With the advent of immune checkpoint blockade, cancer immunotherapy has gained much more attention [1-3]. Cancer immunotherapy has emerged as a promising treatment strategy with many benefits, including inspiring efficacy and relative low toxicity. In the competition between the immune system and the tumor, three immune steps must be achieved successively in order to elicit effective anti-tumor immunity: dendritic cells (DCs) successful deliver antigen signal to T cells; DCs trigger protective T cell responses and cancer-specific T cells realize their function in tumor foci [4,5]. Other strategies reveal that activating innate immune cells responses, notably natural killer (NK) cells responses, also contribute to anti-tumor immunity [6-8]. Interleukin 15 (IL-15), a member of γc cytokines, is a pleiotropic immunomodulator, which is critical for lymphoid development and homeostasis of immune cell populations [9,10]. IL-15 binds to its high affinity receptor α (IL-15Rα) to form superagonist, predominantly performing its function on target cells that expressed receptor β and γ chains in complex [11-13]. By improving DCs function, activating NK and T cells, and facilitating protective memory T cells production, IL-15 shows obvious tumor inhibition capability in both preclinical and clinical trials [14-20].
Although gene therapy is an effective anti-cancer strategy, defects including poor targeting, deleterious insertion into human chromosome, immunogenicity, low gene packaging capacity, complicated process of vector production [21,22] and unpredictable gene expression restrain its translation to clinic. Polymer nanoparticles were employed as a versatile drug or gene delivery platform for cancer therapy due to excellent properties including easy to modify, simple synthesis process, biocompatibility, biodegradation and enhanced blood retention time [23-27]. Biological characteristics of tumor related to damaged lymphatic drainage as well as dilated and leaky vessels could facilitate nanoparticles to achieve passive targeting, enhanced drugs penetration to tumor tissues and reduced system toxicity [28,29].
Herein, superagonist IL-15 plasmid (psIL-15) consisting of mIL-15Rα sushi-linker-mIL-15 was constructed. Nanocarrier composed of methylated polyethylene glycol-b-polylactic acid-b-methylated polyethylene glycol (mPEG-PLA-mPEG) triblock polymer and 1, 2-dioleoyl-3-trimethylammonium-propane (DOTAP) was self-assembled into nanoparticles, named DMAM, to deliver psIL-15. Local delivery of DMAM/psIL-15 in animal models successfully secret soluble superagonist IL-15 (sIL-15) that significantly inhibit tumor growth through enhancing immune cells responses, reducing angiogenesis, promoting tumor cell apoptosis and inhibiting proliferation (Scheme 1).

|
Download:
|
| Scheme 1. Illustration depicts that structure constitution of DMAM/psIL-15 nanocomplexes and its anti-tumor mechanisms in tumor models of mice. After being administrated, local delivery of psIL-15 by DMAM achieved the tumor-specific secretion of sIL-15, which induces the proliferation and activation of immune cells, increases IFN-γ secretion and cell apoptosis as well as inhibits tumor angiogenesis and cell proliferation. | |
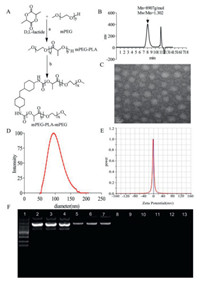
In this study, mPEG-PLA-mPEG triblock polymer was prepared through two reactions as illustrated in Fig. 1A. We further determined the molecular weight of mPEG-PLA-mPEG by gel permeation chromatography (GPC) (Fig. 1B). The result shows that number average molecular weight (Mn) is 8907 g/mol (Mw/Mn = 1.302; Mw, weight average molecular weight). DMAM gene carrier was obtained through film dispersion method of mPEG-PLA-mPEG and DOTAP. The morphology and particle properties of DMAM/psIL-15 were characterized by transmission electron microscopy (TEM) and particle sizer/zeta potential analyzer, respectively. As determined by TEM (Fig. 1C), DMAM condensed psIL-15 to form uniform and spherical structure. Furthermore, particle size (Fig. 1D) and zeta potential (Fig. 1E) of DMAM/psIL-15 were 130.27 ± 3.98 nm (polydispersity index = 0.214) and –2.29 ± 0.64 mV, respectively. To determine gene carry capacity of DMAM, gel electrophoresis was performed. As nanoparticles/plasmid mass ratio increases, plasmid band is finally retarded in agarose gel (Fig. 1F), which illustrates that plasmid can be fully encapsulated by DMAM.
|
Download:
|
| Fig. 1. Preparation and characterization of DMAM nanoparticles. (A) Illustration shows the synthesis of mPEG-PLA-mPEG polymer (a: Tin(II), 140 ℃ oil bath, 12 h; b: HMDI, 140 ℃ oil bath, 8 h). (B) GPC analysis of mPEG-PLA-mPEG polymer (C) TEM image of DMAM/psIL-15. The particle size distribution (D) and zeta potential (E) of DMAM/psIL-15. (F) Agarose gel electrophoresis of DMAM nanoparticles loading psIL-15. Lane 1, marker; lane 2–4, naked plasmids; lane 5–13 indicated different mass ratios of DMAM with psIL-15 (lane 5–7, 25:1; lane 8–10, 50:1; lane 11–13, 75:1). | |
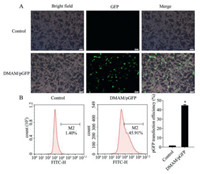
Since effective transfection is the basis of gene therapy, we evaluated transfection efficiency of green fluorescent protein plasmid (pGFP) delivered by DMAM in tumor cells. Obvious expression of GFP was observed in CT26 (Figs. 2A and B) and B16-F10 (Figs. S1A and B in Supporting information) cell lines as compared to Control, demonstrating DMAM could efficiently deliver genes into tumor cells. To activate anti-tumor immune response, tumor cells transfected with DMAM/psIL-15 was expected to efficiently secret sIL-15. Medium (Control), DMAM and DMAM/pc3.1 (pc3.1, pcDNA3.1 plasmid) treatment were considered as contrast groups. Result shows that in compared to DMAM/pc3.1, B16-F10 cells and CT26 cells treated with DMAM/psIL-15 could obviously secret sIL-15 at levels of 197.70 pg/mL (Fig. S2A in Supporting information) and 54.22 pg/mL (Fig. S2B in Supporting information), respectively. We further detected whether sIL15 secreted by tumor cells could promote lymphocyte proliferation and activation. Supernatants from tumor cells that transfected with DMAM/psIL-15 for 48 h were collected and used to incubate the primary isolated lymphocytes. Compared to other groups, supernatant of B16-F10 cells treated with DMAM/psIL-15 significantly induces lymphocytes proliferation after incubation for 24 and 48 h (Figs. 3A and B). Similar result is observed in supernatants of CT26 cells after incubation for 24 h (Fig. 3C). Since IFN-γ and TNF-α are effector molecules of activated lymphocytes including NK and CD8+ T cells [30,31], we also measured the secretion levels of IFN-γ and TNF-α from lymphocytes that co-cultured with transfected tumor cells for 48 h to assess immune cells responses. Our results demonstrate that DMAM/psIL-15 obviously enhances the IFN-γ secretion in co-culture supernatants (Fig. S3A in Supporting information), with no evident effect on TNF-α secretion (Fig. S3B in Supporting information). Therefore, these results illustrate the successfully secreted sIL-15 from tumor cells that transfected with DMAM/psIL-15 could further promote the responses of lymphocytes against tumor cells.
|
Download:
|
| Fig. 2. Transfection efficiency detection of DMAM/pGFP in CT26 cells after 48 h transfection. (A) Images of GFP expression. Scale bar is 50 µm. (B) Transfection efficiency was assessed by flow cytometry (n = 3, P < 0.05). | |

|
Download:
|
| Fig. 3. Treatment with DMAM/psIL-15 stimulates lymphocytes proliferation in vitro. After being stimulated with the supernatants from transfected B16-F10 cells (A: for 24 h; B: for 48 h) and CT26 cell (C: for 24 h), the proliferation activity of lymphocytes was evaluated by CCK8 assay (n = 3, P < 0.05, **P < 0.01, ****P < 0.0001). | |
We next evaluated the anti-tumor effects of psIL-15 delivered by DMAM locally in a colon cancer peritoneal dissemination model. Tumor-bearing mice were divided into four groups, and treated with 5% glucose solution (GS), DMAM, DMAM/pc3.1 and DMAM/psIL-15, respectively. Compared with control treatments, intraperitoneal administration of DMAM/psIL-15 could significantly reduce the ascites volume (Fig. 4A) and improve the survival status of tumor-bearing mice. More importantly, DMAM/psIL-15 significantly suppressed the peritoneal dissemination of colon cancer, as indicated by distinctly decreased average tumor weight and metastatic tumor nodules, with no evident changes in average body weight (Figs. 4A–D). We further identified the therapeutic effects of DMAM/psIL-15 in melanoma subcutaneous model. Compared with control treatments, intratumoral injection of DMAM/psIL-15 could obviously inhibit tumor growth and reduce tumor burden, as indicated by significantly decreased average tumor weight, with no evident changes of average body weight (Figs. S4A–D in Supporting information).

|
Download:
|
| Fig. 4. Anti-tumor effect of DMAM/psIL-15 in metastatic model of colon cancer. The abdominal CT26 tumor-bearing mice were intraperitoneally injected with different formulations as indicated. (A) Image of tumor bearing mice after treatment termination and corresponding tumor nodules (n = 6). (B) Body weight curve during the treatment. (C) Average tumor weight. (D) Tumor nodules were counted for ≥3 mm (left) and < 3 mm (right). P < 0.05, ***P < 0.001 and ****P < 0.0001. | |
We further investigated whether local administration of DMAM/psIL-15 could successfully secret sIL-15 and activate the anti-tumor immunity in vivo. Result indicated that enhanced sIL-15 secretion was observed in ascites derived from DMAM/psIL-15 group as compared to DMAM/pc3.1 group (Fig. S5A in Supporting information). Moreover, DMAM/psIL-15 treatment also significantly increased IFN-γ secretion in ascites as compared to DMAM/pc3.1 group (Fig. S5B in Supporting information), demonstrating that sIL15 induced immune cells activation. In addition, serum sIL-15 levels were comparable in four groups (Fig. S5D in Supporting information). Although serum IFN-γ increased slightly in DMAM/psIL-15 group, no significant difference was found among GS, DMAM and DMAM/pc3.1 groups (Fig. S5E in Supporting information). Moreover, no obvious difference of TNF-α secretion is found among four groups in ascites and serum (Figs. S5C and F in Supporting information).
To further determine the anti-tumor mechanisms of DMAM/psIL-15, TUNEL assay and immumohistochemical staining of CD31 and Ki67 were conducted to assess intratumoral cell apoptosis, neovascularization and cell proliferation, respectively. As shown in Figs. S6 and S7 (Supporting information), compared to control groups, DMAM/psIL-15 treatment causes significantly increased intratumoral apoptosis as well as decreased tumor angiogenesis and proliferation. Together with increased IFN-γ secretion in ascites in DMAM/psIL-15 group, these results collectively indicated that sIL-15 improves tumor microenvironment and enhances anti-tumor immune responses. To evaluate the safety of DMAM/psIL-15 gene therapy, blood biochemical analysis was performed after treatment. No significant abnormalities were observed as all biochemical indexes were comparable in four groups (Fig. S8 in Supporting information), demonstrating that DMAM/psIL-15 is a feasible strategy for cancer immunotherapy with no evidence of system toxicities.
In summary, we have successfully designed biocompatible and biodegradable polymer nanoparticles-DMAM to deliver sIL-15 plasmid. DMAM/psIL-15 obviously promotes lymphocytes proliferation and activation in vitro. Local administration of DMAM/psIL-15 in different tumor models demonstrated obvious anti-tumor effect through enhancing immune cells responses, reducing angiogenesis, promoting tumor cell apoptosis and inhibiting proliferation, with no evidence of system toxicities. Since local delivery of IL-15 superagonist gene through polymer nanoparticles produces sIL-15 in tumor site and circumvent protein degradation problem, DMAM/psIL-15 therapy could provide an alternative approach for cancer immunotherapy. The limitation of our study is that further investigations are needed to clarify the anti-tumor mechanisms of DMAM/psIL-15 more precisely, for example, what kind of immune cells is responsible for anti-tumor immunity and how they response. Based on the multidrug loading capability of polymer nanoparticles system, the potential therapeutic effect of DMAM/psIL-15 combined with other drugs against cancer warrants further research.
Declaration of competing interestThe authors declare no conflicts of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 82172630), the Key R & D Projects of the Science and Technology Department of Sichuan Province (No. 2020YFS0256) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Nos. ZYJC21022, ZYYC21001 and 2019HXFH017). All animal experiments were approved by the Animal Experimental Ethics Committee of State Key Laboratory Biotherapy (SKLB), Sichuan University.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.06.026.
| [1] |
A. Finck, S.I. Gill, C.H. June, Nat. Commun. 11 (2020) 3325. DOI:10.1038/s41467-020-17140-5 |
| [2] |
A.D. Waldman, J.M. Fritz, M.J. Lenardo, Nat. Rev. Immunol. 20 (2020) 651-668. DOI:10.1038/s41577-020-0306-5 |
| [3] |
A.V.R. Kornepati, R.K. Vadlamudi, T. Curie, Nat. Rev. Cancer 22 (2022) 174-189. DOI:10.1038/s41568-021-00431-4 |
| [4] |
I. Mellman, G. Coukos, G. Dranoff, Nature 480 (2011) 480-489. DOI:10.1038/nature10673 |
| [5] |
X. Lei, Y. Lei, J.K. Li, et al., Cancer Lett. 470 (2020) 126-133. DOI:10.1016/j.canlet.2019.11.009 |
| [6] |
H.S. Kim, J.Y. Kim, B. Seol, et al., Nat. Biomed. Eng. 5 (2021) 1360-1376. DOI:10.1038/s41551-021-00768-z |
| [7] |
J.A. Myers, J.S. Miller, Nat. Rev. Clin. Oncol. 18 (2021) 85-100. DOI:10.1038/s41571-020-0426-7 |
| [8] |
Y. Hu, X. Liu, M. Ran, et al., Mater. Today Nano 17 (2022) 100151. DOI:10.1016/j.mtnano.2021.100151 |
| [9] |
P.F. Fiore, S. Di Matteo, N. Tumino, et al., J. Immunother. Cancer 8 (2020) e001428. DOI:10.1136/jitc-2020-001428 |
| [10] |
Z.J. Bernstein, J.B. Spangler, J. Clin. Invest. 131 (2021) e152857. DOI:10.1172/JCI152857 |
| [11] |
S. Dubois, J. Mariner, T.A. Waldmann, Y. Tagaya, Immunity 17 (2002) 537-547. DOI:10.1016/S1074-7613(02)00429-6 |
| [12] |
T.O. Robinson, K.S. Schluns, Immunol. Lett. 190 (2017) 159-168. DOI:10.1016/j.imlet.2017.08.010 |
| [13] |
J.M.J. Van den Bergh, E. Lion, V.F.I. Van Tendeloo, E. Smits, Pharmacol. Ther. 170 (2017) 73-79. DOI:10.1016/j.pharmthera.2016.10.012 |
| [14] |
J.C. Steel, T.A. Waldmann, J.C. Morris, Trends Pharmacol. Sci. 33 (2012) 35-41. DOI:10.1016/j.tips.2011.09.004 |
| [15] |
W. Jiang, C. Zhang, Z.G. Tian, J. Zhang, Immunobiology 219 (2014) 547-553. DOI:10.1016/j.imbio.2014.03.007 |
| [16] |
K. Pilipow, A. Roberto, M. Roederer, et al., Cancer Res. 75 (2015) 5187-5193. |
| [17] |
H.H. Van Acker, S. Anguille, H. De Reu, et al., Front. Immunol. 9 (2018) 658. DOI:10.3389/fimmu.2018.00658 |
| [18] |
D. Alizadeh, R.A. Wong, X. Yang, et al., Cancer Immunol. Res. 7 (2019) 759-772. DOI:10.1158/2326-6066.cir-18-0466 |
| [19] |
A. Berger, S.J. Colpitts, M.S.S. Seabrook, et al., J. Immunother. Cancer 7 (2019) 355. DOI:10.1186/s40425-019-0777-8 |
| [20] |
W. Chen, R.N. Bamford, E.F. Edmondson, T.A. Waldmann, Clin. Cancer Res. 28 (2022) 2082-2093. DOI:10.1158/1078-0432.ccr-21-0496 |
| [21] |
H. Yin, R.L. Kanasty, A.A. Eltoukhy, et al., Nat. Rev. Genet. 15 (2014) 541-555. DOI:10.1038/nrg3763 |
| [22] |
L. Kang, Z.G. Gao, W. Huang, et al., Acta Pharm. Sin. B 5 (2015) 169-175. DOI:10.1016/j.apsb.2015.03.001 |
| [23] |
W.T. Song, S.N. Musetti, L. Huang, Biomaterials 148 (2017) 16-30. DOI:10.1016/j.biomaterials.2017.09.017 |
| [24] |
X.X. Liu, B.L. Wang, Y.Y. Li, et al., ACS Cent. Sci. 5 (2019) 277-289. DOI:10.1021/acscentsci.8b00688 |
| [25] |
J.Y. Wang, H. Wang, H.Y. Cui, et al., Chin. Chem. Lett. 31 (2020) 3143-3148. DOI:10.1016/j.cclet.2020.07.027 |
| [26] |
Y.Y. Hu, L. Lin, Z.P. Guo, et al., Chin. Chem. Lett. 32 (2021) 1770-1774. DOI:10.1016/j.cclet.2020.12.055 |
| [27] |
T. Yu, W. Nie, Z.H. Hong, et al., Adv. Funct. Mater. 31 (2021) 2100715. DOI:10.1002/adfm.202100715 |
| [28] |
H. Maeda, Drug Deliv. Oncol. (2011) 65-84. DOI:10.1002/9783527634057.ch3 |
| [29] |
J. Fang, W. Islam, H. Maeda, Adv. Drug Deliv. Rev. 157 (2020) 142-160. DOI:10.1016/j.addr.2020.06.005 |
| [30] |
C.F. Lin, C.M. Lin, K.Y. Lee, et al., J. Biomed. Sci. 24 (2017) 10. DOI:10.1002/2017JA024185 |
| [31] |
C.J. Kearney, S.J. Vervoort, S.J. Hogg, et al., Sci. Immunol. 3 (2018) eaar3451. DOI:10.1126/sciimmunol.aar3451 |
 2023, Vol. 34
2023, Vol. 34 

