b College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou 311121, China;
c Shanghai Key Laboratory of Functional Materials Chemistry, Key Laboratory for Advanced Materials and Institute of Fine Chemicals, School of Chemistry & Molecular Engineering, East China University of Science & Technology, Shanghai 200237, China
In recent years, fluorescent probes have gained significant prominence in the field of substance analysis and detection [1-4]. The development of high-performance volatile amine gas sensors is crucial for applications in food quality control, disease monitoring, and environmental protection [2]. Traditional methods for amine vapor detection mainly involve complex procedures and expensive instrumentation, while fluorescent probes offer a simple and rapid method for real-time monitoring of amines. Organic thin-film fluorescent probes (OTFFPs) are expected to emerge as a new and efficient method for detecting volatile organic amines (VOAs) with the advantages of rapid response, high sensitivity, minimal contamination to the analyte, and ease of preparation for portable instruments.
Organic amines can be classified into various categories, such as primary amines, secondary amines, and tertiary amines. These different types of amines exhibit subtle differences in their properties, making them difficult to distinguish. Because of the different diffusion modes presented in the solution and film phases, many conventional fluorescent sensing mechanisms that are effective in solutions cannot be directly applied in the solid-state analysis by OTFFPs. Hence, with the purpose of designing film probes, new signaling mechanisms like photoinduced electron transfer, chemical reactions, and organometallic complexes have been developed in recent years.
In this respect, Cheng's group from the Chinese Academy of Sciences has developed a series of OTFFPs for the detection of VOAs. These probes are based on different signaling mechanisms, allowing them to discriminate different amines and thus greatly enrich the family of volatile amine sensors [3,4]. In a recent study, Cheng, Fu, and co-workers designed and synthesized a novel fluorescent probe (IDT-CN) utilizing an indacenodithiophenyl moiety. The results demonstrated that the films prepared using this probe exhibited rapid and distinct fluorescence quenching when exposed to saturated vapors of primary, secondary, and tertiary amines, showing high sensitivity, selectivity, and no pollution from the analyte, making it suitable for amine vapor sensing [5].
The design of IDT-CN incorporated the indacenodithiophene moiety, which is well known for its robust fluorescence emission and tunable properties. By introducing long alkyl chains and an alkenyl malonitrile unit, the researchers successfully overcame the aggregation-induced quenching effect and improved the luminous performance of the IDT-CN film (Fig. 1a). The probe exhibited strong fluorescence emission in the film state, making it suitable for gas-sensing applications.
|
Download:
|
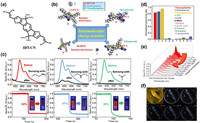
| Fig. 1. (a) Synthesis procedure of IDT-CN. (b) Dynamic simulation of molecular interaction between IDT-CN and diethylamine/trimethylamine. (c) Fluorescence changes and the corresponding quenching rates of IDT-CN films upon exposure to saturated vapors of n-propylamine, diethylamine, and trimethylamine, respectively. (d) Fluorescence quenching rates of IDT-CN films upon exposure to saturated vapors of n-propylamine, diethylamine, trimethylamine, and other interferents, respectively. (e) Fluorescence intensity gradient changes of IDT-CN films after sensing different concentrations of trimethylamine vapors. (f) Photographs of paper-based IDT-CN probe placed with fish during 12 h under irradiation of 365 nm UV light. | |
The sensing mechanism of the IDT-CN probe varied depending on the specific type of amines being detected. For primary amines, a specific chemical reaction occurred between IDT-CN and the amine, leading to a rapid fluorescence quenching. Thus, the IDT-CN film exhibited a quenching rate of 84% within 10 s upon exposure to n-propylamine. In contrast, secondary and tertiary amines resulted in a blue shift of the absorption and a fluorescence quenching via an intramolecular charge transfer process (Fig. 1b). Thus, the films showed a fluorescence quenching rate of 87% within 10 s and 96% within 30 s upon exposure to diethylamine and trimethylamine, respectively. The distinct sensing mechanisms facilitated the selective and sensitive detection of different types of amines (Fig. 1c).
The selectivity of a probe is crucial for accurate and reliable sensing in complex environments. In the case of the IDT-CN film, its selectivity was rigorously evaluated against various laboratory interferents, including water, organic acids, alcohols, tetrahydrofuran, dichloromethane, toluene, acetone, and acetonitrile. The results demonstrated the film's pronounced selectivity towards organic amine vapors. Notably, the film exhibited negligible responses to other tested interferents, highlighting its specificity for amine detection (Fig. 1d).
As we know, trimethylamine (TMA) is an important indicator of food spoilage. Notably, IDT-CN exhibited an impressive detection limit of 4.610 ppt (Fig. 1e). This remarkable level of sensitivity holds great significance in assessing the freshness of various food items, particularly fish and meat. Thus, the capability of the IDT-CN film for detecting TMA was successfully validated through on-site testing using a small yellow croaker, demonstrating its potential for food freshness detection (Fig. 1f).
In conclusion, the research presented a practical and efficient OTFFP for the detection of VOAs. In addition to its rapid response time and high selectivity, the IDT-CN film exhibits excellent optical properties, making it a valuable tool for food freshness monitoring. The study contributes to the advancement of sensor technology and offers a cost-effective and convenient method for highly sensitive amine vapor detection.
| [1] |
S. Li, F.J. Huo, Y.K. Yue, et al., Chin. Chem. Lett. 32 (2021) 3870-3875. DOI:10.1016/j.cclet.2021.05.026 |
| [2] |
Y.Y. Fu, W. Xu, Q.G. He, et al., Sci. China Chem. 59 (2016) 3-15. DOI:10.1007/s11426-015-5498-3 |
| [3] |
J.M. Chena, H.F. Wu, Y.Y. Fu, et al., Talanta 199 (2019) 698-704. DOI:10.1016/j.talanta.2019.03.009 |
| [4] |
K.K. Li, L.X. Wang, J.M. Chen, et al., Sens. Actuators B: Chem. 334 (2021) 129629. DOI:10.1016/j.snb.2021.129629 |
| [5] |
J.H. Zhao, W.X. Xu, B. Li, et al., Chin. Chem. Lett. (2023) 108579. DOI:10.1016/j.cclet.2023.108579 |
 2024, Vol. 35
2024, Vol. 35 

