b University of Chinese Academy of Sciences, Beijing 100049, China
Molecular oxygen is an abundant, green and atom-economical oxidant, which should be an ideal oxidant in organic transformations [1-3]. However, the application of oxygen is limited by the high activation energies in reactions. Living organisms use transition metal-based enzymes to activate oxygen in metabolic processes [2-5]. Natural monooxygenases can also effectively utilize O2 in various organic transformations such as hydroxylation, epoxidation, Baeyer-Villiger oxidation, and sulfonation oxidation [6,7]. Despite the ultrahigh activities, the catalysis of most natural metalloenzymes can only take place under very narrow condition range, and natural enzymes always suffer from their poor stability and difficulty in large scale preparation [8-10]. Compared to natural enzymes, nanozymes or artificial enzymes have the advantages of easy mass preparation and relatively stable catalytic performance, and some of nanozymes have shown enhanced thermal and chemical stability [11-14]. Mimicking natural oxidases or monooxygenases with nanozymes is thus a promising approach to explore effective catalysts for oxygen activation.
Nanozymes can be classified into several categories: metal/metal oxide-doped carbon-based nanozymes, single-atom nanozymes, and metal-organic framework (MOF) nanozymes [10,14,15]. Metal-organic frameworks are an emerging class of organic-inorganic hybrid materials with porous network structures constructed from coordination bonds between organic linkers and metal or metal cluster nodes [16-19]. Most MOFs are highly crystalline with their structures determined at atomic-precise level by single crystal or powder X-ray diffraction techniques. Moreover, the properties of MOFs can be well-designed and fine-tuned by carefully choosing the organic linkers and metal nodes to obtain networks with suitable pore sizes and functionalities [20,21]. Compared with other type of nanozymes, MOF nanozymes are of unique advantages because their predictable and controllable structures allow systematic design of catalytic active sites as well as comprehensive studies on the catalytic mechanisms [22-24]. In this scenario, MOFs and MOF-derived materials have been used to mimic a variety of enzymes [25-29]. For example, the iron porphyrin-containing MOF PCN-222(Fe) have been reported as a peroxidase mimic [30]. More impressively, the multinuclear metal nodes or secondary building units (SBUs) are also good models for multi-metallic catalytic sites of natural enzymes [31,32]. In a very recent report, the Fe3 and Cu3 SBUs of MOF-919 were reported to mimic the functions of peroxidase and oxidase, respectively [33].
Although several MOFs have been shown to be good mimics of natural oxidases, not much effort have been devoted to utilization of MOF catalysts for synthetically meaningful oxidation processes. In this communication, we report the synthesis of FICN-6, a MOF that contains Cu3(OH)(pyz)3 (pyz = pyrazolate) SBUs, and its catalytic activity for oxidative C-C coupling reaction. While such a Cu3 SBU has been shown as a good oxidase mimic in literature, we are showing that it can also serve as a good catalyst for oxidative organic transformations.
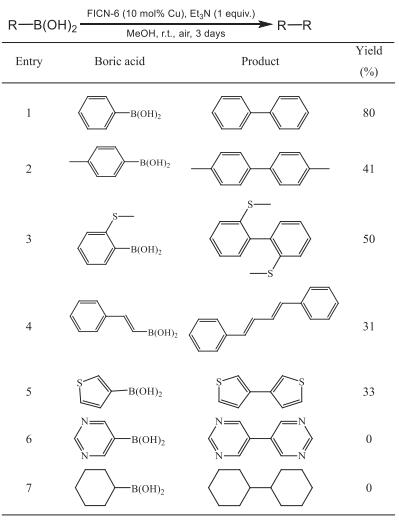
FICN-6 was synthesized via a solvothermal reaction of copper sulfate and the H2pba ligand. Through the morphology analysis by scanning electron microscope (SEM), it was found that the hexagonal shape of FICN-6 can be clearly seen at 10 µm size (Fig. 1a). Single crystal X-ray diffraction studies revealed that FICN-6 crystallizes in the orthorhombic Cmca space group. FICN-6 has a two-fold interpenetration structure (Fig. 1b). For each network, there are two different types of secondary building units (SBUs), one is the four-connected Cu2(O2CR)4 paddle-wheel dimer and the other is the Cu3(OH)(pyz)3(O2CR) planar triangular trimer. The Cu3 trimer is coordinated with three pyrazolate groups and a carboxylate group from four pba2− ligands, so both SBUs are four-connected. Topological analysis found that each isolated network adopts in nbo topology (Fig. 1d). Remarkably, the two interpenetrated networks are not only packed by non-covalent interactions, but also bridged with sulfate anions on the Cu3 trimer SBU. The Cu3 trimer is capped by a sulfate anion from the axial direction, which also coordinates to another Cu3 trimer from the other network. Therefore, the Cu3 trimers from two distinct networks are bridged by two sulfate anions to form an eight-connected Cu6(OH)2(pyz)6(SO4)2(O2CR)2 cluster (Fig. 1c). The parallel distance between the two Cu3 trimers is about 3.20 Å. The two-fold interpenetration structure reduce the size of the pore and cavity in the framework. Viewed from the b axis, the MOF has quadrilateral channels with diagonals of approximately 1.1 nm × 0.8 nm (Fig. 1b). The powder X-ray diffraction pattern (PXRD) of FICN-6 is consistent with that simulated from single-crystal structure, indicating the phase purity of bulk sample (Fig. 1e). Although the single crystal structure of FICN-6 gives a solvent accessible volume of 65.0% as calculated by PLATON, nitrogen and CO2 sorption experiments indicate a low BET surface area of about 18 m2/g (Fig. S6 in Supporting information). The low surface area may be attributed to the strong breathing effect, which is common for MOFs with large quadrilateral channels [34,35]. Thermogravitic analysis indicated that FICN-6 remains stable below 300 ℃ (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 1. (a) An SEM image of FICN-6 (scale bar: 10 µm). (b) Crystal structure of FICN-6 (viewed from b axis). (c) Coordination environment of Cu6 cluster assembled from two Cu3 SBUs. (d) A schematic figure showing the formation of two-fold intercatenated network through SO42− bridging. (e) Simulated (black) and experimental (red) PXRD patterns of FICN-6. | |
Formation of biaryl compounds through the construction of C-C bonds is an important method for the synthesis of natural products, drugs, pesticides and functional materials [36]. At present, symmetric and non-symmetric biaryl compounds are mainly obtained by using transition metal-catalyzed Suzuki coupling [37-39], modified Ullmann reaction [40,41], or Kumada-Corriu-Tamao reaction [42,43]. Harsh conditions such as heating and inert atmosphere are usually required for these processes. Alternatively, oxidative homocoupling of arylboronic acids have been shown to achieve symmetric biphenyl compounds under mild conditions. However, the reaction usually requires noble metal catalysts based on palladium [44-46], gold [47,48], or rhodium [49,50], and sometimes high-cost oxidants such as TEMPO or Ag2O. Recently, Cu-based materials were also reported as effective catalysts for the reaction [51-53]. With the presence of Cu3(OH)(pyz)3 clusters in the structure, we propose that FICN-6 can be used to mimic oxidase-like activity and activate oxygen for aerobic oxidation of organic compounds, and oxidative homocoupling of organic boronic acids was chosen as a model reaction.
The reaction conditions were first optimized with phenylboronic acid as the substrate. As shown in Table 1, homocoupling of phenylboronic acid proceeded smoothly in presence of FICN-6 catalyst and triethylamine (TEA) in methanol solution to give the biphenyl product with a yield of 80% in 3 days (Table 1, entry 4). The reaction was carried out in a mild condition without heating and with air as the oxidant. Changing the solvent into aprotic ones such as dichloromethane or DMF led to reduced yields (Table 1, entries 5 and 6). The reaction yield reduced from 80% to 23% without the addition of TEA (Table 1, entry 3), suggesting that addition of base may promote the reaction. When FICN-6 was not added, the reaction did not proceed (Table 1, entries 1 and 2), showing that FICN-6 is crucial for the reaction. Under nitrogen atmosphere, the yield of biphenyl product was only 21%, significantly lower than that under ambient condition, showing that the environmental oxygen does involve in the reaction as the terminal oxidant (Table 1, entry 10).
|
|
Table 1 Optimization of reaction conditions for FICN-6-catalyzed oxidative homocoupling of phenylboronic acid. |
Since FICN-6 consists of two different types of Cu-based SBUs, both of which may serve as active sites for oxygen activation, it is meaningful to investigate and compare their catalytic activity. Therefore, we conducted control experiments with HKUST-1 and MOF-818 as catalysts. HKUST-1 is constructed from Cu2(O2CR)4 paddle wheel SBUs but contains no Cu3(OH)(pyz)3 planar trimers. MOF-818 has Cu3(OH)(pyz)3 planar trimer SBUs, but no Cu2(O2CR)4 paddle wheel dimer presents in its structure [54]. With a Cu loading of 10%, both HKUST-1 and MOF-818 catalyzed the homologous coupling of phenylboronic acid, but the conversions were much lower with the same conditions and reaction time (Table 1, entries 7 and 8). Therefore, it can be concluded that either isolated Cu2(O2CR)4 or Cu3(OH)(pyz)3 SBU is not an effective catalytic active site. We next compared the catalytic activity of FICN-6 with rht-MOF-pyr, which also consists of both Cu2(O2CR)4 and Cu3(OH)(pyz)3 SBUs but with a non-interpenetrate structure [21]. It was found that rht-MOF-pyr also exhibits low catalytic activity (Table 1, entry 9). Based on these results, we speculate that network interpenetration in FICN-6 introduces synergistic effect between SBUs, which plays an important role on the enhanced catalytic activity. Presumably, the proximity of Cu3(OH)(pyz)3 planar trimers through sulfate bridging may allow binding of oxygen and boronic acid substrate at separate open metal sites that sit closely, which will lead to facile oxidation and promote the reaction.
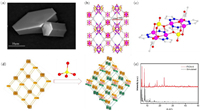
We next tested the recyclability of FICN-6 catalyst. Under the same conditions, the yield of biphenyl product dropped sharply to only 26% with the recovered FICN-6 catalyst from the first run (Fig. S8 in Supporting information). PXRD pattern (Fig. 2a) of recovered catalyst exhibited much lower peak intensity, suggesting that the loss of crystallinity may be the main cause of deteriorated performance. To demonstrate that FICN-6 is the real catalyst for the reaction, a "heterogeneity" test or "hot filtration" experiment was performed (Fig. S10 in Supporting information). For the oxidative homocoupling of phenylboronic acid, the yield of biphenyl product was tested after 12 h as 36%. After that, the FICN-6 solid was removed from the reaction mixture, and the supernatant was further reacted for another 2.5 days. No further conversion was detected after FICN-6 removal (Fig. 2b). Meanwhile, a 3-day reaction gave 80% yield of the biphenyl product. Therefore, it can be concluded that FICN-6 is the real catalyst for homologous coupling reactions.
|
Download:
|
| Fig. 2. (a) The PXRD patterns of FICN-6 before (black) and after (red) catalysis. (b) Testing the "heterogeneity" of FICN-6-catalyzed oxidative homocoupling reaction. | |
We next investigated the compatibility of FICN-6 catalyzed homocoupling reactions with various boronic acid substrates. As shown in Table 2, substituted phenylboronic acids (Table 2, entries 2 and 3) or thiophene-2-boronic acid (Table 2, entry 5) gave reasonable yields of homocoupling products. Olefinic boronic acid such as trans-2-phenylvinylboronic acid was also reactive (Table 2, entry 4). However, boronic acids with highly electron-poor aromatic rings such as pyrimidine-5-boronic acid (Table 2, entry 6) or aliphatic boronic acids such as cyclohexylboronic acid (Table 2, entry 7) did not react under the standard reaction conditions.
|
|
Table 2 Substrate scope for oxidative homocoupling of boronic acids. |
In summary, a Cu-based metal-organic framework with two distinct SBUs, Cu2(O2CR)4 and Cu3(OH)(pyz)3(O2CR), was synthesized. The reported MOF FICN-6 adopts in a two-fold interpenetration structure with Cu3 SBU from one network connecting to another SBU from the other network, leading to an unusual intercatenated structure. Moreover, FICN-6 served as a good heterogeneous catalyst for oxygen activation and oxidative C-C coupling of boronic acids.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe acknowledged the National Natural Science Foundation of China (No. 22005306) for financial support.
Supplementary materialsThe crystallographic data of FICN-6 is available through the Cambridge Crystallographic Data Centre (CCDC No. 2174600). Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.06.058.
| [1] |
D.E. Torres Pazmino, M. Winkler, A. Glieder, M.W. Fraaije, J. Biotechnol. 146 (2010) 9-24. DOI:10.1016/j.jbiotec.2010.01.021 |
| [2] |
X. Huang, J.T. Groves, Chem. Rev. 118 (2018) 2491-2553. DOI:10.1021/acs.chemrev.7b00373 |
| [3] |
A.J. Jasniewski,, Chem. Rev. 118 (2018) 2554-2592. DOI:10.1021/acs.chemrev.7b00457 |
| [4] |
M. Guo, T. Corona, K. Ray, W. Nam, ACS Cent. Sci. 5 (2019) 13-28. DOI:10.1021/acscentsci.8b00698 |
| [5] |
M. Huang, W. Xiang, C. Wang, et al., Chin. Chem. Lett. 31 (2020) 2769-2773. DOI:10.1016/j.cclet.2020.06.040 |
| [6] |
W.J. van Berkel, N.M. Kamerbeek, M.W. Fraaije, J. Biotechnol. 124 (2006) 670-689. DOI:10.1016/j.jbiotec.2006.03.044 |
| [7] |
E.I. Solomon, D.E. Heppner, E.M. Johnston, et al., Chem. Rev. 114 (2014) 3659-3853. DOI:10.1021/cr400327t |
| [8] |
P.V. Iyer, L. Ananthanarayan, Process Biochem. 43 (2008) 1019-1032. DOI:10.1016/j.procbio.2008.06.004 |
| [9] |
Y. Lin, J. Ren, X. Qu, Acc. Chem. Res. 47 (2014) 1097-1105. DOI:10.1021/ar400250z |
| [10] |
J. Wu, X. Wang, Q. Wang, et al., Chem. Soc. Rev. 48 (2019) 1004-1076. DOI:10.1039/c8cs00457a |
| [11] |
H. Wei, E. Wang, Chem. Soc. Rev. 42 (2013) 6060-6093. DOI:10.1039/c3cs35486e |
| [12] |
F. Schwizer, Y. Okamoto, T. Heinisch, et al., Chem. Rev. 118 (2018) 142-231. DOI:10.1021/acs.chemrev.7b00014 |
| [13] |
M. Liang, X. Yan, Acc. Chem. Res. 52 (2019) 2190-2200. DOI:10.1021/acs.accounts.9b00140 |
| [14] |
W. Yang, X. Yang, L. Zhu, et al., Coord. Chem. Rev. 448 (2021) 214170. DOI:10.1016/j.ccr.2021.214170 |
| [15] |
H. Wang, K. Wan, X. Shi, Adv. Mater. 31 (2019) e1805368. DOI:10.1002/adma.201805368 |
| [16] |
O.M. Yaghi, M. O'Keeffe, N.W. Ockwig, et al., Nature 423 (2003) 705-714. DOI:10.1038/nature01650 |
| [17] |
H.C. Zhou, J.R. Long, O.M. Yaghi, Chem. Rev. 112 (2012) 673-674. DOI:10.1021/cr300014x |
| [18] |
R.J. Wei, H.G. Zhou, Z.Y. Zhang, et al., CCS Chem. 3 (2021) 2045-2053. DOI:10.31635/ccschem.020.202000401 |
| [19] |
T.C. Zhuo, Y. Song, G.L. Zhuang, et al., J. Am. Chem. Soc. 143 (2021) 6114-6122. DOI:10.1021/jacs.0c13048 |
| [20] |
H. Furukawa, K.E. Cordova, M. O'Keeffe, O.M. Yaghi, Science 341 (2013) 1230444. DOI:10.1126/science.1230444 |
| [21] |
W.Y. Gao, R. Cai, T. Pham, et al., Chem. Mater. 27 (2015) 2144-2151. DOI:10.1021/acs.chemmater.5b00084 |
| [22] |
X.J. Hu, Z.X. Li, H. Xue, et al., CCS Chem. 2 (2020) 616-622. DOI:10.31635/ccschem.019.201900040 |
| [23] |
X. Niu, X. Li, Z. Lyu, et al., Chem. Commun. 56 (2020) 11338-11353. DOI:10.1039/d0cc04890a |
| [24] |
D. Wang, D. Jana, Y. Zhao, Acc. Chem. Res. 53 (2020) 1389-1400. DOI:10.1021/acs.accounts.0c00268 |
| [25] |
I. Nath, J. Chakraborty, F. Verpoort, Chem. Soc. Rev. 45 (2016) 4127-4170. DOI:10.1039/C6CS00047A |
| [26] |
J. Baek, B. Rungtaweevoranit, X. Pei, et al., J. Am. Chem. Soc. 140 (2018) 18208-18216. DOI:10.1021/jacs.8b11525 |
| [27] |
M. Li, J. Chen, W. Wu, et al., J. Am. Chem. Soc. 142 (2020) 15569-15574. DOI:10.1021/jacs.0c07273 |
| [28] |
X. Feng, Y. Song, J.S. Chen, et al., J. Am. Chem. Soc. 143 (2021) 1107-1118. DOI:10.1021/jacs.0c11920 |
| [29] |
Y. Tu, H. Li, T. Tu, Q. Zhang, CCS Chem. 4 (2022) 872-879. DOI:10.31635/ccschem.021.202000759 |
| [30] |
D. Feng, Z.Y. Gu, J.R. Li, et al., Angew. Chem. Int. Ed. 51 (2012) 10307-10310. DOI:10.1002/anie.201204475 |
| [31] |
Y. Matoba, T. Kumagai, A. Yamamoto, et al., J. Biol. Chem. 281 (2006) 8981-8990. DOI:10.1074/jbc.M509785200 |
| [32] |
C.A. Ramsden, P.A. Riley, Bioorg. Med. Chem. 22 (2014) 2388-2395. DOI:10.1016/j.bmc.2014.02.048 |
| [33] |
S. Kulandaivel, C.H. Lin, Y.C. Yeh, Chem. Commun. 58 (2022) 569-572. DOI:10.1039/d1cc05908d |
| [34] |
C. Serre, F. Millange, C. Thouvenot, et al., J. Am. Chem. Soc. 124 (2002) 13519-13526. DOI:10.1021/ja0276974 |
| [35] |
G. Ferey, C. Serre, Chem. Soc. Rev. 38 (2009) 1380-1399. DOI:10.1039/b804302g |
| [36] |
O. Baudoin, M. Cesario, D. Guenard, F. Gueritte, J. Org. Chem. 67 (2002) 1199-1207. DOI:10.1021/jo0160726 |
| [37] |
G.P. McGlacken, L.M. Bateman, Chem. Soc. Rev. 38 (2009) 2447-2464. DOI:10.1039/b805701j |
| [38] |
F.S. Han, Chem. Soc. Rev. 42 (2013) 5270-5298. DOI:10.1039/c3cs35521g |
| [39] |
S. Fu, S. Yao, S. Guo, et al., J. Am. Chem. Soc. 143 (2021) 20792-20801. DOI:10.1021/jacs.1c08908 |
| [40] |
J. Hassan, M. Sevignon, C. Gozzi, et al., Chem. Rev. 102 (2002) 1359-1470. DOI:10.1021/cr000664r |
| [41] |
E. Sperotto, G.P. van Klink, G. van Koten, J.G. de Vries, Dalton Trans. 39 (2010) 10338-10351. DOI:10.1039/c0dt00674b |
| [42] |
J.P. Corbet, G. Mignani, Chem. Rev. 106 (2006) 2651-2710. DOI:10.1021/cr0505268 |
| [43] |
O. Vechorkin, V. Proust, X. Hu, J. Am. Chem. Soc. 131 (2009) 9756-9766. DOI:10.1021/ja9027378 |
| [44] |
C. Adamo, C. Amatore, I. Ciofini, et al., J. Am. Chem. Soc. 128 (2006) 6829-6836. DOI:10.1021/ja0569959 |
| [45] |
L. Zhou, Q.X. Xu, H.F. Jiang, Chin. Chem. Lett. 18 (2007) 1043-1046. DOI:10.1016/j.cclet.2007.06.023 |
| [46] |
X. Xu, S. Gao, W. Chen, et al., ChemistrySelect 3 (2018) 8863-8866. DOI:10.1002/slct.201801714 |
| [47] |
C. Gonzalez-Arellano, A. Corma, M. Iglesias, F. Sanchez, Chem. Commun. (2005) 1990-1992. |
| [48] |
P.N. Eyimegwu, J.A. Lartey, J.H. Kim, ACS Appl. Nano Mater. 2 (2019) 6057-6066. DOI:10.1021/acsanm.9b01594 |
| [49] |
T. Vogler, A. Studer, Adv. Syn. Catal. 350 (2008) 1963-1967. DOI:10.1002/adsc.200800300 |
| [50] |
D. Tyagi, C. Binnani, R.K. Rai, et al., Inorg. Chem. 55 (2016) 6332-6343. DOI:10.1021/acs.inorgchem.6b01115 |
| [51] |
A.S. Demir, O. Reis, M. Emrullahoglu, J. Org. Chem. 68 (2003) 10130-10134. DOI:10.1021/jo034680a |
| [52] |
G. Cheng, M. Luo, Eur. J. Org. Chem. (2011) 2519-2523. DOI:10.1002/ejoc.201001729 |
| [53] |
B. Kaboudin, Y. Abedi, T. Yokomatsu, Eur. J. Org. Chem. (2011) 6656-6662. DOI:10.1002/ejoc.201100994 |
| [54] |
Q. Liu, Y. Song, Y. Ma, et al., J. Am. Chem. Soc. 141 (2019) 488-496. DOI:10.1021/jacs.8b11230 |
 2023, Vol. 34
2023, Vol. 34