b College of Nuclear Science and Technology, Harbin Engineering University, Harbin 150001, China;
c School of Energy and Power Engineering, Xi'an Jiaotong University, Xi'an 710049, China;
d Laboratory of Nuclear Energy Chemistry, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China
Nuclear power is essential in meeting energy demands while tackling climate change crisis. However, one of the main challenges in safely utilizing nuclear energy lies in preventing the environmental pollution from uranium mining to nuclear waste disposal [1-3]. It is thus of great importance to develop targeted waste forms that can efficiently immobilize various types of radionuclides due to their radioactivity and toxicity [4].
Uranyl silicates are one of the most abundant minerals found in the oxidized zone of U deposits worldwide and are typical alteration products formed during weathering of natural uraninite [5-8]. When interacting with spent nuclear waste in deep ground environments, various structures can be formed due to the flexibility of silicate connectivity [9]. Hence uranium silicates have been explored in its application in nuclear waste immobilization [10-13]. Some uranium silicate compounds have been reported to be capable of immobilizing multiple components of spent nuclear fuel, such as cesium, which is one of the most abundant fission products [9]. Uranium(VI) compounds are dominantly presented in the crust environment, but more mobile than uranium(IV) ones, making it less suitable for immobilizing radionuclides [14]. For instance, coffinite is the only known natural U4+ silicate that form as a primary mineral [15, 16].
Most synthetic explorations have been made towards uranium(VI) compounds in the last few decades [17-20]. Nonetheless, efforts have led to a few novel uranium silicates or germanates (due to their similarities in chemical properties) with oxidation states of uranium other than 6+. For example, Liu et al. [21] obtained Cs2UIVSi6O15 by high-pressure, high-temperature hydrothermal synthesis. On the other hand, two uranium(V) silicates, K(UVO)Si2O6 [22] and K3(U3VO6)(Si2O7) [23], and two uranium(V) germanates, Cs3UVGe7O18 [24] and Rb3(U3VO6)(Ge2O7) [23]. Some mixed-valence uranium silicates and germanates have been reported as well, including [Na9F2][(UVO2)(UVIO2)2(Si2O7)2] [25], Na7UIVO2(UVO)2(UV/VIO2)2SiO6 [26], and Cs8UIV(UVIO2)3(Ge3O9)3·3H2O [27]. The great contrast of the scarcity of these compounds and the abundance of their uranyl counterparts calls for more discovery through synthetic efforts to enrich its crystal chemistry for immobilizing nuclear waste.
Uranium silicates and germanates both exhibit rich connectivity capabilities. Due to the flexibility of orthosilicate ion SiO44−, various silicon units can be formed, such as Si2O7 dimers, Si4O12 squares, chains, sheets, and 3D frameworks [28]. Furthermore, various 0D [29, 30], 1D [31, 32] and 2D [33] silicate units can bond with typical UO6 polyhedra to form diverse uranium silicate compounds. Recently, G. Morrison et al. [9] has successfully synthesized four uranium silicate crystals with salt-inclusion, [AmBnX][(UO2)p(SiqOr)t], by CsCl/CsF flux method. In stacking of uranyl and silicon units, there are diverse U-Si structure with different 10-(ABBBBABBBB), 12-(ABBABBABBABB) and 14-(ABBBBABABBBBAB) membered uranyl silicate rings, indicating the flexibility of this structure. Compared with uranium silicate structure, however, the reports of uranium germanates are relatively limited. The most common GeO4 tetrahedra bond with UO6 polyhedra like SiO4 units, and there are various other modes of germanium coordination with oxygen, such as square pyramids, trigonal bipyramids and octahedra [34]. Moreover, Li et al. [34] synthetized a unique series of open-framework uranyl germanates from different mixed molten fluxes, consisting of 3D 8-, 10-ring channels built upon [UGe4] pentamers and/or [UGe6] heptamers. A pentavalent uranium germanate containing 4- and 6-coordinate germanium was reported by Quang et al. by high-temperature, high-pressure hydrothermal method [24].
Most of the reported uranium(IV) and uranium(V) silicates and germanates are synthesized by high-temperature, high-pressure hydrothermal method [35], and there are a few crystals synthesized by molten salt method. Molten salt electrolysis is a promising technology for reprocessing spent nuclear fuels [36, 37]. Most actinides are recovered and decontaminated from the fission products in the molten salt electrorefining process [38]. Molten salt method has also been proposed to synthesize unique host materials for waste salt after the refining process [9]. On the other hand, molten salt method can provide benefits in novel compound synthesis allowing for the preservation of unusual valence states and ionic species, which would be destabilized in conventional solvents [39]. Moreover, the reaction time can be significantly reduced by using the molten salt method [39, 40]. So far, no uranium(IV) silicate and germanate has been reported using molten salt method. Herein, the synthesis of uranium silicates and germanates with less common valence states (IV, V) by molten salt method is worthy of experimental exploration.
In this work, we report two tetravalent uranium silicate and germanate compounds containing T3O9 trimer by flux method, M2UIVT3O9 (M = K, Cs; T = Si, Ge).
Single crystals M2UIVT3O9 were grown by a mixture of 0.5 mmol U3O8 and 4 mmol SiO2 or GeO2 under a mixture of 20 mmol KBr and 20 mmol KF, and 9 mmol CsF and 11 mmol CsCl, respectively. The starting materials were placed in a silver crucible that was 2.5 cm (top), 1.5 cm (bottom) in diameter and 5.7 cm in height. The silver crucibles were loosely covered with sliver lids and placed in a muffle furnace inside the glove box under inert gas atmosphere and were gradually heated to 800 ℃ in 1.5 h, isothermed for 12 h, and cooled slowly to 400 ℃ or 500 ℃ at a rate of 6 ℃/h, at which temperature the muffle furnace was shut off. After removing redundant salt by washing using deionized water, the crystals were obtained via crystal picking and shown in Fig. S1 (Supporting information). However, the resulting crystals have a low yield (< 10%) and not enough amount can be harvested for further investigation such as powder X-ray diffraction for phase purity determination.
The crystal structure confirmations were executed via intensity data collection on a Bruker D8 CCD diffractometer equipped with a Mo Kα radiation (λ = 0.7107 Å) at 270 K and a CMOS PHOTON 100 detector. An original structure was acquired using Olex2 directly. Further refined structure to determine the final structural model (R1 = 0.0698, 0.0144). A summary of the basic crystallographic data for the two crystals is provided in Table 1, with specific details provided in the Supporting information (Table S1). Moreover, Scanning Electron Microscopy (SEM) equipped with Energy Dispersive Spectroscopy (EDS) confirmed the presence of Cs, K, Ge, Si, U and O in M2UIVT3O9, respectively, shown in Fig. S2 (Supporting information).
|
|
Table 1 Crystallographic data for K2USi3O9 and Cs2UGe3O9. |
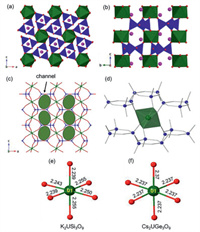
K2USi3O9 (1), crystallizes in the monoclinic space group P121/n1 with lattice parameters a = 7.1076 Å, b = 10.4776 Å, c = 12.2957 Å, γ = 120° and V = 915.67 Å3. The asymmetric unit consist of four U sites, three Si sites, two K sites, and nine O sites. All UO6 octahedra have six long U–O bonds (2.239–2.256 Å), which agrees with the other U(IV) inorganic compound [27]. Using the bond valences parameters R = 2.1 Å and b = 0.373 Å for U(IV)-O the valence of U is calculated to be 4.04, which agrees with the occurrence of U4+ in these sites [41]. The Si atoms coordinate with four O atoms to form a SiO4 tetrahedra. The Si-O bond distances in Si3O9 trimers range between 1.594 and 1.645 Å. These values are in good agreement with values reported in previous studies describing the family of uranium silicate compounds [22, 29]. The specific bond distances are listed in Table S2 (Supporting information). The structure of K2USi3O9 is shown in Figs. 1a and b. Each SiO4 tetrahedron shares two corners with two other tetrahedra to form a silicon oxide trimer (Si3O96−). Each silicon oxide trimer shares its corners with six UO6 octahedra along c direction, forming a 3D uranium silicate framework [USi3O9]2- (Figs. 1b and d). This framework is charge-balanced by K+ cations located in voids formed by the sequence of UO6−SiO4 connectivity. Another channel along the a and b axis of [USi3O9]− framework is ~ 6.79 × 4.29 Å in dimensions, as shown in Fig. 1c.
|
Download:
|
| Fig. 1. Structure of M2UIVT3O9 (a-d) showing the connectivity between UO6 and T3O9, and the bond distances of U-O (Å) (e-f). Color code: U, green; Si/Ge, blue; K/Cs, pink; and O, red. | |
Cs2UGe3O9 (2) crystallizes in a hexagonal space group P-6 with lattice constants of a = 7.5138 Å, b = 7.5138 Å, c = 11.0114 Å, γ = 120° and V = 538.38 Å3. The structure of Cs2UGe3O9 consists of a unique U site, one Ge site, two unique Cs sites and two unique O sites. In this structure, U(1)O6 octahedron contains six long regular U-O bonds (2.237 Å × 6), and the valence of U(1) is calculated to be 4.15 of according to its bond distance, using parameters R = 2.1 Å and b = 0.373 Å [41]. The Ge atoms are tetrahedrally coordinated by O atoms with short Ge-O bond distances, 1.711–1.767 Å. Its specific bond distances are listed in Table S2 (Supporting information). The structure of Cs2UGe3O9 is shown in Figs. 1a and b, which is isostructural to K2USi3O9 described above. Germanium oxide trimer (Ge3O96−) coordinate with UO6 octahedra to form a 3D uranium germanate framework [UGe3O9]2− which is charge-balanced by Cs+ cations. Its channel along the a and b axis of [UGe3O9]− framework is ~6.82 × 5.32 Å in dimensions, as shown in Fig. 1c.
Table S2 lists selected specific interatomic bond distances of the two M2UIVT3O9 (M = K, Cs; T = Si, Ge) structures. Figs. 1e and f showed the U-O bond distances of each unique U atom in both structures. The channel of [USi3O9]2− framework (~6.79 × 4.29 Å) is smaller than that of [UGe3O9]2− one (~6.82 × 5.32 Å). This is consistent with the result that the smaller cation K+ is immobilized in the [USi3O9]2− framework while the [UGe3O9]2− framework traps Cs+ cation.
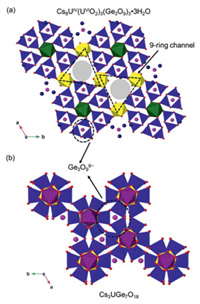
The structures of K2USi3O9 and Cs2UGe3O9 consist of three-membered single-ring Si3O96− and Ge3O96− trimers. These T3O96− trimers strike resemblance with previously reported structures [24]. Nguyen et al. [27] reported mixed-valence uranium germanate Cs8UIV(UVIO2)3(Ge3O9)3·3H2O, in which Ge3O96− trimers connect UIVO6 and UVIO6 to form a three-dimensional framework with 9-ring channels occupied by Cs cations and H2O molecules (Fig. 2a); whereas in Cs2UGe3O9, the channels are only occupied by Cs cations. On the other hand, they also [24] reported a Cs3UGe7O18 structure, where Ge3O96− trimers coordinate with UVO6 and GeO6 to form a 3D framework, with Ge−O−Ge bond angles of 130. 39 (17)°, 124. 55 (16)° and 127. 69 (17)°, respectively (Fig. 2b). Both previously reported structures (Cs8UIV(UVIO2)3(Ge3O9)3·3H2O and Cs2UGe7O18) were, however, obtained by high-temperature, high-pressure hydrothermal reactions, in which the reactions were heated at 585 ℃ for 2 and 4 days with a slow cooling rate of 2 ℃/h. Furthermore, there were no previous reports on Si3O96− related frameworks.
|
Download:
|
| Fig. 2. Structures of (a) Cs8UIV(UVIO2)3(Ge3O9)3·3H2O and (b) Cs2UVGe7O18. Color code: UO6 octahedra: yellow and green polyhedra, GeO6 octahedra: dark purple polyhedra, GeO4 tetrahedra: blue polyhedra, Cs cations: purple circles, O atoms: dark blue circles. Crystal structure images redrawn from the cif files of [24, 27]. | |
The two M2UIVT3O9 (M = K, Cs; T = Si, Ge) structures were optimized by density functional theory (DFT) calculations. More details are given in Supporting information. Compared to the experimentally measured bond distance, the calculated bond distances of U-O are between 2.27 and 2.29 Å. The similar bond distances indicate that both structures are stable. The optimized CIF file can be found in the Supporting Information. The calculated formation enthalpy is −6.30 and −5.36 and eV/atom for K2USi3O9 and Cs2UGe3O9, respectively. Their relatively large absolute values demonstrate the high stability of these two kinds of compounds. The total and projected density of states (DOS) of each element for these three compounds are illustrated in Fig. 3. For the structures of K2USi3O9 and Cs2UGe3O9, their orbitals of U and O atoms are hybridized in the range of about −21 to −16, and −10 to 0 eV, respectively, indicating the strong U-O bonds. Similarly, there are overlaps between the Si and O orbitals of K2USi3O9 and between the Ge and O orbitals of Cs2UGe3O9 in a wide range, indicating the strong Si-O and Ge-O bonds. In a word, the strong interaction between metal atoms and oxygen atoms leads to the high stability of these two compounds.

|
Download:
|
| Fig. 3. The total and projected density of states (DOS) of each element for (a) Cs2UGe3O9, (b) K2USi3O9. | |
In summary, we have successfully synthesized two tetravalent uranium silicate and germanate crystals containing a three-membered single-ring T3O96− under flux growth method under inert gas atmosphere. Bond valence sum calculations have confirmed the valence states of uranium to be 4+. K2USi3O9 is the first uranium silicate that contains the Si3O96− trimers. Molten salt method under inactive gas have demonstrated its potential in synthesizing a variety of uranium compounds with less common valence states, and immobilizing different types of radionuclides such as uranium and cesium in one crystal structure. More work is being conducted in exploring novel uranium structures by this group.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Science Fund for Young Scholars (No. 22106165), the National Science Fund for Distinguished Young Scholars (No. 21925603), the Major Program of the National Natural Science Foundation of China (No. 21790373) and the National Natural Science Foundation of China (No. U20B2020).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.026.
| [1] |
Y.K. Kim, T. Kim, Y. Kim, et al., J. Hazard. Mater. 340 (2017) 130-139. DOI:10.4062/biomolther.2016.075 |
| [2] |
Z.W. Huang, K.Q. Hu, L. Mei, et al., Dalton. Trans. 49 (2020) 983-987. DOI:10.1039/c9dt04158c |
| [3] |
K.Q. Hu, Z.W. Huang, Z.H. Zhang, et al., Inorg. Chem. 24 (2018) 16766-16769. DOI:10.1002/chem.201804284 |
| [4] |
P.C. Zhang, L. Wang, Z.W. Huang, et al., ACS Appl. Mater. Interfaces 12 (2020) 15579-15587. DOI:10.1021/acsami.0c00861 |
| [5] |
C. Frondel, U.S. Geol, Surv. Bull. 1064 (1958) 1400. DOI:10.1212/WNL.46.2.379 |
| [6] |
R.J. Finch, R.C. Ewing, J. Nucl. Mater. 190 (1992) 133-156. DOI:10.1016/0022-3115(92)90083-W |
| [7] |
R.J. Finch, T. Murakami, Rev. Mineral. 38 (1999) 91-180. DOI:10.1515/9781501509193-008 |
| [8] |
E.C. Pearcy, J.D. Prikryl, W.M. Murphy, B.W. Leslie, Appl. Geochem. 9 (1994) 713-732. DOI:10.1016/0883-2927(94)90030-2 |
| [9] |
G. Morrison, M.D. Smith, H.C. Zur Loye, J. Am. Chem. Soc. 138 (2016) 7121-7129. DOI:10.1021/jacs.6b03205 |
| [10] |
C.N. Wilson, MRS OPL 212 (1990) 197-204. DOI:10.1557/PROC-212-197 |
| [11] |
P. Finn, J. Hoh, S. Wolf, S. Slater, J. Bates, Radiochim. Acta 74 (1996) 65-71. DOI:10.1524/ract.1996.74.special-issue.65 |
| [12] |
D.J. Wronkiewicz, J.K. Bates, S.F. Wolf, E.C. Buck, J. Nucl. Mater. 238 (1996) 78-95. DOI:10.1016/S0022-3115(96)00383-2 |
| [13] |
D.J. Wronkiewicz, J.H. Lee, Scientific Basis for Nuclear Waste Management XXII, Vol. 556, Materials Research Society, 1999, pp. 431-438.
|
| [14] |
L. Zhang, S.M. Aksenov, A.M. Kokot, et al., Inorg. Chem. 59 (2020) 5813-5817. DOI:10.1021/acs.inorgchem.0c00385 |
| [15] |
L.H. Fuchs, E. Gebert, Am. Miner. 43 (1958) 243-248. DOI:10.1007/BF01489039 |
| [16] |
V. Dubinchuk, I. Naumova, I.Y. Kravtsova, G. Sidorenko, DMiner. Zhurn. 3 (1981) 81-85. |
| [17] |
C.A. Juillerat, E.E. Moore, G. Morrison, et al., Inorg. Chem. 57 (2018) 11606-11615. DOI:10.1021/acs.inorgchem.8b01729 |
| [18] |
G. Morrison, H.C. zur Loye, Cryst. Growth. Des. 16 (2016) 1294-1299. DOI:10.1021/acs.cgd.5b01408 |
| [19] |
G. Morrison, M.D. Smith, H.C. Zur Loye, Inorg. Chem. 56 (2017) 1053-1056. DOI:10.1021/acs.inorgchem.6b02931 |
| [20] |
Z.W. Huang, K.Q. Hu, L. Mei, et al., Inorg. Chem. 60 (2021) 651-659. DOI:10.1021/acs.inorgchem.0c02473 |
| [21] |
H.K. Liu, K.H. Lii, Inorg. Chem. 50 (2011) 5870-5872. DOI:10.1021/ic200771p |
| [22] |
C.S. Chen, F.S. Lee, K.H. Lii, J. Am. Chem. Soc. 127 (2005) 12208-12209. DOI:10.1021/ja0543853 |
| [23] |
C.H. Lin, C.S. Chen, A.A. Shiryaev, Y.V. Zubavichus, K.H. Lii, Inorg. Chem. 47 (2008) 4445-4447. DOI:10.1021/ic800300v |
| [24] |
Q.B. Nguyen, C.L. Chen, Y.W. Chiang, K.H. Lii, Inorg. Chem. 51 (2012) 3879-3882. DOI:10.1021/ic3000872 |
| [25] |
Y.C. Chang, W. J.Chang, S. Boudin, K.H. Lii, Inorg. Chem. 52 (2013) 7230-7235. DOI:10.1021/ic400854j |
| [26] |
C.S. Lee, C.H. Lin, S.L. Wang, K.H. Lii, Angew. Chem. Int. Ed. 49 (2010) 4254-4256. DOI:10.1002/anie.201001095 |
| [27] |
Q.B. Nguyen, H.K. Liu, W.J. Chang, K.H. Lii, Inorg. Chem. 50 (2011) 4241-4243. DOI:10.1021/ic200508v |
| [28] |
G. Morrison, T.T. Tran, P.S. Halasyamani, H.C. zur Loye, Inorg. Chem. 55 (2016) 3215-3217. DOI:10.1021/acs.inorgchem.6b00242 |
| [29] |
X.H. Mo, S.J. Hwu, Inorg. Chem. 44 (2005) 3121-3126. DOI:10.1021/ic050228t |
| [30] |
Y.H. Chen, H.K. Liu, W.J. Chang, D.L. Tzou, K.H. Lii, J. Solid. State Chem. 236 (2016) 55-60. DOI:10.1016/j.jssc.2015.07.034 |
| [31] |
C.L. Liu, H.K. Liu, W.J. Chang, K.H. Lii, Inorg. Chem. 54 (2015) 8165-8167. DOI:10.1021/acs.inorgchem.5b01390 |
| [32] |
F.L. Forray, A.M.L. Smith, A. Navrotsky, et al., Geochim. Cosmochim. Acta 127 (2014) 107-119. DOI:10.1016/j.gca.2013.10.043 |
| [33] |
H.K. Liu, W.J. Chang, K.H. Lii, Inorg. Chem. 50 (2011) 11773-11776. DOI:10.1021/ic201857e |
| [34] |
H. Li, E.M. Langer, P. Kegler, G. Modolo, E.V. Alekseev, Inorg. Chem. 57 (2018) 11201-11216. DOI:10.1021/acs.inorgchem.8b01781 |
| [35] |
J. Qiu, P.C. Burns, Chem. Rev. 113 (2013) 1097-1120. DOI:10.1021/cr300159x |
| [36] |
Y.C. Liu, Y.L. Liu, Y. Zhao, et al., J. Nucl. Mater. 532 (2020) 152057. |
| [37] |
Y.C. Liu, Y.L. Liu, L. Wang, et al., J. Nucl. Mater. 542 (2020) 152483. DOI:10.1016/j.jnucmat.2020.152483 |
| [38] |
Y.L. Liu, H. Ren, T.Q Yin, et al., Electrochim. Acta 326 (2019) 134980. |
| [39] |
P. Mahjoor, S.E. Latturner, Inorg. Chem. 49 (2010) 4486-4490. DOI:10.1021/ic902211c |
| [40] |
G. Morrison, C.M. Read, M.D. Smith, H.C. zur Loye, CrystEngComm 17 (2015) 1968-1974. DOI:10.1039/C4CE02430C |
| [41] |
O.C. Gagne, F.C. Hawthorne, Acta Crystallogr. B 71 (2015) 562-578. DOI:10.1107/S2052520615016297 |
 2022, Vol. 33
2022, Vol. 33 


