b Institut de Chimie et Biochimie Moléculaires et Supramoléculaires, Laboratoire de Chimie Organique 2-Glycochimie, UMR 5246, CNRS, Université Claude Bernard Lyon 1, Université de Lyon, Villeurbanne 69622, France;
c CNRS, Univ. Grenoble Alpes, CERMAV, Grenoble 38000, France;
d College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China;
e CNRS, Institut de Chimie des Substances Naturelles, Université Paris-Saclay, UPR 2301, Gif-sur-Yvette 91198, France
Carbohydrate–protein interactions are involved in a large panel of biological events including, but not limited to, cell–cell communication [1], inflammation [2], viral or bacterial infection [3], immune responses [4] and tumor cell metastasis [5]. Current advances in glycobiology have shown that individual interactions between carbohydrates and proteins are weak in energy, generally showing dissociation constants in the millimolar range. Oligosaccharide ligands, on the other hand, possess much higher binding affinities towards lectin receptors on account of the "glycoside cluster effect" [6]. Inspired by the multivalency concept, a vast array of polysaccharides [7], glycopolymers [8], glycodendrimers [9] and supramolecular glycopolymers [10] have been successfully exploited, offering opportunities to design anti-adhesins [11], drug delivery systems [12, 13], and vaccines [14] on account of their abilities to recognize and bind specific biological targets.
Recently, multivalent glycoconjugates have been developed as anti-adhesive molecules against pathogen infections. Pseudomonas aeruginosa, an opportunistic Gram-negative bacterial pathogen, is of special interest because of its morbidity and mortality risks for immuno-compromised and cystic fibrosis patients [15]. This bacterium expresses two soluble lectins (LecA and LecB), involved in host recognition, adhesion to tissue and formation of biofilms [15, 16]. Their tetrameric structures allow for the cross-linking between the bacterial biofilm polysaccharides and the host cells [17]. Among strategies that have been developed to competitively interfere with the recognition processes between host cells and pathogens, multivalent glycoconjugates have the potential to prevent colonization or even reverse the formation of biofilms for fighting bacterial infection. Therefore, a large number of glycoclusters have been constructed based on various scaffolds including proteins [18, 19], polymers [20, 21], dendrimers [22], calixarenes [23-31], resorcinarenes [32], cyclodextrins [33], porphyrins [34-37], fullerenes [38-47], and pillararenes [48-51], to prevent or treat diseases caused by bacteria.
Pillar[n]arenes, as a novel family of macrocyclic host molecules [52-54], have been well-received by the supra(bio)molecular chemistry community since their first report in 2008 by Ogoshi [55]. Different from meta-bridged calixarenes, the highly symmetrical scaffolds of pillararenes consist of para-linked hydroquinone units [56]. In particular, per-functionalized pillar[5]arenes (P[5]) with ten identical substituents on both rims are especially easy to be synthesized and functionalized, allowing for the construction of a wide range of supramolecular architectures [57-60]. Intensive investigations on the lectin binding properties of deca-glycosylated P[5]s, as well as glyco[2]rotaxanes featuring per-glycosylated P[5] ring components, have been reported. The ability of these multivalent glycocluters to compete with carbohydrate−lectin interactions could provide a promising anti-adhesive strategy for fighting pathogen infections [48-51].
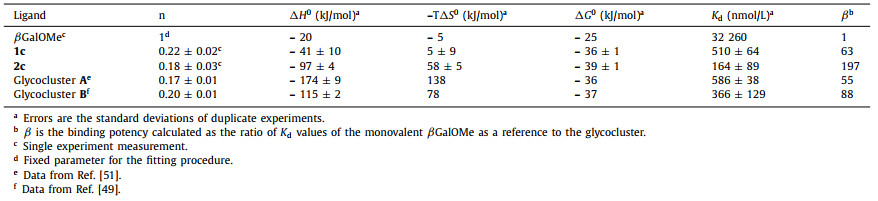
In a previous study, one of us showed that penta-substituted P[5] macrocyclic platforms attached with five glycosides also provide excellent multivalent interactions with bacterial lectins [51]. Nonetheless, these P[5]-based pentavalent glycoclusters are not pure compounds but instead mixtures of four constitutional isomers, which arose as a result of the statistical cyclization of asymmetrically-alkylated hydroquionone monomers during the P[5] synthesis (Fig. 1). Although the overall binding affinities of these pentavalent glycoclusters are good for bacterial lectins, the influence of the constitutional integrity of the P[5] scaffolds on their lectin binding abilities could not be investigated as a result of the non-trivial separation of these isomers. Thanks to the recent improvements on the design and syntheses of penta-functionalizable rim-differentiated P[5]s (RD-P[5]s) [61-63], herein we describe the syntheses and characterizations of a series of carbohydrate functionalized RD-P[5]s (1a-1f and 2a-2d). In each of them, one rim is O-methylated, and the other is decorated with glycosides. The binding affinities between these newly synthesized constitutionally-pure pentavalent glycoclusters and three types of lectins (ConA, LecA and LecB) were investigated successfully by isothermal titration microcalorimetry (ITC) methods. In addition, the influence of the TEG spacers between the RD-P[5] scaffolds and the carbohydrate epitopes on the water solubility and binding affinity were evaluated.
|
Download:
|
| Fig. 1. Schematic illustration of the molecular design of pentavalent glycoclusters based on (a) rim-differentiated P[5] and (b) mixture of four penta-substituted P[5] constitutional isomers. | |
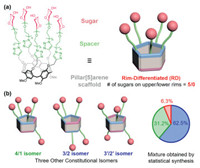
In order to obtain the desired RD-P[5]-based pentavalent glycoclusters (Scheme 1), Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) "click" chemistry was exploited for the effective conjugations of assorted carbohydrate epitopes onto the P[5] scaffold under mild conditions [64]. Penta-propargyl RD-P[5] 3 was first obtained by following our previously reported protocol [61] as a single constitutional isomer. The azido-functionalized carbohydrate derivatives 4a-4f, e.g., glucosyl (Glc), galactosyl (Gal), mannosyl (Man) and fucosyl (Fuc) residues, as well as lactosyl (Lac) [65] and maltosyl (Mal) moieties, were readily prepared (Supporting information for detailed synthetic procedures). By executing CuAAC between 3 and 4a-4f, acetate protected intermediates 6a-6f were synthesized in good yields. The targeted RD-P[5]-based glycoclusters 1a-1f were then obtained after acetate deprotection under Zemplén conditions (MeOH/MeONa). The solubility of these glycoclusters 1a-1f in aqueous media varies depending on the nature of the glycosides. To obtain improved water solubility, azido-functionalized glycosides 5a-5d with triethyleneglycol (TEG) spacers, which provide flexibility to the designed glycoclusters, were prepared according to the literature procedures. Similarly, another series of acetate-protected intermediates 7a-7d and RD-P[5]-based glycoclusters 2a-2d with TEG linkers was synthesized (Scheme 1) to evaluate the influence of the spacer arms towards water solubility and lectin binding.
|
Download:
|
| Scheme 1. Synthesis of RD-P[5]-based glycoclusters using RD-(propargyl)5-P[5] scaffold. | |
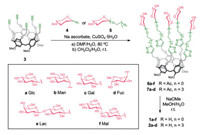
Subsequently, all RD-P[5]-based glycoclusters were fully characterized by NMR spectroscopy, high-resolution mass spectrometry, and analytical HPLC (Supporting information). The relatively simple and sharp 1H NMR spectra of the penta-propargyl 3 and the glycocluster derivatives (Fig. 2), on account of their averaged five-fold structures, rendering the corresponding protons on the repeating hydroquinone units homotopic, confirm that these samples do not contain the other three types of P[5] constitutional isomers showing lower symmetries.
|
Download:
|
| Fig. 2. (a) Partial 1H NMR spectra (400 MHz, CDCl3, 298 K) of compounds 3, 6a and 7a. (b) The stereochemical inversion between conformers of RD-P[5]-based glycoclusters. | |
The anomeric stereochemistry of the carbohydrate moieties in these RD-P[5] glycoclusters were also determined by 1H NMR analyses of the H1 anomeric protons. The Glc 1a and 2a, Gal 1c and 2c, Lac 1e and Mal 1f derivatives, with and without TEG spacer arms, respectively, are all β-anomers [66-70], whereas the Man glycoclusters 1b and 2b were assigned as α-anomers [67, 71]. On the other hand, the Fuc glycocluster 1d features pure β-anomer [72], and the TEG-Fuc analogue 2d is the α-anomer [73].
After the incorporation of glycoside moieties onto the inherently chiral and conformationally-labile P[5] macrocycle, these glycosylated RD-P[5]s exist as ensembles of diastereoisomeric conformers in solution. As the result of the "OMe-through-the-annlus" stereochemical inversion of the P[5] scaffold, all conformers are interchangeable to each other [74]. Most 1H NMR signals of intermediates 6a-6f are coalesced and display single sets of sharp peaks on account of the rapid diastereoisomerization on the NMR time scale, e.g. the aromatic P[5], triazole and saccharide protons, while the diastereotopic -OCH2- methylene protons next to the triazole unit and the -CH2- methylenes on the P[5] units show more complicated splitting patterns. On the other hand, the removal of the acetate group, or rotations of the P[5] scaffold, resulting in more broadened 1H NMR spectra.
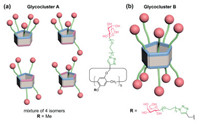
Finally, the binding properties of these newly synthesized constitutionally-pure RD-P[5]-based glycoclusters were then investigated by isothermal titration calorimetry (ITC). In this study, the glucosylated glycoclusters 1a and 2a were synthesized as non-binding control ligands for lectins. Concanavalin A (ConA), a mannose-binding lectin displaying multivalent effect, was used as a model lectin, against which the mannosylated glycoclusters 1b and 2b were assayed. The galactosylated P[5] glycoclusters 1c and 2c were evaluated as ligands of LecA, and the results were compared with previously reported P[5] glycoclusters with either five or ten epitopes on the macrocycle, e.g., the mixture of penta-galactosylated P[5] constitutional isomers glycoclusters A and deca-functionalized P[5] glycocluster B (Fig. 3). The fucosylated glycoclusters 1d and 2d were evaluated as potential ligands of LecB and AFL. In addition, the lactosylated glycocluster 1e was synthesized to evaluate the influence of a second hexose moiety on the solubility of the overall glycocluster, and the maltosylated glycocluster 1f was used as a negative control toward lectins.
|
Download:
|
| Fig. 3. Structure of the (a) penta- or (b) deca-substituted pillararene-based glycoclusters previously studied. | |
In comparison to the monovalent reference, methyl α-d-mannopyranoside (αManOMe), which shows a dissociation constant against ConA in the micromolar range, the penta-substituted glycoclusters 1b and 2b, both showing enough solubility for the ITC measurements, display excellent affinities in the nanomolar range with potency β values of 67 and 38, respectively (Table 1 and Figs. S52 and S53 in Supporting information). In order to confirm that these high affinities observed are attributed directly to specific interactions between the mannose epitopes and the ConA binding pockets, galactosylated glycocluster 1c was used (Fig. S56 in Supporting information) as a negative control to rule out the non-specific binding due to the P[5] scaffold. Furthermore, the stoichiometries of the glycocluster/lectin complex (n) are close to 0.25, implying that four lectins are bound to the pentavalent ligands 1b and 2b. The fifth potential lectin cannot be accommodated onto the remaining mannose epitope presumably owing to steric hindrance.
|
|
Table 1 ITC data for the binding of mannosylated RD-P[5]-based glycoclusters 1b (without spacer arm) and 2b (with TEG spacer arm) towards ConA. |
While ConA was used as a model lectin for these binding studies, the design of high affinity ligands for LecA was proven to be more useful toward potential antibacterial applications [12]. Glycoclusters 1c and 2c were then assayed (Table 2 and Figs. S54 and S55 in Supporting information) against LecA by ITC, both showing very strong affinities for the lectin with dissociation constants in the nanomolar range. The data collected is in line with the affinities reported for the previously studied penta-substituted glycocluster A and the deca-substituted analogue B.
|
|
Table 2 ITC data for the binding of galactosylated RD-P[5]-based glycoclusters 1c (without spacer arm) and 2c (with TEG spacer arm) towards LecA. |
The glycoside cluster effect is strong with binding potency β values in the range of 100, and the glycocluster 2c with the TEG spacer (β = 197) is preferred over 1c with short arm (β = 63). This preference is not due to steric hindrance since in both cases, the stoichiometries (n) of the ligand/lectin complex are very similar and close to 0.20, meaning that all five carbohydrate epitopes are bound to a LecA monomer. Increasing the valency to the decavalent glycocluster B did not trigger a sharp increase in binding potency since dissociation constant is in the same nanomolar range. Glycocluster 1a was used as a negative control for binding to LecA (Fig. S57 in Supporting information) to demonstrate the absence of non-specific binding of the RD-P[5] scaffold to the lectin.
As for the effect of P[5] constitutional isomerism, the isomer-free glycoclusters 1c and 2c displayed very similar binding properties to corresponding glycocluster A consisting of all four constitutional isomers. The only difference between the RD-P[5] glycoclusters 1c and 2c and the corresponding mixture A only reside in the different thermodynamics parameters (ΔH0 and –TΔS0) while the overall change in free energy (ΔG0) remains virtually unchanged.
The fucosylated glycoclusters 1d and 2d were designed and synthesized as potential ligands for LecB but also AFL, a multivalent lectin isolated from the fungi Aspergillus fumigatus. While the syntheses did not cause any trouble, we found that the water solubility of the short arm based glycocluster 1d was too low to proceed with binding studies. The hydroxyl group missing at the 6-position of fucose in comparison to Glc, Man and Gal glycoclusters was enough to produce an insoluble compound in water. Nevertheless, the TEG spacer arm incorporated in glycocluster 2d could provide enough solubility to perform the ITC binding measurements in aqueous media.
Surprisingly, the ITC data collected did not display any heat of interaction when titrating the glycocluster/lectin complex (Fig. S58 in Supporting information) with neither LecB nor AFL. Several attempts were performed with increased concentrations of lectin to ligand ratio to no avail. In parallel, a surface plasmon resonance (SPR) method was also investigated (data not shown) but again without success. The ITC and SPR control experiments, on the other hand, could be successfully performed with the monovalent reference methyl α-l-fucopyranoside, demonstrating that the lack of binding observed for glycocluster 2d was not due to experimental artifacts. As far as we are concerned, there is no clear explanation of why this pentavalent fucosylated glycocluster with good water solubility did not bind to the LecB and AFL fucose-binding lectins.
In addition, the desired water-soluble lactosylated glycocluster 1e was evaluated as LecA ligand. A more detailed description of the thermodynamic parameter governing the binding mode was obtained by ITC (Fig. S59 in Supporting information). Nonetheless, while a titration curve could be observed with genuine heat measured, the curve was not sigmoidal and therefore could not provide any useful data.
In conclusion, pillar[5]arenes, as the ideal candidates, provide decavalent scaffolds on a minimal molecular architecture to presentation of carbohydrate epitopes on their periphery. The developed multivalent glycosylated P[5] has been proved beneficial on targeting bacterial adhesion lectins. Calling for the design of P[5]-based glycocluster to understand the influence of the constitutional integrity of the P[5] scaffold, as well as the length and solubility of the spacer arms, on their binding to lectins, the syntheses of a series of RD-P[5] based glycoclusters were achieved by applying CuAAC conjugations from the azido-functionalized glycosides and alkyne-functionalized RD-P[5] precursors. The binding properties towards lectins of the glycosylated RD-P[5]-based multivalent ligands with and without TEG spacer arm were studied, respectively. The mannosylated RD-P[5]-based glycoclusters showed good binding properties toward ConA, and similarly the galactosylated RD-P[5]-based glycoclusters displayed high affinities to LecA. However, the fucosylated glycoclusters with TEG spacer arm did not bind to the LecB and AFL fucose-binding lectins at all. These results show clearly that the pure RD-P[5]-based glycoclusters displayed similar lectin binding properties as the corresponding mixture of constitutional isomers. The only possible difference between them could reside in the different pattern of the thermodynamics parameters in terms of both enthalpy and entropy, while the overall free energy change remains virtually unaffected. In addition, glycosylated multivalent ligands with the spacer arm also displayed binding properties similar to those without spacer arm.
In the present study, we have employed a stereolabile system to demonstrate that the constitutional integrity of the pillar[5]arene scaffold influenced only marginally the binding properties toward lectin. In the future, stereostable P[5] glycoclusters of one handedness are, in principle, also of significant interest. As a matter of fact, when designing multivalent glycoclusters, the chirality of the core scaffold must be considered as a parameter potentially influencing, either positively or negatively, the binding properties to the lectins. All these studies, once accomplished, will jointly elucidate novel design principles for future developments of multivalent glycosylated P[5] derivatives in treating infections.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial supports from the National Natural Science Foundation of China (No. 21801184), the Tianjin Municipal Applied Basic and Key Research Scheme of China (No. 18JCQNJC06400), Xiamen University, Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (No. SN-ZJU-SIAS-006), Université de Lyon, the French Agence Nationale de la Recherche (DynaSweet, ANR-08-BLAN-0305), Glyco@Alps (ANR-15-IDEX-02), and Labex Arcane/CBH-EUR-GS (ANR-17-EURE-0003) are gratefully acknowledged.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.107872.
| [1] |
A. Varki, R. Cummings, J. Esko, H. Freeze, G.W. Hart, J. Marth, Essentials of Glycobiology. 2nd ed.. New York: Cold Spring Harbor Laboratory Press, 1999.
|
| [2] |
P.C. Pang, P.C. Chiu, C.L. Lee, et al., Science 333 (2011) 1761-1764. DOI:10.1126/science.1207438 |
| [3] |
A. Varki, Glycobiology 3 (1993) 97-130. DOI:10.1093/glycob/3.2.97 |
| [4] |
Y.C. Lee, R.T. Lee, Acc. Chem. Res. 28 (1995) 321-327. DOI:10.1021/ar00056a001 |
| [5] |
B. Ernst, G.W. Hart, P. Sinaÿ, Carbohydrates in Chemistry and Biology, Wiley-VCH, Weinheim, 2000.
|
| [6] |
S. Andre, F. Sansone, H. Kaltner, et al., ChemBioChem 9 (2008) 1649-1661. DOI:10.1002/cbic.200800035 |
| [7] |
A.S.A. Mohammed, M. Naveed, N. Jost, J. Polym. Environ. 29 (2021) 2359-2371. DOI:10.1007/s10924-021-02052-2 |
| [8] |
I. Pramudya, H. Chung, Biomater. Sci. 7 (2019) 4848-4872. DOI:10.1039/c9bm01385g |
| [9] |
Y. Kim, J.Y. Hyun, I. Shin, Chem. Soc. Rev. 50 (2021) 10567-10593. DOI:10.1039/d0cs01606c |
| [10] |
A. Imberty, Y.M. Chabre, R. Roy, Chem. Eur. J. 14 (2008) 7490-7499. DOI:10.1002/chem.200800700 |
| [11] |
F. Seidi, R. Jenjob, T. Phakkeeree, D. Crespy, J. Control. Release 284 (2018) 188-212. DOI:10.1016/j.jconrel.2018.06.026 |
| [12] |
A. Bernardi, J. Jiménez-Barbero, A. Casnati, et al., Chem. Soc. Rev. 42 (2013) 4709-4727. DOI:10.1039/C2CS35408J |
| [13] |
S. Cecioni, A. Imberty, S. Vidal, Chem. Rev. 115 (2015) 525-561. DOI:10.1021/cr500303t |
| [14] |
V. Verez-Bencomo, V. Fernández-Santana, E. Hardy, et al., Science 305 (2004) 522-525. DOI:10.1126/science.1095209 |
| [15] |
A. Imberty, M. wimmerová, E.P. Mitchell, N. Gilboa-Garber, Microbes Infect. 6 (2004) 221-228. DOI:10.1016/j.micinf.2003.10.016 |
| [16] |
N. Gilboa-Garber, Methods Enzymol. 83 (1982) 378-385. |
| [17] |
G. Cioci, E.P. Mitchell, C. Gautier, et al., FEBS Lett. 555 (2003) 297-301. DOI:10.1016/S0014-5793(03)01249-3 |
| [18] |
T. Branson, T. McAllister, J. Garcia-Hartjes, et al., Angew. Chem. Int. Ed. 53 (2014) 8323-8327. DOI:10.1002/anie.201404397 |
| [19] |
S. Kuhaudomlarp, L. Cerofolini, S. Santarsia, et al., Chem. Sci. 11 (2020) 12662-12670. DOI:10.1039/d0sc03741a |
| [20] |
D. Boffoli, F. Bellato, G. Avancini, et al., Drug Deliv. Transl. Res. 12 (2021) 1-23. |
| [21] |
C. O'Reilly, S. Blasco, B. Parekh, et al., RSC Adv. 11 (2021) 16318-16325. DOI:10.1039/d0ra05107a |
| [22] |
G. Paulíková, J. Houser, M. Kašáková, et al., Sci. Rep. 9 (2019) 1-12. DOI:10.1038/s41598-018-37186-2 |
| [23] |
K. Chakroun, M. Taouai, V. Porkolab, et al., J. Med. Chem. 64 (2021) 14332-14343. DOI:10.1021/acs.jmedchem.1c00818 |
| [24] |
G.M.L. Consoli, G. Granata, V. Cafiso, S. Stefani, C. Geraci, Tetrahedron Lett. 52 (2011) 5831-5834. DOI:10.1016/j.tetlet.2011.08.142 |
| [25] |
S. Cecioni, R. Lalor, B. Blanchard, et al., Chemistry 15 (2009) 13232-13240. DOI:10.1002/chem.200901799 |
| [26] |
S. Cecioni, S. Faure, U. Darbost, et al., Chem. Eur. J. 17 (2011) 2146-2159. DOI:10.1002/chem.201002635 |
| [27] |
D. Sicard, S. Cecioni, M. Iazykov, et al., Chem. Commun. 47 (2011) 9483-9485. DOI:10.1039/c1cc13097h |
| [28] |
S. Cecioni, S.E. Matthews, H. Blanchard, et al., Carbohydr. Res. 356 (2012) 132-141. DOI:10.1016/j.carres.2012.02.006 |
| [29] |
A.M. Boukerb, A. Rousset, N. Galanos, et al., J. Med. Chem. 57 (2014) 10275-10289. DOI:10.1021/jm500038p |
| [30] |
S. Cecioni, J.P. Praly, S.E. Matthews, et al., Chem. Eur. J. 18 (2012) 6250-6263. DOI:10.1002/chem.201200010 |
| [31] |
A. Dondoni, A. Marra, Chem. Rev. 110 (2010) 4949-4977. DOI:10.1021/cr100027b |
| [32] |
Z.H. Soomro, S. Cecioni, H. Blanchard, et al., Org. Biomol. Chem. 9 (2011) 6587-6597. DOI:10.1039/c1ob05676j |
| [33] |
T. Furuike, S. Aiba, S.I. Nishimura, Tetrahedron 56 (2000) 9909-9915. DOI:10.1016/S0040-4020(00)00962-5 |
| [34] |
Y.M. Chabre, R. Roy, Chem. Soc. Rev. 42 (2013) 4657-4708. DOI:10.1039/c3cs35483k |
| [35] |
Y. Chen, H. Vedala, G.P. Kotchey, et al., ACS Nano 6 (2012) 760-770. DOI:10.1021/nn2042384 |
| [36] |
H. Vedala, Y. Chen, S. Cecioni, et al., Nano Lett. 11 (2011) 170-175. DOI:10.1021/nl103286k |
| [37] |
D. Sicard, Y. Chevolot, E. Souteyrand, et al., J. Mol. Recognit. 26 (2013) 694-699. DOI:10.1002/jmr.2333 |
| [38] |
J.F. Nierengarten, J. Iehl, V. Oerthel, et al., Chem. Commun. 46 (2010) 3860-3862. DOI:10.1039/c0cc00034e |
| [39] |
M. Durka, K. Buffet, J. Iehl, et al., Chem. Commun. 47 (2011) 1321-1323. DOI:10.1039/C0CC04468G |
| [40] |
I. Nierengarten, J.F. Nierengarten, Chem. Asian J. 9 (2014) 1436-1444. DOI:10.1002/asia.201400133 |
| [41] |
A. Muñoz, D. Sigwalt, B.M. Illescas, et al., Nat. Chem. 8 (2016) 50-57. DOI:10.1038/nchem.2387 |
| [42] |
J. Luczkowiak, A. Munoz, M. Sanchez-Navarro, et al., Biomacromolecules 14 (2013) 431-437. DOI:10.1021/bm3016658 |
| [43] |
M. Durka, K. Buffet, J. Iehl, et al., Chem. Eur. J. 18 (2012) 641-651. DOI:10.1002/chem.201102052 |
| [44] |
M. Sánchez-Navarro, A. Muñoz, B.M. Illescas, J. Rojo, N. Martín, Chem. Eur. J. 17 (2011) 766-769. DOI:10.1002/chem.201002816 |
| [45] |
P. Compain, C. Decroocq, J. Iehl, et al., Angew. Chem. 122 (2010) 5889-5892. DOI:10.1002/ange.201002802 |
| [46] |
R. Rísquez-Cuadro, J.M. Garcia Fernandez, J.F. Nierengarten, C. Ortiz Mellet, Chem. Eur. J. 19 (2013) 16791-16803. DOI:10.1002/chem.201303158 |
| [47] |
S. Cecioni, V. Oerthel, J. Iehl, et al., Chem. Eur. J. 17 (2011) 3252-3261. DOI:10.1002/chem.201003258 |
| [48] |
T. Mohy El Dine, R. Jimmidi, A. Diaconu, et al., J. Med. Chem. 64 (2021) 14728-14744. DOI:10.1021/acs.jmedchem.1c01241 |
| [49] |
K. Buffet, I. Nierengarten, N. Galanos, et al., Chem. Eur. J. 22 (2016) 2955-2963. DOI:10.1002/chem.201504921 |
| [50] |
S.P. Vincent, K. Buffet, I. Nierengarten, A. Imberty, J.F. Nierengarten, Chem. Eur. J. 22 (2016) 88-92. DOI:10.1002/chem.201504110 |
| [51] |
N. Galanos, E. Gillon, A. Imberty, S.E. Matthews, S. Vidal, Org. Biomol. Chem. 14 (2016) 3476-3481. DOI:10.1039/C6OB00220J |
| [52] |
L.T. Wu, C. Han, X.B. Jing, Y. Yao, Chin. Chem. Lett. 32 (2021) 3322-3330. DOI:10.1016/j.cclet.2021.04.046 |
| [53] |
G.P. Sun, L.T. Pu, S. Pangannaya, et al., Front. Chem. 7 (2019) 743. DOI:10.3389/fchem.2019.00743 |
| [54] |
K. Wang, X. Tian, J.H. Jordan, et al., Chin. Chem. Lett. 33 (2022) 89-96. DOI:10.1016/j.cclet.2021.06.026 |
| [55] |
T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [56] |
M. Xue, Y. Yang, X.D. Chi, Z.B. Zhang, F.H. Huang, Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [57] |
I. Nierengarten, M. Holler, M. Rémy, et al., Molecules 26 (2021) 2358. DOI:10.3390/molecules26082358 |
| [58] |
K. Shang, Y. Wang, Y.C. Lu, Isr. J. Chem. 58 (2018) 1241-1245. DOI:10.1002/ijch.201800080 |
| [59] |
X.W. Wu, Y. Zhang, Y.C. Lu, et al., J. Mater. Chem. B 5 (2017) 3483-3487. DOI:10.1039/C7TB00752C |
| [60] |
X. Liu, W. Shao, Y.J. Zheng, et al., Chem. Commun. 53 (2017) 8596-8599. DOI:10.1039/C7CC04932C |
| [61] |
P. Demay-Drouhard, K. Du, K. Samanta, et al., Org. Lett. 21 (2019) 3976-3980. DOI:10.1021/acs.orglett.9b01123 |
| [62] |
K. Du, A.C.H. Sue, Synlett 30 (2019) 2209-2215. DOI:10.1055/s-0037-1611921 |
| [63] |
M.J. Guo, X.M. Wang, C.H. Zhan, et al., J. Am. Chem. Soc. 140 (2018) 74-77. DOI:10.1021/jacs.7b10767 |
| [64] |
J.Y. Zhao, W.W. Yang, C.Y. Liang, et al., Chem. Commun. 57 (2021) 11193-11196. DOI:10.1039/d1cc04840f |
| [65] |
Y.C. Chang, Y.H. Lv, P. Wei, et al., Adv. Funct. Mater. 27 (2017) 1703083-1703095. DOI:10.1002/adfm.201703083 |
| [66] |
A. Bianchi, A. Bernardi, J. Org. Chem. 71 (2006) 4565-4577. DOI:10.1021/jo060409s |
| [67] |
L. Mahal, A. Sanki, Synlett 2006 (2006) 0455-0459. DOI:10.1055/s-2006-926264 |
| [68] |
L.M. Artner, L. Merkel, N. Bohlke, et al., Chem. Commun. 48 (2012) 522-524. DOI:10.1039/C1CC16039G |
| [69] |
K.S. Bücher, P.B. Konietzny, N.L. Snyder, L. Hartmann, Chem. Eur. J. 25 (2019) 3301-3309. |
| [70] |
C. Pető, G. Batta, Z. Györgydeák, F. Sztaricskai, Liebigs Ann. Chem. 1991 (1991) 505-507. DOI:10.1002/jlac.199119910192 |
| [71] |
L. Han, W. Sheng, X. Li, et al., MedChemComm 10 (2019) 598-605. DOI:10.1039/c9md00036d |
| [72] |
C. Palomo, J.M. Aizpurua, E. Balentová, et al., Org. Lett. 10 (2008) 2227-2230. DOI:10.1021/ol8006259 |
| [73] |
S. Wang, N. Galanos, A. Rousset, et al., Carbohydr. Res. 395 (2014) 15-18. DOI:10.1016/j.carres.2014.06.002 |
| [74] |
K. Du, P. Demay-Drouhard, K. Samanta, et al., J. Org. Chem. 85 (2020) 11368-11374. DOI:10.1021/acs.joc.0c01464 |
 2023, Vol. 34
2023, Vol. 34