Compartmentalization in biological systems can facilitate subcellular process by concentrating different components to confined spaces where many complicated biological behaviours occur. Inspired by nature, chemists have been constantly searching for confined spaces that mimic natural binding sites. So far, a plethora of different hosts [1] have been successfully constructed and extensively applied in various fields such as catalysis [2], signalling [3], sensing [4] and separations [5]. Cavitands, which were coined by Nobel Laureate Donald J. Cram as ‘enforced cavities large enough to accommodate simple molecules or ions’ have grown expansively over the past few decades [6,7]. By definition, cavitands can refer to any hosts with enforced cavities, such as crown ether, cyclodextrin, cucurbituril and calixarene. Nevertheless, this term has gradually evolved to refer resorcinarene (RA) scaffold-based hosts over the last three decades. Occasionally, it can be seen in literatures discussing aryl-extended calixpyrrole receptor [8,9], gated molecular basket [10,11] or some other hosts with a specific name [12]. Here, within this context, the term cavitand is limited to refer as RA-based hosts. Owing to the bowl-like conformation, there is only one open end within cavitand, whereas other hosts like pillararenes or cucurbiturils have two ends that allow guest molecules to bind and egress, which give cavitand unique properties.
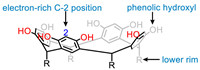
The definitive structure of RA was first reported by Erdtman and co-workers in 1968 [13], although its initial investigation can date back to 1872 [14]. RAs are generally synthesized by acid-catalyzed condensation of aldehyde with resorcinol or pyrogallol. Under alkaline conditions, the reaction of formaldehyde and resorcinol will give RAs with no alkyl group at the C-junctions [15]. The clockwise or counterclockwise interannular hydrogen bonding between the phenolic hydroxyl groups makes RA a bowl-like conformation, in which there is a shallow cavity that can accommodate some small entities [16]. Although the original RA molecules have limited applications, it is noteworthy that the active sites within RA can be easily modified which significantly expanded their applications in various fields (Scheme 1). First, the phenolic pairs on adjacent rings of RA cores can be linked by alkylene [17], alkylsilicon [18], phosphoryl [19], phenylene [20] and so on. The approach of bridging inhibits the flipping of benzene rings, endowing the RA with rigidified cavities. Second, the electron-rich C-2 position of RA can be functionalized by electrophilic aromatic substitution reaction. Third, the lower rims can also be connected via feet-to-feet to create a bis-RA or to decorate with specific groups to make RA soluble in different solvent [21]. These versatile functionalization approaches significantly enriched the application of RA-based cavitands.
|
Download:
|
| Scheme 1. Active sites that can be modified in RA: Phenolic hydroxyl, electron-rich C-2 position of aromatic unit and lower rim. | |
In this review, we expect to give a concise overview over the structural diversities realized in RA-based cavitands to date. We will introduce and discuss the different type of cavitands realized in this field. Different strategies for synthesizing diverse cavitands, such as small cavity cavitands, wider cavity cavitands, deep cavity cavitands, biscavitands, asymmetric cavitands, are discussed in details. The examples given in this review will be limited to the last five years or so, readers who are interested in those previous seminal works are referred to aforementioned reviews [22,23]. To the best of our knowledge, there has never been a review of cavitand that is discussed in this classification. Furthermore, we hope that this review can provide a systematic and brief summary to researchers who will study RA-based cavitand in the future.
2. Small cavity cavitandsAs previously described, RA itself has a shallow cavity, but it can be further functionalized by linking adjacent phenols. One of the most commonly employed methods is to bridge with CH2BrCl in functionalizing R[4]As, leading to a relatively small cavitand. Besides, ethylene, silyl, phosphoryl could also act as linkages to generate cavitands with a small cavity [24], in which unique and interesting binding behaviours are observed [25].
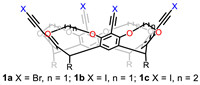
Rissanen and co-workers built a series of small tetrahaloethynyl cavitands (1a–c, Fig. 1) by connecting two adjacent phenolic hydroxyl groups with methylene and ethylene [26-28]. These cavitands feature four bromo- and four iodoethynyl groups on the upper rims of RA, which show strong halogen bonding with oxygen, nitrogen and bromide acceptors. Methylene bridged 1a and 1b formed self-included dimers, while more flexible ethylene bridged 1c had a much weaker tendency dimerize in the solid state. Not only these cavitands function as scaffolds for generating dimeric capsules, but also complicated one-, two-, and three-dimensional frameworks were observed by co-crystallization with different acceptors and solvents in the solid phase. These various higher-order networks, assembled through host-host, host-acceptor or host-solvent, are highly dependent upon the particular nature of acceptor and solvent and present the emergent complexity of these RA-based complexes [29,30].
|
Download:
|
| Fig. 1. Chemical structures of tetrahaloethynyl cavitands 1a-c. | |
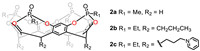
Tetraphosphonate cavitands are powerful synthetic receptors and widely used in specific molecular recognition toward N-methylammonium salts by a combination of cation-dipole, cation-π and hydrogen bonding interactions [19,31]. Enrico and co-workers determined the crystal structure of 13 kinds of complexes based on the small tetraphosphonate cavitands 2a-c with amino acids (Fig. 2) [32]. They investigated the ability of these receptors to discriminate between different amino acids and accomplished a fundamental understanding on the molecular recognition properties including binding constant Ka and thermodynamic parameters of the complexation process. Besides, solvent influence on the complexation selectivity was evaluated in water and methanol. Their study confirms that phosphonate cavitands can be used as synthetic receptor for the detection of epigenetic modifications of histones under physiological conditions [33].
|
Download:
|
| Fig. 2. Chemical structures of tetraphosphonate cavitands 2a-c. | |
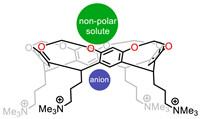
To get more specific comprehension of binding effects in aqueous supramolecular chemistry, Gibb et al. reported a water-soluble small cavitand 3 and compared halide affinities using traditional unscreened model and a screened (Debye–Hückel) model [34]. Their findings will help address the uncertainty often encountered with binding constant determinations in water and buffered solutions. More recently, they explored its bonding with non-polar guests and anion complexation (Fig. 3) [35]. On the one hand, the shallow pocket (non-polar site) can bind hydrophobic guest with assistance of hydrogen bonding to the acetal groups. Guest molecules without halogen atoms bind weakly, while guests such as iodomethane and 1-bromopropane substituent group showed sufficient binding association. On the other hand, research on anion association properties with the crown of ammonium groups of 3 was carried out; anion binding to the crown was relatively strong, despite the high dielectric of water. These findings shed light on both hydrophobic and Hofmeister effect.
|
Download:
|
| Fig. 3. Water-soluble cavitand 3 and its different binding abilities with non-polar guests and anion. | |
Great efforts have been made to use R[4]A to create new host molecules with larger cavities, such as extending the walls through bridging units [36]. Other than that, an easier way is to use its higher homologues [37,38]. In the traditional sense, cavitands are usually synthesized based on R[4]As. When RAs with more than four repeating units are employed, these obtained wider cavity cavitands will definitely pave the way for the construction of supramolecular systems with intriguing recognition and self-assembly properties [39]. For example, Wang’s team constructed a series of nanobelt 4 with a diameter of ~10 Å based on R[6]A (Fig. 4), which would be the potential precursors to various hydrocarbon nanobelts [40,41].
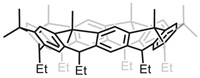
|
Download:
|
| Fig. 4. R[6]A based nanobelt 4 from Wang’s group. | |
Placido et al. reported the improved synthesis of larger R[6]A by a Lewis acid-catalyzed condensation of 1,3-dimethoxy-2-methylbenzene with paraformaldehyde in o-dichlorobenzene solvent [42]. Demethylation was operated via BBr3 to get R[6]A with more easily modified phenolic hydroxyl groups. Subsequently, the first example of R[6]A based cavitand 5 was obtained, where sulfate bridges were introduced between the adjacent phenolic hydroxyl groups (Fig. 5) [43]. The obtained tetrasulfate cavitand 5 was able to interact with the 1,8-diammoniumoctane cation in solution via a two-point double-hydrogen bonding between the ammonium groups and the sulfate bridges. 5 was also able to accommodate the dimethylviologen cation by ion–dipole interaction. The R[6]A cavitand was considered as the first examples of supramolecular application of larger RA macrocycles which stimulated great interest in constructing cavitand with wider cavity.
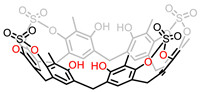
|
Download:
|
| Fig. 5. Chemical structure of tetrasulfate-bridged R[6]A cavitand 5. | |
Recently, Agnieszka Szumna and co-workers have shown an easy and scalable syntheses of various higher analogues of R[4]A, such as R[5]A, (2-nitro/carboxyl) R[5]A, pyrogallol[5]arene and R[7]A [44], among which the R[5]A have demonstrated potential applications in the construction of capsules and molecular cages due to their unique symmetry and proven tuneability [45]. Moreover, R[5]A was utilized to synthesize O-methylene bridged R[5]A-based cavitand 6 with bromochloromethane in DMA (Fig. 6). The 1H NMR spectrum confirmed that the ring has been locked in C5v-symmetric vase conformation. In addition, they also realized other chemical modifications to these homologues through different reactions such as Mannich reaction.
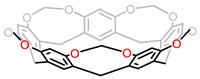
|
Download:
|
| Fig. 6. Chemical structure of R[5]A based cavitand 6. | |
The aforementioned small and wider cavitands are too shallow to accommodate large guest molecules, severely limiting their development as molecular containers. Therefore, chemists began to introduce functional “walls” to fabricate deep-cavity cavitand to make them suitable containers. For instance, Diederich’s group investigated the switchable supramolecular nanosystems in depth, such as the pH- [46], redox- [47,48], and photo-responsive [49,50] RA-based receptors. Enrico Dalcanale’s group studied the application of deep cavitands in constructing coordination cages [51] and supramolecular sensors [52-55]. Hooley’s group focused on biosensing with arrayed deep cavitand hosts [56,57]. Apart from these above, deep cavitands were mainly used for separation of isomers, studying conformation of trapped guests and catalysis. Up to now, there are two main classes of deep cavity cavitand: Rebek’s dynamic cavitands [58-61] and Gibb’s rigid cavitands [62-64].
Rebek and Yu’s group has delved into cavitands and investigated their binding behaviour with guest molecules [65-67]. Recently, they synthesized four container hosts (tetraurea cavitand 7a, tetramethylbenzimidazole cavitand 7b, tetrabenzimidazole cavitand 7c and tetrabenzotriazole cavitand 7d, Fig. 7). Their binding with the molecular "dumbbell" guests in water were investigated and the contribution of the hydrophobic effect and the cation-π interaction were studied in depth [68]. The results showed that the container molecule always preferred binding to the uncharged tert-butyl group, regardless of the presence of charged groups on the container periphery. This preference was largely determined by the solvation of polar trimethylammonium in water, which outcompeted the attraction between the positive charge and the π-surface of the container wall. Furthermore, cavitand 7d was found to be able to form velcraplex in water and bind with both hydrophobic and amphiphilic guests [69]. Recently, they applied their water-soluble capsule with benzoselenodiazole walls for the isomer separation of alkyl-O-methyl oximes derivatives. The results showed that the capsule preferred binding Z-isomers and the binding affinity varies with the terminal substituents. Furthermore, the presence of capsular cavity can promote the E-Z isomerization, and was accelerated 10-fold by sonication [70].
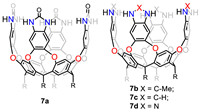
|
Download:
|
| Fig. 7. Chemical structures of cavitand 7a-7d. | |
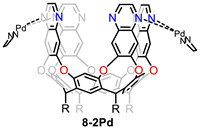
While Rebek’s group is well-known for their dynamic deep-cavity cavitand, they reported rigid cavitands where the vase conformation was stabilized by metal coordination. They added increasing quantities of Pd(EDA)(H2O)2·2NO3 to a quinoxaline cavitand and metallo-cavitand 8-2Pd was successfully obtained [71]. 8-2Pd could serve as a selective container to efficiently separate p-functionalized toluene derivatives from a mixture of isomers (Fig. 8) [72]. Moreover, 8-2Pd could also separate n-alkanes from their mixtures with isooctane, and trans-1,4-dimethyl cyclohexane from its mixture with cis-isomer [73]. Later, they prepared another new metallo-cavitands which showed selectivity for o-difluorobenzene over its isomers with >99.9% purity and binding towards hydrophilic molecules [74,75]. In addition to metal coordination, they used covalent linkages to bridge adjacent walls and formed two new cavitands which could separate p-cresol from its m-isomer and separate o-xylene from its p- and m-isomers, respectively [76].
|
Download:
|
| Fig. 8. Chemical structure of metallo-cavitand 8-2Pd. | |
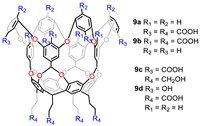
In 2004, Gibb’s group synthesized a rigid cavitand 9a with an external coating of carboxylic acid groups (Fig. 9) [77]. To further understand the important factors in context dependent guest binding, they challenged the molecular dynamics (MD) simulations to examine the affinity and binding thermodynamics of positive and negative charged guests to two constitutionally isomeric cavitand hosts octa-acid 9a and exo-octa-acid 9b [78]. The binding of cationic guests to 9b was stronger than that of anionic guests, whereas 9a shows opposite trends. Their test revealed that the asymmetry in affinity of the host-guest pair could not be explained by simple Coulomb interaction. It could also be arisen from complex ion-ion and ion-couple interactions. Moreover, they explored how the orientation of R1 and R2 groups on the wettability of the non-polar pocket [79,80]. Ramamurthy et al. synthesized two new cavitands tetra-acid tetra-alcohol 9c and 9d based on Gibb’s work [81]. Due to different substituents on the upper-rim, it can be observed that 9c was a desired inert container as sensitizers for triplet exciton utilization, but 9d was not. They also demonstrated that the benzoate moiety at the periphery was the key factor to the sensitizer.
|
Download:
|
| Fig. 9. Chemical structures of cavitands 9a-9d. | |
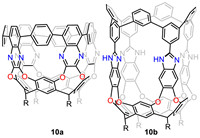
In order to further increase bending strain and complexity of aromatic systems, Raúl Hernández Sánchez’s group was committed to exploring the possibility of using RA to template the synthesis of strained conjugated aromatics. Firstly, they synthesized octabromo-derivative using RA and 2,3-dichloro-5,8-dibromoquinoxaline under basic conditions, which was followed by Suzuki coupling with 1,4-benzenediboronic to afford tubularene 10a (Fig. 10) [82]. Later, they treated octamine materials with 3,5-dibromobenzaldehyde to obtain another octabromo derivative, which was then coupled with 1,3-phenyldiborate bis(pinacol) ester to generate cavitand 10b [83]. The architecture of these tubularenes is highly tunable, which could be further modified. The expansion of tubularene family will open up a new landscape for discovery of novel properties and applications of contorted aromatic systems [84].
|
Download:
|
| Fig. 10. Chemical structures of RA-based tubularenes 10a and 10b. | |
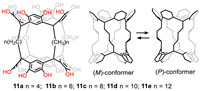
Biscavitands refer to a type of dimer cavitands bridged via feet-to-feet connected alkyl chains. Lower rim-connected ditopic cavitand will not be included in this section [85]. Different from (hemi)carcerands [86], biscavitands usually encapsulate guest molecules by their two isolated cavities. It is difficult for guests to be trapped in the flexible cavity formed by alkyl linkages. Haino’s group has done lots of work based on bisresorcinarenes (Bis-RA, Fig. 11) [87], which were optimally synthesized via condensation of resorcinol and even-numbered bisdioxolanes under acid-catalyzed conditions. As precursor of biscavitands, bis-RAs processed similar structures, which were initially synthesized in order to achieve supramolecular polymerization via intermolecular hydrogen bonding between phenolic groups [88]. In 2017, they reported an improved synthesis of bis-RAs 11a-e [89]. Single crystal analysis revealed that 11a and 11b adopted two helical conformations (P and M) that could dynamically exchange in solution, whereas the long aliphatic chains of 11c-e preferred parallel orientations for their two RA units. Interestingly, when odd-numbered bisdioxolanes were applied to reaction, triresorcinarenes were generated [90].
|
Download:
|
| Fig. 11. Bis-RAs 11a-e and the interconversion of helical conformations. | |
On the basis of previous work about bis-RAs, Haino’s group began to focus on introducing interannular bridges to form a series of biscavitands. In 2016, octaphosphonate biscavitands 12a-b with phosphonate interaromatic bridges were successfully synthesized (Fig. 12) [91]. Similar to bis-RAs, their flexible conformations were largely attributed to the presence of the length of alkyl chain linker. However, different lengths of alkyl chains led to distinct allosteric effects during the formation of host-guest complexes. 12a-b were able to bind ammonium guest through hydrogen bond and CH-π interactions in a host-to-guest ratio of 1:2. The complex of 12a with the first guest significantly facilitated the second guest binding, which proved the positive allosteric effect while 12b indicated a negative cooperativity [92]. Later, the group fabricated other two biscavitands 13a and 13b with SiMe2 or SiBu2 groups in the interannular bridge [93]. Both two biacavitands adopted D4h symmetric conformation at room temperature and the energy barrier for conformational conversion was obviously higher than bis-RAs due to restriction of interannular bridges.
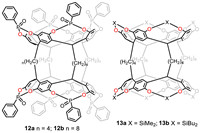
|
Download:
|
| Fig. 12. Chemical structures of biscavitands 12a-b and 13a-b. | |
Lately, to further deepen the cavity of biscavitands for more fascinating function, they first synthesized octaiodobiscavitand 14a through aromatic Finkelstein iodination (Fig. 13), then Suzuki-Miyaura and Sonogashira coupling reactions were applied to make modification on the upper its rims to obtain 14b and 14c, in which the latter was able to self-assemble into supramolecular polymer in solid state [94]. Then, they introduced new kinds of biscavitand carrying Rebek’s deep cavitand building block. Hexadecanitro biscavitand 15a was synthesized and modified by reduction and acylation to afford 15b [95]. The cooperativity of host-guest complexation was influenced by the solvation of the cyclic hydrogen bonds. Thermodynamic parameters for complexation process implied the homotropic negative cooperativity in toluene and chloroform as well as non-cooperativity in THF.
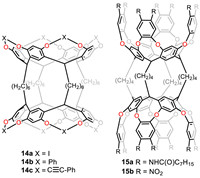
|
Download:
|
| Fig. 13. Chemical structures of 14a-c and 15a-b. | |
The symmetrical cavitands are the ones that the hydroxyl groups of the RA are bridged by identical functional groups. The effectiveness of RA in catalysis and separation can often be improved by pre-organization of the guest methods [96]. The cavitands themselves have limited catalytic ability. Therefore, some researchers tried to fabricate cavitands for catalytic use by incorporating various catalytic functional groups in asymmetric cavitands [97].
Tetsuo Iwasawa’s group has done a series of work to synthesize asymmetric catalytic cavitands according to the above strategies [98-102]. Recently, they successfully synthesized three cavitands with cis-arrangement of two quinoxaline walls (cis-16a-c) where the two catalytic centers were inwardly oriented. Homogeneous gold-catalyzed dimerization, hydration of alkynes and rhodium-catalyzed hydroformylation of styrene were carried out compared with isomers trans-16a-c (Fig. 14) [103]. The structure-activity relationship analysis revealed that the less impeded cis-environment around the metal center reduced or modified the product distribution in comparison with trans-16a-c. The shorter distance between the cis isomers promoted cooperation of the two metal catalytic centers. Their study revealed that the cis- or trans-positioning of the catalyst centers directly influenced cooperation between the two metallic atoms to control catalytic activity, reaction profile, and product selectivity.
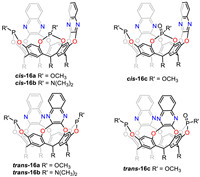
|
Download:
|
| Fig. 14. The structures of cavitands cis-16a-c and their trans-isomers. | |
Antonio et al. designed and synthesized a new family of achiral and chiral gold(I)-cavitand complexes (Fig. 15) [104]. The effects of these gold(I)-cavitands complexes as catalysts on the exo/endo selectivity and enantioselective alkoxycyclization of 1,6-enynes were studied. The yield and enantiomeric selectivity were affected by stoichiometric amounts of AuCl, the types of aromatic wall, temperature as well as charge factors. Their study revealed that chiral gold(I)-cavitand could develop a new enantioselective alkoxycyclization of 1,6-enynes. Subsequently, they applied chiral gold(I) catalyst (S,S,S,S)-17 to the total synthesis of (+)- and (-)-mafaicheenamine C, assigning the absolute configuration of the natural compounds. The cavity effect leading to the high regio- and stereoselectivities was supported by theoretical study.
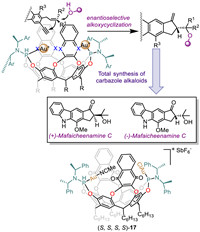
|
Download:
|
| Fig. 15. Chiral gold(I) catalyst (S,S,S,S)-17 and its application in total synthesis of (+)- and (-)-mafaicheenamine C. | |
Agustí et al. created a new deep cavitand receptor 18 functionalized with a chiral bis(pyridyl)dipyrrolidine tetradentate ligand, which could be coordinated to divalent metal Fe and Mn (Fig. 16) [105]. These supramolecular functional complexes were used for catalyzing selective C—H/C═C oxidation reactions with hydrogen peroxide under mild conditions. It is notable that the manganese catalyst exhibits a better performance than the iron analogue; both of them are relatively stable and show no obvious decomposition. However, one of disadvantages of using metallo-cavitand 18 as catalyst is the free rotation of the ligand scaffold, which limited the system for further application.
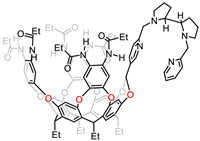
|
Download:
|
| Fig. 16. Chemical structure of self-folding cavitand 18. | |
The functional groups of the above-mentioned cavitands are modified by the adjacent phenolic hydroxyl groups or lower rims. Apart from those, there are some other cavitands which do not fall into the above categories discussed here. The electron-rich C-2 position of aromatic units of RA can also be functionalized. For instance, Kenji’s group pioneered in employing cavitands in making dimeric capsules [106-108]. Warmuth’s group focused on designing multicomponent nanocages by dynamic covalent chemistry [109].
Olivia and co-workers prepared a series of water-soluble RA-based metallo-cavitand 19aZnⅡ and its dodecylphosphocholine (DPC) micelle-encapsulated equivalent 19bZnⅡ (Fig. 17) [110]. These biomimetic structures could recognize small organic guests such as acetate, acetylacetone and acetamide. Binding constants of acetylacetonate in water with 19aZnⅡ and 19bZnⅡ was measured to be 2.4 (± 0.7) ×104 L/mol and 2.8 (± 0.7) ×104 L/mol at pH 7.4, respectively. The micellar environment has subtle impact on either metal ion binding or guest accommodation. It represents an easy alternative to tedious synthetic work and opens new perspectives for molecular recognition in water. Moreover, they expand the application of 19aZnⅡ to recognize alkyl phosphates in water [111]. The results showed that the 19aZnⅡ system could efficiently bind linear monoalkyl phosphates at physiological pH. The reason for these results was the synergistic effect of the metal ion binding site for phosphate anionic head, the hydrophobic cavity for alkyl moiety embedment, side-arms that could undergo protonation, thus allowing further electrostatic stabilization, spacers between the recognition points that allowed directional and spatial control of guest binding.
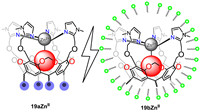
|
Download:
|
| Fig. 17. Two strategies for the water solubilization of a RA-based metallo-receptor: water-soluble 19aZnⅡ and its micelle-encapsulated equivalent 19bZnⅡ. | |
Additionally, self-assembled hierarchical supramolecular architectures could be constructed by RA-based cavitand. Yi-Tsu Chan and co-workers reported three types of metallo-supramolecular nanocapsules, including dimeric capsules (20a-c), a Sierpiński triangular prism (20d), and a cubic star (20e) (Fig. 18) [112]. They investigated the self-sorting behaviour of the three RA-based ligand L1, 120°-bent bis-terpyridine (bis-tpy) ligands L2-4 and cadmium salts. The results showed that the cavity size of the dimeric capsules could be readily modulated by the bis-tpy ligands of varying spacer length, and their self-sorting behaviour was proven to be spacer-length dependent. Besides, they established an unprecedented Sierpiński triangular prism 20d through the heteroleptic complexation of L1 with tetrakis-tpy ligand L5, which will allow to further develop the precise self-assembly of elaborate 3D fractal structures such as a Sierpiński tetrahedron. Finally, they produced a cubic star 20e through assembly of L1 and trigonal connector L6. TEM and AFM experiments gained more structural insights into their structures and the obtained dimensions data consistent with the corresponding theoretical ones. This work presents an efficient self-assembly method for preparation of cavitand-functionalized nanocapsules with tunable size and geometry, which are potential candidates for applications in molecular self-recognition and catalysis.
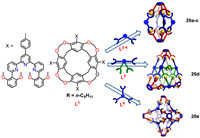
|
Download:
|
| Fig. 18. Molecular structures and cartoon representations of the ligands L1−L6 and corresponding self-assembled capsules 20a-e. Reproduced with permission [112]. Copyright 2020, American Chemical Society. | |
The aforementioned cavitands involved simple molecular binding or the construction of complex structures. In addition, cavitands could coordinate with metal ions for crystal engineering [113-115]. Jiang’s group synthesized two tetrapyridine-functionalized cavitands decorated with S,N-donors at upper rims and successfully constructed two [CumIn] cluster-based cavitands including one dimensional MOF 21a (m = 6 and n = 5) and supramolecular cluster 21b (m = 8 and n = 8) (Fig. 19) [116]. Subsequently, they found that 21b showed better catalytic performance than 21a for Cu-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. They attributed this to the nature of the porous network array of 21b. The exposed Cu8I8 cluster motif of 21b was supported by cavitands and thus easy to coordinate with substrates (alkynes and azides) resulting in the formation of a polynuclear catalysis center, which could cooperatively catalyze CuACC reaction. On the contrary, the Cu6I5 active site of 21a was completely trapped by six cavitands in a closed stacking structure so that the access of substrates to cluster center will be difficult.
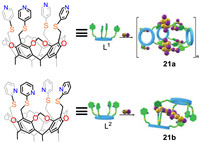
|
Download:
|
| Fig. 19. The structures of 21a and 21b. Reproduced with permission [116]. Copyright 2021, Royal Society of Chemistry. | |
In conclusion, we have provided a comprehensive overview over the structural diversity of five major classes of RA-based cavitands developed so far, as well as a discussion on their potential applications. For the first and second types of cavitands, smaller and wider cavities endow them with unique host-guest chemistry properties and interesting binding behaviours. For the third type of cavitand, functionalized aromatic walls give them deep cavities to bind varieties of guest molecules, which can be used for catalysis of chemical reactions or separation of isomers. For bis-cavitands, two separate cavitands are connected feet-to-feet by alkyl chain bridge to produce bis-cavitands that are excellent candidate for constructing supramolecular polymer or studying allosteric effect. For asymmetric cavitands, introducing catalytic sites endows them with excellent catalytic ability. Despite of these fascinating achievements, many possibilities still exist in cavitand chemistry. RA-based cavitand could potentially be employed as building block for porous materials [117,118], such as porous organic polymers(POP), covalent organic framework (COF) and metal organic framework (MOF), which could apply for heterogeneous catalysis or environment remediation [119]. In addition to these, their potential application in other fields, such as drug and gene delivery, light-harvesting, are waiting to be uncovered. Designing novel structure of cavitand offers great intellectual challenges and inspiration for intriguing applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Natural Science Foundation of Jiangsu Province (Nos. BK20200432, BK20211179), National Natural Science Foundation of China (Nos. 22271154, M-0411), and the Fundamental Research Funds for the Central Universities (No. NG2022003).
| [1] |
L. Escobar, P. Ballester, Chem. Rev. 121 (2021) 2445-2514. DOI:10.1021/acs.chemrev.0c00522 |
| [2] |
K. Wang, X. Tian, J.H. Jordan, et al., Chin. Chem. Lett. 33 (2022) 89-96. DOI:10.1016/j.cclet.2021.06.026 |
| [3] |
K. Wang, M. Zuo, T. Zhang, H. Yue, X.Y. Hu, Chin. Chem. Lett. 34 (2023) 107848. DOI:10.1016/j.cclet.2022.107848 |
| [4] |
S. Niu, L.L. Mao, H. Xiao, et al., Chin. Chem. Lett. 33 (2022) 1970-1974. DOI:10.1016/j.cclet.2021.09.090 |
| [5] |
Y.X. Yan, Y. Zhang, Y. Chen, Y. Liu, ACS Appl, Polym. Mater. 2 (2020) 5641-5645. DOI:10.1021/acsapm.0c00958 |
| [6] |
J.R. Moran, S. Karbach, D.J. Cram, J. Am. Chem. Soc. 104 (1982) 5826-5828. DOI:10.1021/ja00385a064 |
| [7] |
D.J. Cram, Science 219 (1983) 1177-1183. DOI:10.1126/science.219.4589.1177 |
| [8] |
Q. Sun, L. Escobar, P. Ballester, Angew. Chem. Int. Ed. 60 (2021) 10359-10365. DOI:10.1002/anie.202101499 |
| [9] |
L. Escobar, F.A. Arroyave, P. Ballester, Eur. J. Org. Chem. 2018 (2018) 1097-1106. DOI:10.1002/ejoc.201701602 |
| [10] |
K. Hermann, D.A. Turner, C.M. Hadad, J.D. Badjic, Chem. Eur. J. 18 (2012) 8301-8305. DOI:10.1002/chem.201201153 |
| [11] |
S.E. Border, R.Z. Pavlovic, L. Zhiquan, J.D. Badjic, J. Am. Chem. Soc. 139 (2017) 18496-18499. DOI:10.1021/jacs.7b11960 |
| [12] |
S. Mirzaei, V.M. Espinoza Castro, R.H. Sánchez, Chem. Sci. 13 (2022) 2026-2032. DOI:10.1039/d1sc07041j |
| [13] |
H. Erdtman, S. Högberg, S. Abrahamsson, B. Nilsson, Tetrahedron Lett. 9 (1968) 1679-1682. DOI:10.1016/S0040-4039(01)99028-8 |
| [14] |
A. Baeyer, Ber. Dtsch. Chem. Ges. 5 (1872) 280-282. DOI:10.1002/cber.18720050186 |
| [15] |
J.M. Bourgeois, H. Stoeckli-Evans, Helv. Chim. Acta 88 (2005) 2722-2730. DOI:10.1002/hlca.200590211 |
| [16] |
E.R. Abdurakhmanova, P. Cmoch, A. Szumna, Org. Biomol. Chem. 20 (2022) 5095-5103. DOI:10.1039/d2ob00880g |
| [17] |
M. Wehbie, G. Arrachart, I. Karamé, L. Ghannam, S. Pellet-Rostaing, Sep. Purif. Technol. 169 (2016) 17-24. DOI:10.1016/j.seppur.2016.06.003 |
| [18] |
A. Mouradzadegun, S. Elahi, F. Abadast, RSC Adv. 4 (2014) 31239-31248. DOI:10.1039/C4RA03463E |
| [19] |
A. Pedrini, F. Bertani, E. Dalcanale, Molecules 23 (2018) 2670. DOI:10.3390/molecules23102670 |
| [20] |
N.W. Wu, J. Rebek, Jr., J. Am. Chem. Soc. 138 (2016) 7512-7515. DOI:10.1021/jacs.6b04278 |
| [21] |
J.L. Liu, M. Sun, Y.H. Shi, et al., J. Inclus. Phenom. Macrocycl. Chem. 102 (2022) 201-233. DOI:10.1007/s10847-021-01119-w |
| [22] |
T. Heinz, D.M. Rudkevich, J. Rebek, Jr., Nature 394 (1998) 764-766. DOI:10.1038/29501 |
| [23] |
D. Ajami, J. Rebek, Jr., Acc. Chem. Res. 46 (2013) 990-999. DOI:10.1021/ar300038r |
| [24] |
J. Jiao, B. Sun, Y. Ding, et al., Org. Lett. 22 (2020) 8984-8988. DOI:10.1021/acs.orglett.0c03385 |
| [25] |
M.L. Tan, Q.H. Guo, X.Y. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 23649-23658. DOI:10.1002/anie.202013149 |
| [26] |
L. Turunen, F. Pan, N.K. Beyeh, et al., Cryst. Growth Des. 18 (2017) 513-520. |
| [27] |
L. Turunen, F. Pan, N.K. Beyeh, et al., CrystEngComm 19 (2017) 5223-5229. DOI:10.1039/C7CE01118K |
| [28] |
L. Turunen, N.K. Beyeh, F. Pan, A. Valkonen, K. Rissanen, Chem. Commun. 50 (2014) 15920-15923. DOI:10.1039/C4CC07771G |
| [29] |
K. Twum, K. Rissanen, N.K. Beyeh, Chem. Rec. 21 (2021) 386-395. DOI:10.1002/tcr.202000140 |
| [30] |
Y.J. Zhu, Y. Gao, M.M. Tang, J. Rebek, Jr., Y. Yu, Chem. Commun. 57 (2021) 1543-1549. DOI:10.1039/d0cc07784d |
| [31] |
R. Pinalli, M. Suman, E. Dalcanale, Eur. J. Org. Chem. 2004 (2004) 451-462. DOI:10.1002/ejoc.200300430 |
| [32] |
R. Pinalli, G. Brancatelli, A. Pedrini, et al., J. Am. Chem. Soc. 138 (2016) 8569-8580. DOI:10.1021/jacs.6b04372 |
| [33] |
N. Bontempi, E. Biavardi, D. Bordiga, et al., Nanoscale 9 (2017) 8639-8646. DOI:10.1039/C7NR02491F |
| [34] |
J.H. Jordan, H.S. Ashbaugh, J.T. Mague, B.C. Gibb, J. Am. Chem. Soc. 143 (2021) 18605-18616. DOI:10.1021/jacs.1c08457 |
| [35] |
H.R. Aziz, W. Yao, J.H. Jordan, B.C. Gibb, Supramol. Chem. 33 (2021) 226-271. DOI:10.1080/14702436.2021.1888644 |
| [36] |
A. Gissot, J. Rebek, J. Am. Chem. Soc. 126 (2004) 7424-7425. DOI:10.1021/ja049074r |
| [37] |
H. Konishi, T. Nakamura, K. Ohata, K. Kobayashi, O. Morikawa, Tetrahedron Lett. 37 (1996) 7383-7386. DOI:10.1016/0040-4039(96)01683-8 |
| [38] |
C. Naumann, E. Roman, C. Peinador, et al., Chem. Eur. J. 7 (2001) 1637-1645. DOI:10.1002/1521-3765(20010417)7:8<1637::AID-CHEM16370>3.0.CO;2-X |
| [39] |
C. Naumann, S. Place, J.C. Sherman, J. Am. Chem. Soc. 124 (2002) 16-17. |
| [40] |
T.H. Shi, S. Tong, M.X. Wang, Angew. Chem. Int. Ed. 59 (2020) 7700-7705. DOI:10.1002/anie.202002827 |
| [41] |
Q.H. Guo, Y. Qiu, M.X. Wang, J.F. Stoddart, Nat. Chem. 13 (2021) 402-419. DOI:10.1038/s41557-021-00671-9 |
| [42] |
P.D. Sala, C. Gaeta, W. Navarra, et al., J. Org. Chem. 81 (2016) 5726-5731. DOI:10.1021/acs.joc.6b00803 |
| [43] |
C. Gaeta, P.D. Sala, C. Talotta, et al., Org. Chem. Front. 3 (2016) 1276-1280. DOI:10.1039/C6QO00336B |
| [44] |
M. Chwastek, A. Szumna, Org. Lett. 22 (2020) 6838-6841. DOI:10.1021/acs.orglett.0c02357 |
| [45] |
M. Chwastek, P. Cmoch, A. Szumna, Angew. Chem. Int. Ed. 60 (2021) 4540-4544. DOI:10.1002/anie.202013105 |
| [46] |
T. Gottschalk, B. Jaun, F. Diederich, Angew. Chem. Int. Ed. 46 (2007) 260-264. DOI:10.1002/anie.200603366 |
| [47] |
I. Pochorovski, M.O. Ebert, J.P. Gisselbrecht, et al., J. Am. Chem. Soc. 134 (2012) 14702-14705. DOI:10.1021/ja306473x |
| [48] |
I. Pochorovski, F. Diederich, Acc. Chem. Res. 47 (2014) 2096-2105. DOI:10.1021/ar500104k |
| [49] |
J.V. Milic, F. Diederich, Chem. Eur. J. 25 (2019) 8440-8452. DOI:10.1002/chem.201900852 |
| [50] |
V. Garcia-Lopez, M. Zalibera, N. Trapp, et al., Chem. Eur. J. 26 (2020) 11451-11461. DOI:10.1002/chem.202001788 |
| [51] |
L. Pirondini, F. Bertolini, B. Cantadori, et al., Proc. Natl. Acad. Sci. U. S. A. 99 (2002) 4911-4915. DOI:10.1073/pnas.072612199 |
| [52] |
R.M. Yebeutchou, E. Dalcanale, J. Am. Chem. Soc. 131 (2009) 2452-2453. DOI:10.1021/ja809614y |
| [53] |
G. Valenti, E. Rampazzo, E. Biavardi, et al., Faraday Discuss. 185 (2015) 299-309. DOI:10.1039/C5FD00096C |
| [54] |
F. Bertani, N. Riboni, F. Bianchi, et al., Chem. Eur. J. 22 (2016) 3312-3319. DOI:10.1002/chem.201504229 |
| [55] |
A. Aprile, F. Ciuchi, R. Pinalli, E. Dalcanale, P. Pagliusi, J. Phys. Chem. Lett. 7 (2016) 3022-3026. DOI:10.1021/acs.jpclett.6b01300 |
| [56] |
W. Zhong, R.J. Hooley, Acc. Chem. Res. 55 (2022) 1035-1046. DOI:10.1021/acs.accounts.2c00026 |
| [57] |
J. Chen, B.L. Hickey, L. Wang, et al., Nat. Chem. 13 (2021) 488-495. DOI:10.1038/s41557-021-00647-9 |
| [58] |
Q. Shi, D. Masseroni, J. Rebek, Jr., J. Am. Chem. Soc. 138 (2016) 10846-10848. DOI:10.1021/jacs.6b06950 |
| [59] |
S. Mosca, Y. Yu, J.V. Gavette, K.D. Zhang, J. Rebek, Jr., J. Am. Chem. Soc. 137 (2015) 14582-14585. DOI:10.1021/jacs.5b10028 |
| [60] |
J.M. Yang, Y. Yu, J. Rebek, Jr., J. Am. Chem. Soc. 143 (2021) 2190-2193. DOI:10.1021/jacs.0c12302 |
| [61] |
Y.H. Wan, Y.J. Zhu, J. Rebek, Jr., Y. Yu, Molecules 26 (2021) 1922. DOI:10.3390/molecules26071922 |
| [62] |
K. Wang, X. Cai, W. Yao, et al., J. Am. Chem. Soc. 141 (2019) 6740-6747. DOI:10.1021/jacs.9b02287 |
| [63] |
K. Wang, J.H. Jordan, B.C. Gibb, Chem. Commun. 55 (2019) 11695-11698. DOI:10.1039/c9cc06501f |
| [64] |
K. Wang, B.C. Gibb, J. Org. Chem. 82 (2017) 4279-4288. DOI:10.1021/acs.joc.7b00264 |
| [65] |
F.U. Rahman, D. Tzeli, I.D. Petsalakis, et al., J. Am. Chem. Soc. 142 (2020) 5876-5883. DOI:10.1021/jacs.0c01290 |
| [66] |
Y. Yu, J.M. Yang, J. Rebek, Chem 6 (2020) 1265-1274. DOI:10.1016/j.chempr.2020.04.014 |
| [67] |
M. Petroselli, Y.Q. Chen, M.K. Zhao, J. Rebek, Y. Yu, Chin. Chem. Lett. 34 (2023) 107834. DOI:10.1016/j.cclet.2022.107834 |
| [68] |
Y. Zhu, M. Tang, H. Zhang, et al., J. Am. Chem. Soc. 143 (2021) 12397-12403. DOI:10.1021/jacs.1c06510 |
| [69] |
F.U. Rahman, R. Wang, H.B. Zhang, et al., Angew. Chem. Int. Ed. 61 (2022) e202205534. DOI:10.1002/anie.202205534 |
| [70] |
K. Kanagaraj, R. Wang, M.K. Zhao, et al., J. Am. Chem. Soc. 145 (2023) 5816-5823. DOI:10.1021/jacs.2c12907 |
| [71] |
F.U. Rahman, Y.S. Li, I.D. Petsalakis, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 17648-17653. DOI:10.1073/pnas.1909154116 |
| [72] |
F.U. Rahman, J.M. Yang, Y.H. Wan, et al., Chem. Commun. 56 (2020) 6945-6948. DOI:10.1039/d0cc02778b |
| [73] |
Y.H. Wan, Faiz-Ur Rahman, J. Rebek Jr., Y. Yu, Chin. J. Chem. 39 (2021) 1498-1502. DOI:10.1002/cjoc.202000709 |
| [74] |
H.B. Zhang, K. Kanagaraj, J. Rebek, Jr., Y. Yu, J. Org. Chem. 86 (2021) 8873-8881. DOI:10.1021/acs.joc.1c00794 |
| [75] |
Y.Q. Chen, H.W. Guan, K. Kanagaraj, J. Rebek, Y. Yu, Chin. Chem. Lett. 33 (2022) 4908-4911. DOI:10.1016/j.cclet.2022.03.039 |
| [76] |
J.M. Yang, Y.Q. Chen, Y. Yu, P. Ballester, J. Rebek, Jr., J. Am. Chem. Soc. 143 (2021) 19517-19524. DOI:10.1021/jacs.1c09226 |
| [77] |
C.L.D. Gibb, B.C. Gibb, J. Am. Chem. Soc. 126 (2004) 11408-11409. DOI:10.1021/ja0475611 |
| [78] |
P. Suating, T.T. Nguyen, N.E. Ernst, et al., Chem. Sci. 11 (2020) 3656-3663. DOI:10.1039/c9sc06268h |
| [79] |
J.W. Barnett, M.R. Sullivan, J.A. Long, et al., Nat. Chem. 12 (2020) 589-594. DOI:10.1038/s41557-020-0458-8 |
| [80] |
P. Suating, N.E. Ernst, B.D. Alagbe, et al., J. Phys. Chem. B 126 (2022) 3150-3160. DOI:10.1021/acs.jpcb.2c00628 |
| [81] |
P. Jagadesan, S.R. Samanta, R. Choudhury, V. Ramamurthy, J. Phys. Org. Chem. 30 (2017) e3728. DOI:10.1002/poc.3728 |
| [82] |
S. Mirzaei, E. Castro, R.H. Sanchez, Chem. Sci. 11 (2020) 8089-8094. DOI:10.1039/d0sc03384g |
| [83] |
E. Castro, S. Mirzaei, R.H. Sanchez, Org. Lett. 23 (2021) 87-92. DOI:10.1021/acs.orglett.0c03765 |
| [84] |
S. Mirzaei, E. Castro, R.H. Sanchez, Chem. Eur. J. 27 (2021) 8642-8655. DOI:10.1002/chem.202005408 |
| [85] |
A. Lledó, S. Kamioka, A.C. Sather, J. Rebek, Angew. Chem. Int. Ed. 50 (2011) 1299-1301. DOI:10.1002/anie.201006166 |
| [86] |
S. Ro, S.J. Rowan, A.R. Pease, D.J. Cram, J.F. Stoddart, Org. Lett. 2 (2000) 2411-2414. DOI:10.1021/ol005962p |
| [87] |
D. Shimoyama, T. Haino, Asian J. Org. Chem. 9 (2020) 1718-1725. DOI:10.1002/ajoc.202000302 |
| [88] |
H. Yamada, T. Ikeda, T. Mizuta, T. Haino, Org. Lett. 14 (2012) 4510-4513. DOI:10.1021/ol301996q |
| [89] |
D. Shimoyama, T. Ikeda, R. Sekiya, T. Haino, J. Org. Chem. 82 (2017) 13220-13230. DOI:10.1021/acs.joc.7b02301 |
| [90] |
D. Shimoyama, R. Sekiya, H. Kudo, T. Haino, Org. Lett. 22 (2020) 352-356. DOI:10.1021/acs.orglett.9b03693 |
| [91] |
D. Shimoyama, H. Yamada, T. Ikeda, R. Sekiya, T. Haino, Eur. J. Org. Chem. 20 (2016) 3300-3303. DOI:10.1002/ejoc.201600410 |
| [92] |
C.A. Hunter, H.L. Anderson, Angew. Chem. Int. Ed. 48 (2009) 7488-7499. DOI:10.1002/anie.200902490 |
| [93] |
D. Shimoyama, T. Haino, J. Org. Chem. 84 (2019) 13483-13489. DOI:10.1021/acs.joc.9b01730 |
| [94] |
D. Shimoyama, R. Sekiya, T. Haino, Chem. Commun. 56 (2020) 3733-3736. DOI:10.1039/d0cc00933d |
| [95] |
H. Fujimoto, D. Shimoyama, K. Katayanagi, et al., Org. Lett. 23 (2021) 6217-6221. DOI:10.1021/acs.orglett.1c01837 |
| [96] |
K. Wang, J.H. Jordan, X.Y. Hu, L. Wang, Angew. Chem. Int. Ed. 59 (2020) 13712-13721. DOI:10.1002/anie.202000045 |
| [97] |
S. Korom, P. Ballester, J. Am. Chem. Soc. 139 (2017) 12109-12112. DOI:10.1021/jacs.7b05458 |
| [98] |
N. Natarajan, E. Brenner, D. Sémeril, D. Matt, J. Harrowfield, Eur. J. Org. Chem. 2017 (2017) 6100-6113. DOI:10.1002/ejoc.201700725 |
| [99] |
M.P. Schramm, M. Kanaura, K. Ito, M. Ide, T. Iwasawa, Eur. J. Org. Chem. 2016 (2016) 813-820. DOI:10.1002/ejoc.201501426 |
| [100] |
M. Inoue, K. Ugawa, T. Maruyama, T. Iwasawa, Eur. J. Org. Chem. 2018 (2018) 5304-5311. DOI:10.1002/ejoc.201800948 |
| [101] |
Y. Matsumoto, Y. Taguchi, N. Yoshida, et al., Supramol. Chem. 33 (2021) 253-265. DOI:10.1080/10610278.2021.1981323 |
| [102] |
N. Endo, M. Kanaura, M.P. Schramm, T. Iwasawa, Tetrahedron Lett. 57 (2016) 4754-4757. DOI:10.1016/j.tetlet.2016.09.039 |
| [103] |
M. Inoue, S. Kamiguchi, K. Ugawa, et al., Eur. J. Org. Chem. 2019 (2019) 6261-6268. DOI:10.1002/ejoc.201901058 |
| [104] |
I. Martin-Torres, G. Ogalla, J.M. Yang, A. Rinaldi, A.M. Echavarren, Angew. Chem. Int. Ed. 60 (2021) 9339-9344. DOI:10.1002/anie.202017035 |
| [105] |
D. Vidal, M. Costas, A. Lledó, ACS Catal. 8 (2018) 3667-3672. DOI:10.1021/acscatal.7b04426 |
| [106] |
M. Nakamura, K. Kishimoto, Y. Kobori, et al., J. Am. Chem. Soc. 138 (2016) 12564-12577. DOI:10.1021/jacs.6b07284 |
| [107] |
N. Nishimura, K. Kobayashi, Angew. Chem. Int. Ed. 47 (2008) 6255-6258. DOI:10.1002/anie.200802293 |
| [108] |
K. Kobayashi, M. Yamanaka, Chem. Soc. Rev. 44 (2015) 449-466. DOI:10.1039/C4CS00153B |
| [109] |
X. Liu, R. Warmuth, J. Am. Chem. Soc. 128 (2006) 14120-14127. DOI:10.1021/ja0644733 |
| [110] |
S. Collin, A. Parrot, L. Marcelis, et al., Chem. Eur. J. 24 (2018) 17964-17974. DOI:10.1002/chem.201804768 |
| [111] |
S. Collin, N. Giraud, E. Dumont, O. Reinaud, Org. Chem. Front. 6 (2019) 1627-1636. DOI:10.1039/c9qo00263d |
| [112] |
L. He, S.C. Wang, L.T. Lin, et al., J. Am. Chem. Soc. 142 (2020) 7134-7144. DOI:10.1021/jacs.0c01482 |
| [113] |
R. Pinalli, E. Dalcanale, F. Ugozzoli, C. Massera, CrystEngComm 18 (2016) 5788-5802. DOI:10.1039/C6CE01010E |
| [114] |
W.Y. Pei, G. Xu, J. Yang, et al., J. Am. Chem. Soc. 139 (2017) 7648-7656. DOI:10.1021/jacs.7b03169 |
| [115] |
F.F. Wang, Y.Y. Liu, W.Y. Pei, J.F. Ma, Inorg. Chem. 60 (2021) 7329-7336. DOI:10.1021/acs.inorgchem.1c00497 |
| [116] |
C.X. Hu, Y.H. Xuan, Z.H. Jiang, et al., CrystEngComm 23 (2021) 7179-7185. DOI:10.1039/d1ce01069g |
| [117] |
Z. Li, Y.W. Yang, Adv. Mater. 34 (2022) e2107401. DOI:10.1002/adma.202107401 |
| [118] |
R. Tang, Y. Ye, S. Zhu, et al., Chin. Chem. Lett. 34 (2023) 107734. DOI:10.1016/j.cclet.2022.08.014 |
| [119] |
L.P. Skala, A. Yang, M.J. Klemes, L. Xiao, W.R. Dichtel, J. Am. Chem. Soc. 141 (2019) 13315-13319. DOI:10.1021/jacs.9b06749 |
 2023, Vol. 34
2023, Vol. 34 

