b State Key Laboratory of Chemical Engineering, Stoddart Institute of Molecular Science, Department of Chemistry, Zhejiang University, Hangzhou 310027, China;
c Green Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou 450001, China;
d Department of Chemistry, University of Utah, Salt Lake City, UT 84112, United States
Supramolecular self-assemblies have attracted great attention not only because of their methodological importance, but also due to their successful applications in sensors [1-6], catalysis [7-13], medical reagents [14-18] and smart materials [19-26]. Coordination-driven transition-metal mediated self-assembly is one of the highly efficient approaches for the construction of discrete or consecutive metal organic cycles/cages (COCs) with programmed shapes and geometries [27-31]. By incorporating functional macrocycles, such as porphyrin [32-36], calixarene [37-39], cavitand [40-42], and pillararene [43-46] into the discrete assemblies, artificial functional nanoscale devices with precisely controlled geometries can be fabricated. Because of their interesting host-guest properties with metal and organic cations, crown ethers have attracted much attention and have been widely used in cation transport and separation, chemosensors, molecular machines and supramolecular polymers [47-54]. Previous works have demonstrated that crown ethers can also be used as flexible building blocks in the construction of discrete complexes [55-58]. As the earliest and most prominent class of crown ethers, 18-crown-6 (18C6) is well known for its strong binding affinity to various metal and ammonium cations, which promote their applications in many fields [59-65], including phase transfer catalysis, superconductors, crystal engineering and mass spectrometry.
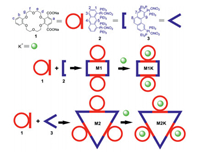
In the present work, we combined a dicarboxylate-functionalized dibenzo crown ether 1 with the well-established platinum(Ⅱ)-oxygen coordination driven self-assembly to report the syntheses, spectroscopic characterization and host-guest chemistry of an A2D2 (A = acceptor and D = donor) rectangle metallacycle M1 and an A3D3 triangle metallacycle M2 based on 18-crown-6, respectively (Scheme 1, Schemes S2 and S3 in Supporting information).
|
Download:
|
| Scheme 1. Self-assembly of dibenzo-18-crown-6-functionalized metallacycles M1 and M2 and their complexation with K+. | |
The dicarboxylate-functionalized dibenzo 18C6 donor 1 was easily synthesized in four steps from commercially available reagents (Scheme S1 in Supporting information). As shown in Scheme 1, the neutral rectangle M1 and triangle M2 were then prepared via two component coordination-driven self-assembly by stirring 1 with either the anthracene-based clip 2 or the 60° phenanthrene-based acceptor 3 respectively, in a 1:1 ratio H2O/CH2Cl2 solution. As the reaction proceeded, the metallacycles were extracted into the organic phase, while the inorganic byproduct remained in the aqueous solution. After washing with water to remove the residual inorganic salts, pure metallacycles were furnished in almost quantitative yields. As far as we know, this is the first report to perform coordination-driven self-assembly in a biphase system.
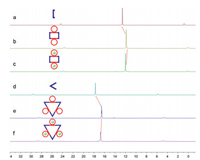
The 31P{1H} spectroscopy supported the formation of single, discrete 2D metallacycles with highly symmetric structures (Fig. 1). For example, the 31P{1H} NMR spectra for M1 and M2 showed a sharp singlet at δ 11.85 (Fig. 1b) and 16.55 ppm (Fig. 1e), respectively, suggesting a single phosphorus environment as expected. The peaks of M1 and M2 shifted upfield from the starting platinum acceptors 2 (δ 12.59 ppm, Fig. 1a) and 3 (δ 17.73 ppm, Fig. 1d). Since 18-crown-6 is an excellent host for selective complexation of potassium cation, we investigated the host-guest behavior of these two neutral metallacycles. After the addition of corresponding KPF6 respectively, the 31P NMR spectra still showed sharp singlets, but they shifted downfield slightly (δ 11.99 ppm for M1K and δ 16.79 ppm for M2K) (Figs. 1c and f). These results indicated that the binding of the K+ slightly changed the chemical environment of the phosphorus atoms.
|
Download:
|
| Fig. 1. 31P{1H} NMR (121.4 MHz, CD2Cl2/CD3COCD3 = 2:1, 22 ℃) spectra: (a) 2; (b) M1; (c) M1 + 2 equiv. KPF6; (d) 3; (e) M2; (f) M2 + 3 equiv. KPF6. | |
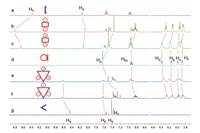
The 1H NMR spectra of M1 and M2 (Figs. 2b and f) also confirmed the highly symmetric discrete structures and displayed significant spectroscopic differences from the precursor building blocks 2 and 3 respectively (Fig. 2). The upfield shifts of Ha, Hb and Hc after the dicarboxylic acid 1-A (Fig. 2) was incorporated into the rectangle M1 or the triangle M2 could be attributed to the formation of new Pt-O coordinate bonds (Pt-OOC- instead of Pt-ONO2). The upfield shifts of Hd-g indicated the metal-coordination could also affect the ethyleneoxy protons on 1 by decreasing the electron density of the crown ether. After the addition of KPF6, except for H5, the protons of M1 and M2 on the clip acceptor 2 shifted downfield somehow (Figs. 2c and e), especially for protons on 1. This is consistent with the complex-induced slight downfield shifts observed in 31P{1H} NMR spectra, and the formation of M1K and M2K with the electron-deficient potassium cations.
|
Download:
|
| Fig. 2. Partial 1H NMR (400 MHz, CD2Cl2/CD3COCD3 = 2:1, 22 ℃) spectra: (a) 2, (b) M1; (c) M1 + 2 equiv. KPF6; (d) dicarboxylic acid 1-A; (e) M2 + 3 equiv. KPF6; (f) M2; (g) 3. | |
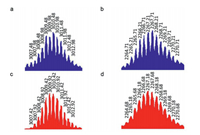
Further evidence for the formation of the desired self-assembled metallacycles was obtained by electrospray ionization mass spectrometry (ESI-MS) studies. For M1, peaks were observed corresponding to [M1 + H]+ (m/z 2971.51), [M1 + K]+ (m/z 3010.48), [M1 + H + K]2+ (m/z 1505.75) and [M1 + 2 K]2+ (m/z 1524.23). These were all isotopically resolved and in excellent agreement with their theoretical distributions (Figs. 3a and c, Fig. S4 in Supporting information). For M2, peaks were also found in excellent agreement with their theoretical isotopical distributions: [M2 + K]+ (m/z 4495.14), [M2 + 2H]2+ (m/z 2229.63), [M2 + H + K]2+ (m/z 2248.64), [M2 + Na + K]2+ (m/z 2259.62) and [M2 + 2 K]2+ (m/z 2267.71), as shown in Figs. 3b and d, Figs. S5 and S6 (Supporting information). We also found that no matter if univalent or bivalent components in their mass spectra, compared with other complexes, the highest peak is the one with K+. For instance, [M1 + K]+ (m/z 3010.48) in Fig. S4 and [M2 + 2 K]2+ (m/z 2267.71) in Fig. S6. This further indicated the selective host-guest complexation of the neutral rectangle M1 and triangle M2 with K+.
|
Download:
|
| Fig. 3. ESI mass spectra of (a) [M1 + K]+ and (b) [M2 + 2 K]2+ and their simulated spectra (c and d, respectively). | |
In summary, a new dibenzo 18-crown-6 ether dicarboxylate donor 1 with 180° coordination angle was designed and synthesized. By employing this new unit, a discrete A2D2 rectangle M1 and an A3D3 triangle M2 were synthesized via coordination-driven self-assembly and characterized by 31P{1H} NMR, 1H NMR and electrospray ionization mass spectrometry. The complexation of the two metallacycles with K+ was further studied to present that the host-guest recognition of crown ethers did not interfere with the coordination-driven self-assembly. The convenient preparation and simple purification of these metallacycles make it possible to prepare crown ether functionalized self-assemblies for catalysis, molecular devices or chemical sensors.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsS. Li thanks the National Natural Science Foundation of China (Nos. 22071040 and 21773052), the Natural Science Foundation of Zhejiang Province (No. LY22B040001) and the Science & Technology Innovation Program of Zhejiang Province (No. 2018R52051). F. Huang thanks the National Natural Science Foundation of China (Nos. 22035006 and 21620102006) for financial support.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.05.035.
| [1] |
M.H. Keefe, K.D. Benkstein, J.T. Hupp, Coord. Chem. Rev. 205 (2000) 201-228. DOI:10.1016/S0010-8545(00)00240-X |
| [2] |
Y. Guo, S. Shao, J. Xu, Y. Shi, S. Jiang, Tetrahedron Lett. 45 (2004) 6477-6480. DOI:10.1016/j.tetlet.2004.06.109 |
| [3] |
H. Wang, X. Yan, C.E. Hauke, et al., J. Am. Chem. Soc. 137 (2015) 15276-15286. DOI:10.1021/jacs.5b10130 |
| [4] |
S. Zhang, I. Boussouar, H. Li, Chin. Chem. Lett. 32 (2021) 642-648. DOI:10.1016/j.cclet.2020.06.035 |
| [5] |
Y. Liu, T. Shen, J. Li, et al., ACS Sens. 2 (2017) 1430-1434. DOI:10.1021/acssensors.7b00313 |
| [6] |
Y. Sun, P.J. Stang, Aggregate 2 (2021) e94. |
| [7] |
M. Fujita, Y.J. Kwon, S. Washizu, K. Ogura, J. Am. Chem. Soc. 116 (1994) 1151-1152. DOI:10.1021/ja00082a055 |
| [8] |
J. Kang, J. Rebek, Nature 385 (1997) 50-52. DOI:10.1038/385050a0 |
| [9] |
M. Yoshizawa, M. Tamura, M. Fujita, Science 312 (2006) 251-254. DOI:10.1126/science.1124985 |
| [10] |
V. Vajpayee, H. Kim, A. Mishra, et al., Dalton Trans. 40 (2011) 3112-3115. DOI:10.1039/c0dt01481h |
| [11] |
A.C. Reyes, T.L. Amyes, J.P. Richard, J. Am. Chem. Soc. 138 (2016) 15251-15259. DOI:10.1021/jacs.6b09936 |
| [12] |
J. Jiang, B.D. Sherman, Y. Zhao, et al., ACS Appl. Mater. Interfaces 9 (2017) 19529-19534. DOI:10.1021/acsami.7b05173 |
| [13] |
T. Hong, Z. Zhang, Y. Sun, et al., J. Am. Chem. Soc. 142 (2020) 10244-10249. DOI:10.1021/jacs.0c01563 |
| [14] |
V. Vajpayee, Y.H. Song, Y.J. Yang, et al., Organometallics 30 (2011) 6482-6489. DOI:10.1021/om200908c |
| [15] |
T.R. Cook, V. Vajpayee, M.H. Lee, P.J. Stang, K.W. Chi, Acc. Chem. Res. 46 (2013) 2464-2474. DOI:10.1021/ar400010v |
| [16] |
C. Mari, V. Pierroz, S. Ferrari, G. Gasser, Chem. Sci. 6 (2015) 2660-2686. DOI:10.1039/C4SC03759F |
| [17] |
J.H. Jo, N. Singh, D. Kim, et al., Inorg. Chem. 56 (2017) 8430-8438. DOI:10.1021/acs.inorgchem.7b01101 |
| [18] |
G.Y. Wu, X. Shi, H. Phan, et al., Nat. Commun. 11 (2020) 3178. DOI:10.1038/s41467-020-16940-z |
| [19] |
S. Tashiro, M. Tominaga, Y. Yamaguchi, K. Kato, M. Fujita, Angew. Chem. Int. Ed. 45 (2006) 241-244. DOI:10.1002/anie.200502802 |
| [20] |
F. Wang, J. Zhang, X. Ding, et al., Angew. Chem. Int. Ed. 49 (2010) 1090-1094. DOI:10.1002/anie.200906389 |
| [21] |
Z. Zhang, Y. Luo, J. Chen, et al., Angew. Chem. Int. Ed. 50 (2011) 1397-1401. DOI:10.1002/anie.201006693 |
| [22] |
S. Dong, B. Zheng, F. Wang, F. Huang, Acc. Chem. Res. 47 (2014) 1982-1994. DOI:10.1021/ar5000456 |
| [23] |
Y. Sun, C. Chen, P.J. Stang, Acc. Chem. Res. 52 (2019) 802-817. DOI:10.1021/acs.accounts.8b00663 |
| [24] |
T. Xiao, L. Zhou, X.Q. Sun, et al., Chin. Chem. Lett. 31 (2020) 1-9. DOI:10.1016/j.cclet.2019.05.011 |
| [25] |
Z. Zhang, T. Hong, S. Li, et al., Organometallics 40 (2021) 1-5. DOI:10.1021/acs.organomet.0c00604 |
| [26] |
C. Mu, Z. Zhang, Y. Hou, et al., Angew. Chem. Int. Ed. 60 (2021) 12293-12297. DOI:10.1002/anie.202100463 |
| [27] |
D.L. Caulder, K.N. Raymond, Acc. Chem. Res. 32 (1999) 975-982. DOI:10.1021/ar970224v |
| [28] |
R. Chakrabarty, P.S. Mukherjee, P.J. Stang, Chem. Rev. 111 (2011) 6810-6918. DOI:10.1021/cr200077m |
| [29] |
T.R. Cook, Y.R. Zheng, P.J. Stang, Chem. Rev. 113 (2013) 734-777. DOI:10.1021/cr3002824 |
| [30] |
S. Datta, M.L. Saha, P.J. Stang, Acc. Chem. Res. 51 (2018) 2047-2063. DOI:10.1021/acs.accounts.8b00233 |
| [31] |
Y. Hou, Z. Zhang, L. Ma, et al., CCS Chem. 3 (2021) 3153-3160. DOI:10.1016/s0968-0896(01)00186-9 |
| [32] |
B. Zhu, H. Chen, W. Lin, et al., J. Am. Chem. Soc. 136 (2014) 15126-15129. DOI:10.1021/ja507531b |
| [33] |
Y. Ye, T.R. Cook, S.P. Wang, et al., J. Am. Chem. Soc. 137 (2015) 11896-11899. DOI:10.1021/jacs.5b07529 |
| [34] |
A.N. Oldacre, A.E. Friedman, T.R. Cook, J. Am. Chem. Soc. 139 (2017) 1424-1427. DOI:10.1021/jacs.6b12404 |
| [35] |
Y. Kim, J. Koo, I.C. Hwang, et al., J. Am. Chem. Soc. 140 (2018) 14547-14551. DOI:10.1021/jacs.8b08030 |
| [36] |
G. Yu, B. Zhu, L. Shao, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 6618-6623. DOI:10.1073/pnas.1902029116 |
| [37] |
H. Jude, H. Disteldorf, S. Fischer, et al., J. Am. Chem. Soc. 127 (2005) 12131-12139. DOI:10.1021/ja053050i |
| [38] |
K. Su, F. Jiang, J. Qian, et al., Inorg. Chem. 53 (2014) 18-20. DOI:10.1021/ic4024184 |
| [39] |
S. Wang, X. Gao, X. Hang, et al., J. Am. Chem. Soc. 140 (2018) 6271-6277. DOI:10.1021/jacs.7b13193 |
| [40] |
M. Yamanaka, Y. Yamada, Y. Sei, K. Yamaguchi, K. Kobayashi, J. Am. Chem. Soc. 128 (2006) 1531-1539. DOI:10.1021/ja0555365 |
| [41] |
M. Nakamura, K. Kishimoto, Y. Kobori, et al., J. Am. Chem. Soc. 138 (2016) 12564-12577. DOI:10.1021/jacs.6b07284 |
| [42] |
A. Ovsyannikov, S. Solovieva, I. Antipin, S. Ferlay, Coord. Chem. Rev. 352 (2017) 151-186. DOI:10.1016/j.ccr.2017.09.004 |
| [43] |
Z.Y. Li, Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
| [44] |
G.Y. Wu, X.Q. Wang, L.J. Chen, et al., Inorg. Chem. 57 (2018) 15414-15420. DOI:10.1021/acs.inorgchem.8b02712 |
| [45] |
M. He, L. Chen, B. Jiang, et al., Chin. Chem. Lett. 30 (2019) 131-134. DOI:10.1016/j.cclet.2018.10.035 |
| [46] |
H. Zhu, Q. Li, B. Shi, et al., J. Am. Chem. Soc. 142 (2020) 17340-17345. DOI:10.1021/jacs.0c09598 |
| [47] |
M. Zhang, X. Yan, F. Huang, Z. Niu, H.W. Gibson, Acc. Chem. Res. 47 (2014) 1995-2005. DOI:10.1021/ar500046r |
| [48] |
X. Yan, T.R. Cook, J.B. Pollock, et al., J. Am. Chem. Soc. 136 (2014) 4460-4463. DOI:10.1021/ja412249k |
| [49] |
C. Ren, J. Shen, H. Zeng, J. Am. Chem. Soc. 139 (2017) 12338-12341. DOI:10.1021/jacs.7b04335 |
| [50] |
D. Aoki, G. Aibara, S. Uchida, T. Takata, J. Am. Chem. Soc. 139 (2017) 6791-6794. DOI:10.1021/jacs.7b01151 |
| [51] |
S. Schneider, E.D. Licsandru, I. Kocsis, et al., J. Am. Chem. Soc. 139 (2017) 3721-3727. DOI:10.1021/jacs.6b12094 |
| [52] |
L. Wang, L. Cheng, G. Li, et al., J. Am. Chem. Soc. 142 (2020) 2051-2058. DOI:10.1021/jacs.9b12164 |
| [53] |
X. Li, Y. Deng, J. Lai, G. Zhao, S. Dong, J. Am. Chem. Soc. 142 (2020) 5371-5379. DOI:10.1021/jacs.0c00520 |
| [54] |
J. Yuan, Y. Song, X. Li, et al., Org. Lett. 23 (2021) 9554-9558. DOI:10.1021/acs.orglett.1c03781 |
| [55] |
S. Li, J. Huang, T.R. Cook, et al., J. Am. Chem. Soc. 135 (2013) 2084-2087. DOI:10.1021/ja3118812 |
| [56] |
S. Li, J. Huang, F. Zhou, et al., J. Am. Chem. Soc. 136 (2014) 5908-5911. DOI:10.1021/ja502490k |
| [57] |
Y. Ye, S.P. Wang, B. Zhu, et al., Org. Lett. 17 (2015) 2804-2807. DOI:10.1021/acs.orglett.5b01211 |
| [58] |
Z. Zhou, X. Yan, T.R. Cook, M.L. Saha, P.J. Stang, J. Am. Chem. Soc. 138 (2016) 806-809. DOI:10.1021/jacs.5b12986 |
| [59] |
T. Akutagawa, K. Shitagami, S. Nishihara, et al., J. Am. Chem. Soc. 127 (2005) 4397-4402. DOI:10.1021/ja043527a |
| [60] |
Y. Han, H.Y. Lu, Q.S. Zong, J.B. Guo, C.F. Chen, J. Org. Chem. 77 (2012) 2422-2430. DOI:10.1021/jo3000755 |
| [61] |
Y. Chen, M.T. Rodgers, J. Am. Chem. Soc. 134 (2012) 2313-2324. DOI:10.1021/ja2102345 |
| [62] |
Y.H. Wang, Z.Y. Cao, J. Zhou, J. Org. Chem. 81 (2016) 7807-7816. DOI:10.1021/acs.joc.6b01457 |
| [63] |
L. Martin, A.L. Morritt, J.R. Lopez, et al., Inorg. Chem. 56 (2017) 717-720. DOI:10.1021/acs.inorgchem.6b02708 |
| [64] |
R. Biswas, P. Ghosh, T. Banerjee, S.M. Ali, A.K. Singha Deb, ACS Omega 3 (2018) 1663-1674. DOI:10.1021/acsomega.7b01828 |
| [65] |
M.W. Shi, S.P. Thomas, V.R. Hathwar, et al., J. Am. Chem. Soc. 141 (2019) 3965-3976. DOI:10.1021/jacs.8b12927 |
 2023, Vol. 34
2023, Vol. 34 

