness, D.J. Galas, et al., Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 4504-4509. DOI:10.1073/pnas.0730811100 |
| [60] |
J. Van, C. Gerlier, B. Pilmis, et al., J. Clin. Virol. 145 (2021) 105021. DOI:10.1016/j.jcv.2021.105021 |
| [61] |
M. Graham, S. Muhi, T. Hoang, et al., Pathology 53 (2021) 912-914. |
| [62] |
K. Pardee, A.A. Green, M.K. Takahashi, et al., Cell 165 (2016) 1255-1266. DOI:10.1016/j.cell.2016.04.059 |
| [63] |
E. Xiong, L. Jiang, T. Tian, et al., Angew. Chem. Int. Ed. Engl. 60 (2021) 5307-5315. DOI:10.1002/anie.202014506 |
| [64] |
J.H. Tsou, H. Liu, S.A. Stass, et al., Biomedicines 9 (2021) 239. DOI:10.3390/biomedicines9030239 |
| [65] |
J.S. Gootenberg, O.O. Abudayyeh, J.W. Lee, et al., Science 356 (2017) 438-442. DOI:10.1126/science.aam9321 |
| [66] |
M. Patchsung, K. Jantarug, A. Pattama, et al., Nat. Biomed. Eng. 4 (2020) 1140-1149. DOI:10.1038/s41551-020-00603-x |
| [67] |
O.O. Abudayyeh, J.S. Gootenberg, M.J. Kellner, et al., CRISPR J. 2 (2019) 165-171. DOI:10.1089/crispr.2019.0011 |
| [68] |
H.M. Yoo, S. Kim, et al., Int. J. Mol. Sci. 22 (2021) 6150. DOI:10.3390/ijms22116150 |
| [69] |
H. de Puig, R.A. Lee, D. Najjar, et al., Sci. Adv. 7 (2021) eabh2944. DOI:10.1126/sciadv.abh2944 |
| [70] |
C. Myhrvold, C.A. Freije, J.S. Gootenberg, et al., Science 360 (2018) 444-448. DOI:10.1126/science.aas8836 |
| [71] |
M.N. Esbin, O.N. Whitney, S. Chong, et al., RNA 26 (2020) 771-783. DOI:10.1261/rna.076232.120 |
| [72] |
T. Hou, W. Zeng, M. Yang, et al., PLoS Pathog. 16 (2020) e1008705. DOI:10.1371/journal.ppat.1008705 |
| [73] |
J Joung, A Ladha, M Saito, et al., N. Engl. J. Med. 15 (2020) 1492-1494. DOI:10.1056/nejmc2026172 |
| [74] |
J.S. Chen, E. Ma, L.B. Harrington, et al., Science 360 (2018) 436-439. DOI:10.1126/science.aar6245 |
| [75] |
Z. Huang, D. Tian, Y. Liu, et al., Biosens. Bioelectron. 164 (2020) 112316. DOI:10.1016/j.bios.2020.112316 |
| [76] |
X. Ding, K. Yin, Z. Li, et al., Nat. Commun. 11 (2020) 4711. DOI:10.1038/s41467-020-18575-6 |
| [77] |
J.P. Broughton, X. Deng, G. Yu, et al., Nat. Biotechnol. 38 (2020) 870-874. DOI:10.1038/s41587-020-0513-4 |
| [78] |
X. Zhu, X. Wang, S. Li, et al., ACS Sens. 6 (2021) 881-888. DOI:10.1021/acssensors.0c01984 |
| [79] |
L.B. Harrington, D. Burstein, J.S. Chen, et al., Science 362 (2018) 839-842. DOI:10.1126/science.aav4294 |
| [80] |
L. Guo, X.H. Sun, X. Wang, et al., Cell Discov. 6 (2020) 34. |
| [81] |
A. Pickar-Oliver, C.A. Gersbach, Nat. Rev. Mol. Cell Biol. 20 (2019) 490-507. DOI:10.1038/s41580-019-0131-5 |
| [82] |
P. Fozouni, S. Son, M. Derby, et al., Cell 184 (2021) 323-333. DOI:10.1016/j.cell.2020.12.001 |
| [83] |
M. Margulis, O. Erster, S. Roth, M. Mandelboim, A. Danielli, J. Mol. Diagn. 23 (2021) 1680-1690. DOI:10.1016/j.jmoldx.2021.08.012 |
| [84] |
R. Paul, E. Ostermann, Q. Wei, Biosens. Bioelectron. 169 (2020) 112592. DOI:10.1016/j.bios.2020.112592 |
| [85] |
L. Falzone, G. Gattuso, A. Tsatsakis, D.A. Spandidos, M. Libra, Int. J. Mol. Med. 6 (2021) 100. |
| [86] |
S. Kasetsirikul, M. Umer, N.T. Nguyen, et al., Analyst 145 (2020) 7680-7686. DOI:10.1039/D0AN01609H |
| [87] |
F. Ludolf, F.F. Ramos, F.F. Bagno, Sci. Adv. 8 (2022) eabn7424. DOI:10.1126/sciadv.abn7424 |
| [88] |
F. Amanat, D. Stadlbauer, S. Strohmeier, et al., Nat. Med. 26 (2020) 1033-1036. DOI:10.1038/s41591-020-0913-5 |
| [89] |
Q. Bayin, L. Huang, C. Ren, et al., Talanta 227 (2021) 122207. DOI:10.1016/j.talanta.2021.122207 |
| [90] |
B.D. Grant, C.E. Anderson, J.R. Williford, et al., Anal. Chem. 92 (2020) 11305-11309. DOI:10.1021/acs.analchem.0c01975 |
| [91] |
D. Duan, K. Fan, D. Zhang, et al., Biosens. Bioelectron. 74 (2015) 134-141. DOI:10.1016/j.bios.2015.05.025 |
| [92] |
D. Liu, C. Ju, C. Han, et al., Biosens. Bioelectron. 173 (2020) 112817. |
| [93] |
S. Zhang, B. Wang, Y. Mu, et al., Lab Chip 20 (2020) 979-986. DOI:10.1039/C9LC01143A |
| [94] |
S. Yu, S.B. Nimse, J. Kim, K.S. Song, T. Kim, Anal. Chem. 92 (2020) 14139-14144. DOI:10.1021/acs.analchem.0c03202 |
| [95] |
S. Kim, Y. Hao, E.A. Miller, et al., ACS Sens. 6 (2021) 1891-1898. DOI:10.1021/acssensors.1c00235 |
| [96] |
L.S. Nguyen, D. Laghlam, E.D. Gonfreville, et al., Crit. Care 24 (2020) 573. DOI:10.1186/s13054-020-03290-x |
| [97] |
Y.L. Wu, C. Li, S. Xia, et al., Cell Host Microbe 27 (2020) 891-898. DOI:10.1016/j.chom.2020.04.023 |
| [98] |
Q. Wang, J. Wang, Y. Huang, et al., Biosens. Bioelectron. 197 (2022) 113739. DOI:10.1016/j.bios.2021.113739 |
| [99] |
Y.F. Dai, C.C. Liu, Angew. Chem. Int. Ed. 58 (2019) 12355-12368. DOI:10.1002/anie.201901879 |
| [100] |
X.L. Liu, Y.P. Wang, Y.F. Gao, Y.J. Song, Analyst 146 (2021) 1115-1126. DOI:10.1039/D0AN02154G |
| [101] |
J. Zhuang, J. Yin, S. Lv, et al., Biosens. Bioelectron. 163 (2019) 112291. |
| [102] |
B. Hoseinpour, H. Ashorynejad, A. Sarreshtehdari, et al., J. Mol. Liq. 325 (2020) 114961. |
| [103] |
M. Tao, X. Yu, S. Shuai, et al., Biosens. Bioelectron. 170 (2020) 112649. DOI:10.1016/j.bios.2020.112649 |
| [104] |
S.E. Conklin, K. Martin, Y.C. Manabe, et al., J. Clin. Microbiol. 59 (2020) e02020. |
| [105] |
H. Yin, Z. Tong, C. Shen, et al., Lab Chip 22 (2022) 2671-2681. DOI:10.1039/D2LC00101B |
| [106] |
C. Zhang, T. Belwal, Z. Luo, B. Su, X. Lin, Small 18 (2022) e2102711. DOI:10.1002/smll.202102711 |
| [107] |
R.Y. Chen, L. Kan, F. Duan, et al., Mikrochim. Acta 188 (2021) 316. DOI:10.1007/s00604-021-04974-z |
| [108] |
M.A. Unal, F. Bayrakdar, H. Nazir, et al., Small 17 (2021) 25. DOI:10.5604/01.3001.0014.6077 |
| [109] |
F. De Maio, V. Palmieri, G. Babini, et al., iScience 24 (2021) 102788. DOI:10.1016/j.isci.2021.102788 |
| [110] |
N. Rabiee, S. Ahmadi, G.J. Soufi, et al., J. Chem. Technol. Biotechnol. 97 (2022) 7. DOI:10.1002/jctb.6961 |
| [111] |
F. Zhang, Y.C. Pan, X.W. Luan, et al., Sens. Actuator B-Chem. 345 (2021) 130382. DOI:10.1016/j.snb.2021.130382 |
| [112] |
S.A. Hashemi, S. Bahrani, S.M. Mousavi, et al., Talanta 239 (2022) 123113. DOI:10.1016/j.talanta.2021.123113 |
| [113] |
I.C. Samper, A. Sanchez-Cano, W. Khamcharoen, et al., ACS Sens. 6 (2021) 4067-4075. DOI:10.1021/acssensors.1c01527 |
| [114] |
S.M. Imani, L. Ladouceur, T. Marshall, et al., ACS Nano 14 (2020) 12341-12369. DOI:10.1021/acsnano.0c05937 |
| [115] |
A. Bisht, A. Mishra, H. Bisht, R.M. Tripathi, J. Anal. Test. 5 (2021) 327-340. DOI:10.1007/s41664-021-00200-0 |
| [116] |
Y.H. Wang, L.L. He, K.J. Huang, et al., Analyst 144 (2019) 2849-2866. DOI:10.1039/C9AN00081J |
| [117] |
J. Li, D. Wu, Y. Yu, et al., Biosens. Bioelectron. 183 (2021) 113206. DOI:10.1016/j.bios.2021.113206 |
| [118] |
M. Guerreiro, D. Freitas, P. Alves, et al., ACS Sens. 4 (2019) 1654-1661. DOI:10.1021/acssensors.9b00489 |
| [119] |
J. Shi, C. Chan, Y. Pang, et al., Biosens. Bioelectron. 67 (2015) 595-600. DOI:10.1016/j.bios.2014.09.059 |
| [120] |
N.K. Singh, P. Ray, A.F. Carlin, et al., Biosens. Bioelectron. 180 (2021) 113111. DOI:10.1016/j.bios.2021.113111 |
| [121] |
L.Q. Wang, X.J. Wang, Y.G. Wu, et al., Nat. Biomed. Eng. 6 (2022) 276-285. DOI:10.1038/s41551-021-00833-7 |
| [122] |
D.R. Kong, X.J. Wang, C.J. Gu, et al., J. Am. Chem. Soc. 143 (2021) 17004-17014. DOI:10.1021/jacs.1c06325 |
| [123] |
Z. Wang, K.Y. Yi, Q.Y. Lin, et al., Nat. Commun. 10 (2019) 1544. DOI:10.1038/s41467-019-09573-4 |
| [124] |
P.T.K. Loan, C. Ye, D. Wu, et al., Biosens. Bioelectron. 99 (2018) 85-91. DOI:10.1016/j.bios.2017.07.045 |
| [125] |
H. Kang, X.J. Wang, M. Guo, et al., Nano Lett. 21 (2021) 7897-7904. DOI:10.1021/acs.nanolett.1c00837 |
| [126] |
R.A. Picca, K. Manoli, E. Macchia, et al., Adv. Funct. Mater. 30 (2020) 20. |
| [127] |
C.H. Dai, Y.G. Wu, B.P. Cao, et al., J. Am. Chem. Soc. 143 (2021) 19794-19801. DOI:10.1021/jacs.1c08598 |
| [128] |
G. Ke, D. Su, Y. Li, et al., Sci. China-Mater. 64 (2021) 739-747. DOI:10.1007/s40843-020-1577-y |
| [129] |
Y.G. Wu, D.Z. Ji, C.H. Dai, et al., Nano Lett. 22 (2022) 3307-3316. DOI:10.1021/acs.nanolett.2c00415 |
| [130] |
F. Qu, Z. Chen, J. You, et al., Spectroscopy 196 (2018) 148-154. |
| [131] |
Y. Bernard, T.J. Andrew, N.L. Jennifer, et al., Nature 406 (2000) 605-608. DOI:10.1038/35020524 |
| [132] |
S. Alhogail, G. Suaifan, F.J. Bikker, et al., ACS Omega 4 (2019) 21684-21688. DOI:10.1021/acsomega.9b02080 |
| [133] |
Y.T. Buyuksunetci, B.E. Citil, U. Tapan, U. Anik, Mikrochim. Acta 188 (2021) 335. |
| [134] |
P. Moitra, M. Alafeef, K. Dighe, M.B. Frieman, D. Pan, ACS Nano 14 (2020) 7617-7627. DOI:10.1021/acsnano.0c03822 |
| [135] |
B.D. Ventura, M. Cennamo, A. Minopoli, et al., ACS Sens. 5 (2020) 3043-3048. DOI:10.1021/acssensors.0c01742 |
| [136] |
Y.K. Gao, Y.K. Han, C. Wang, L. Qiang, L.J.A.C.A. Han, Anal. Chim. Acta 1154 (2021) 338330. DOI:10.1016/j.aca.2021.338330 |
| [137] |
S. Singh, J. Lab. Phys. 12 (2020) 1-2. |
| [138] |
J. Rodel, R. Egerer, A. Suleyman, et al., J. Clin. Virol. 132 (2020) 104616. DOI:10.1016/j.jcv.2020.104616 |
| [139] |
K.R. Peck, Clin. Microbiol. Infect. 26 (2020) 805-807. DOI:10.1016/j.cmi.2020.04.025 |
| [140] |
T. Nicol, C. Lefeuvre, O. Serri, et al., J. Clin. Virol. 129 (2020) 104511. DOI:10.1016/j.jcv.2020.104511 |
| [141] |
D. Ndwandwe, L. Mathebula, R. Kamadjeu, C.S. Wiysonge, Pan Afr. Med. J. 37 (2020) 10. |
| [142] |
X. Mei, H.C. Lee, K.Y. Diao, et al., Nat. Med. 26 (2020) 1224-1228. DOI:10.1038/s41591-020-0931-3 |
| [143] |
J. Abbasi, J. Am. Med. Assoc. 324 (2020) 1386. |
| [144] |
M. Lv, M. Wang, N. Yang, et al., Ann. Transl. Med. 8 (2020) 622. DOI:10.21037/atm-20-3311 |
| [145] |
S. Madhurantakam, S. Muthukumar, S. Prasad, ACS Omega 7 (2022) 12467-12473. DOI:10.1021/acsomega.2c00638 |
 2024, Vol. 35
2024, Vol. 35