b The Key Laboratory of Special Function Materials and Structure Design, Ministry of Education, Lanzhou University, Lanzhou 730000, China;
c Radiochemistry Laboratory, School of Nuclear Science and Technology, Lanzhou University, Lanzhou 730000, China
Since September 2020, China has announced to the international community that China's energy system will continue to accelerate the clean and low-carbon transformation under the goal of peaking carbon emissions and achieving carbon neutrality (the dual-carbon target) [1]. Nuclear energy, a safe, clean and efficient energy, will become the main substitute of traditional fossil energy. By August 2021, there are 51 nuclear power plants under operation in China mainland with an installed capacity of 5326 kW, and there are 18 nuclear power plants under construction with an installed capacity of 1902 kW. China adopts the closed nuclear fuel cycle, in which the spent fuel will be reprocessed and high-level radioactive waste (HLW) was eventually generated during the reprocessing [2]. HLW is the most difficult type of radioactive waste for treatment and disposal because of its high specific activity, long half-life and high biological toxicity. The disposal of HLW has become one of the bottlenecks restricting the sustainable development of nuclear energy in China.
At present, deep geological disposal is considered to be the most safe and feasible disposal method for HLW. Radionuclide (RN) migration studies include both laboratory-scale and in-situ experiments, collected data and established models from which can provide direct data and model support for the design, construction, operation and safety assessment of the HLW repository. Thus, studies on RN migration are indispensable for realizing HLW geological disposal. According to reports by Wang et al. [3], China's RN migration studies included: (1) determination of sorption and diffusion parameters of the key RNs in the back-filling materials and the host-rock in near-field HLW repository; (2) study of speciation and colloidal behavior of the key RNs under near-field disposal conditions; (3) study of long-term corrosion stability of the HLW glass and its outer packaging materials under disposal conditions; (4) mastering of the in-situ RN migration experimental techniques and methods. China's nuclide migration study began in the 1980s. Despite the limited investment in human and financial resources, China has still established a good research foundation in the aforementioned aspects 1–3. China's first underground research laboratory (URL) for geological disposal of HLW is about to start construction in Beishan area (Gansu, China) and in-situ RN migration experiments will be carried out simultaneously.
The development of nuclear power in China is facing unprecedented challenges, which provides a great opportunity for domestic studies of HLW disposal and RN migration. Although there is still a gap with the international advanced level, China's research level in the field of RN migration has been significantly improved in recent years. To some extent, the rapid development of geology, environmental science, chemistry and other disciplines in China has also driven the development of RN migration study. Due to the limited space, this review only summarizes the progress of China's RN migration studies over the past decade regarding three aspects, RN sorption, RN transport and radioactive colloid. Some results from other disciplines are also included in this review, because although these studies were not performed for the HLW geological disposal, their results could be applied as important references for RN migration studies.
2. SorptionThe RN sorption on solid medium is believed to be a significant process controlling the environmental behavior of RN in disposal system [4]. It is considered that RN sorption on the medium surface reaches thermodynamic equilibrium state. The sorption normally is described by distribution coefficient, Kd, the ratio of solid phase concentration to liquid phase concentration of RN. Inconsistent to the sorption percentage, Kd is independent of the amount of adsorbent, resulting in a more convenient comparison of sorption performance between different adsorbents. Batch sorption experiments are carried out to determine Kd under varying chemical conditions, such as pH, ionic strength, RN concentration. In the experiment, a known amount of adsorbent (i.e., clay, oxide) is added to the solution with known RN concentration and experimental condition is controlled. When RN sorption reaches equilibrium, solid and liquid phases are separated by filtration or ultracentrifugation, and RN concentration in solution is then determined. The Kd can be calculated by the following formula:
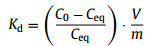
|
(1) |
where C0 is the initial RN concentration; Ceq is the RN concentration; V is the volume of the aqueous solution and m is the mass of the solid adsorbent.
2.1. Batch sorption experimentsAs early as the 1980s, China has carried out studies on RN sorption on different environmental media [5]. Similar to the development trend of adsorption studies abroad, China's studies on adsorption have also experienced a trend from simple oxides to individual clay minerals, and then to complex mineral mixtures.
2.1.1. Sorption on common oxides and clay mineralsRN sorption on single oxide and clay minerals can establish a basis for understanding the sorption behavior of different RN in the environment. Because of simple structure and easy characterization, oxides are more used as model solid phase to clarify the sorption mechanisms as well as the effects of environmental factors (pH, Rn concentration, humic substance) on adsorption. Guo et al. [6-9] and Wang et al. [10] have systematically studied the sorption of Eu(Ⅲ) and U(Ⅵ) on anatase, rutile, silica, γ-alumina and goethite. Mou et al. and Zhang et al. [11, 12] studied the Co(Ⅱ) sorption on γ-alumina and MnO2 under different conditions, respectively.
In the past decade, the experimental data of RN sorption on bentonite/montmorillonite has been further expanded [13-19]. In these studies, bentonite/montmorillonite samples from different regions of China were selected, e.g., Lin'an (Zhejiang), Xinghe (Inner Mongolia) and Jinchuan (Gansu), covering common cations, e.g., Sr(Ⅱ), Ni(Ⅱ), Eu(Ⅲ)/Am(Ⅲ), Np(Ⅴ) and U(Ⅵ). The design of sorption experiments in these studies was systematic, and factors like RN concentration, pH, ionic strength, temperature, CO2, the presence of humic acid (HA)/fulvic acid (FA), etc., were included. These sorption data greatly enriched the understanding of the sorption performance of bentonite and were valuable for the safety assessment of HLW disposal in China. In addition to bentonite/montmorillonite, sorption studies of Sr(Ⅱ), Ni(Ⅱ), Eu(Ⅲ)/Am(Ⅲ), Np(Ⅴ), U(Ⅵ) on other common clay minerals (e.g., kaolinite, rectorite, illite and apatite) have also been reported [20-24]. The batch sorption data on these clays is helpful for the siting of HLW repository which may be built in clayey formation in China.
2.1.2. Sorption on graniteSince the Beishan area (Gansu Province, China) had been investigated as the most possible HLW disposal site in China, domestic research institutions, such as China Institute of Atomic Energy (CIAE), Peking University and Lanzhou University, have successively carried out RN sorption studies on Beishan granite in the context of safety assessment of HLW disposal. As early as 2005, Zhang et al. [25] studied the Pu sorption on granite (collected from 600 m depth underground in Beishan area) as a function of pH, CO32− concentration and O2 concentration. Jiang et al. [26] and Zhou et al. [27] studied the effect of temperature on the sorption of Np(Ⅳ)/Np(Ⅴ) and Am(Ⅲ) on Beishan granite under low oxygen conditions, respectively. It was found that the Am(Ⅲ) sorption reaction was an endothermic, whereas adsorption reactions of Np(Ⅳ)/Np(Ⅴ) may be exothermic because increased temperature did not facilitate the adsorption. Guo et al. [28, 29], Chen et al. [30] and Jin et al. [31, 32] performed systematical experiments to study the sorption of Eu(Ⅲ)/Am(Ⅲ), Se(Ⅳ), Co(Ⅱ)/Ni(Ⅱ) and U(Ⅵ) on Beishan granite samples from different boreholes, discussed the sorption mechanisms of different RNs based on experimental data and XPS analysis, and compared sorption data with the literature data on granite originated from different areas over the world.
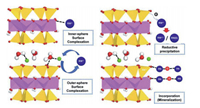
2.2. Sorption mechanismBatch sorption experiment normally does not make any distinction with regard to the mechanism. Overall, the interaction of RN with mineral surfaces includes mainly outer-sphere attachment, inner-sphere surface complexation, incorporation (mineralization) and surface induced reductive precipitation (Fig. 1). In principle, the term "adsorption" refers to outer-sphere attachment and/or inner-sphere surface complexation. For experiment with redox insensitive RN in a short term, "sorption" almost equals to "adsorption". The detailed explanations for these mechanisms are in following.
|
Download:
|
| Fig. 1. Schematic of four sorption reaction mechanisms. | |
Sorption mechanism can be distinguished by advanced spectroscopic techniques, such as X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS), time-resolved laser fluorescence spectroscopy (TRLFS) and X-ray photoelectron spectroscopy (XPS). However, because of the complexity of mineral surfaces (even for single mineral), the discrepancy can be found in the study of surface species with different spectroscopic techniques. Theoretical calculation, which provides detailed structure of the surface complexes at the atomic level, can serve as a useful tool to complement the spectroscopic analysis. In this section, in addition to the application of spectroscopic study and theoretical calculation on defining surface species during adsorption process, progress of experimental and mechanistic studies on incorporation (mineralization) and reductive precipitation are also reviewed.
2.2.1. Adsorption"Outer-sphere attachment" refers to that hydrated cations interact with negatively charged mineral surfaces by purely electrostatic attraction. Such reactions are also well-known as cation exchange to describe cation interactions with permanently charged clay mineral at low pH. "Inner-sphere surface complexation" refers to the formation of direct bonding between cations and mineral surface with significant ionic or covalent contribution (Fig. 1). Compared to outer-sphere adsorption, inner-sphere adsorption on minerals is not affected by varying ionic strength and an apparent hysteresis occurs in desorption process.
Many studies have been performed in combination of batch experiment and advanced spectroscopic techniques to study sorption mechanisms. Sheng et al. [33] studied interaction between Ni(Ⅱ) and diatomite as a function of time, pH, and temperature by batch, XPS, and EXAFS techniques, and found that formation of inner-sphere or outer-sphere surface complexation was dependent to pH, time and Si dissolution. Similar patterns were found in Ni(Ⅱ) adsorption on Ca-montmorillonite, where batch and EXAFS techniques were combined [34]. Ni(Ⅱ) interaction with cryptomelane was studied with multiple techniques (batch, XPS, EXAFS, XRD and FT-IR) by Wu et al. [35], and besides outer-sphere surface complexes, inner-sphere surface complexes in both the edge-shared and double corner-shared modes were found at pH 4.0–7.0. Wang et al. [36] applied TRLFS to study complexation between Eu(Ⅲ), hydrous alumina particles and fulvic acid (FA), and found that Eu(Ⅲ) formed both outer-sphere and inner-sphere surface complexes on alumina surface in the absence of FA, and ternary surface complex (COO−Eu−(O−Al≡)·4H2O) formed in the presence of FA. Niu et al. [37] selected aluminum hydroxide as representative environmental solid to compare effects of fixed and dissolved HA on Eu(Ⅲ)/Yb(Ⅲ) adsorption behaviors and analyzed the adsorption mechanisms with TRLFS, XPS and EXAFS. It was found that although Eu(Ⅲ)/Yb(Ⅲ) formed same type of complexes in the presence of fixed or dissolved HA, fixed and dissolved HA showed apparently different effect on Eu(Ⅲ)/Yb(Ⅲ) adsorption on aluminum hydroxide, implying that effects of HA on trivalent lanthanides/actinides adsorption in the environment might be overestimated.
Theoretical calculation and combinative study of theoretical calculation and spectroscopic analysis were also performed to obtain detailed structure information of surface species by domestic researchers. Detailed structure parameters of uranyl outer-sphere and inner-sphere complexes on hydroxylated SiO2 (001) surface were obtained with periodic density functional theory (DFT) calculation and ab initio molecular dynamics (AIMD) simulation [38, 39]. Wang et al. [40] studied Zn(Ⅱ) adsorption on tetrahedral Si(t) and octahedral Al(o) surfaces of kaolinite, by means of DFT calculations and classical molecular dynamics simulations, and obtained the position and structure for both outer-sphere and mono-/bi-dentate inner-sphere complexes. Coordination structure of U(Ⅵ) on (001) and (012) facets of hematite were investigated by Mei et al. [41] coupling experimental, spectroscopic and theoretical calculation, and an inner-sphere edge-sharing bidentate complex on the hematite (001), and a corner-sharing complex on the (012) facet was deciphered by EXAFS and further verified by DFT calculation. Tan et al. [42] studied structures of uranyl adsorbed on the (100) and (110) surfaces of γ-Al2O3 with DFT calculation, and found that several favorable uranyl structures fit well the available TRLFS and EXAFS data.
2.2.2. Incorporation (mineralization)When mineral dissolution occurs with the geochemical variations, trace amount of RN may form precipitates with chemical species from mineral dissolution and be incorporated into the solid matrix. Besides, the incorporation of RN into some matrix can also be preceded by an adsorption step, i.e., surface complexation can yield nucleation and precipitation of new phases (Fig. 1). However, incorporation into minerals are challenging for some actinides because of incompatible ionic radii.
In recent years, Chinese researchers have made contributions in understanding nucleation and precipitation of new phases. Xu et al. [43] studied the substrate effect of mica and hematite on the nucleation and crystallization of calcite using scanning electron microscope (SEM), X-ray diffraction (XRD), and electron backscatter diffraction (EBSD) methods. They found that calcite crystals initially nucleated from the Ca2+ layers adsorbed on the surfaces. Zhu et al. [44] systemically studied Fe(Ⅱ) sorption in anoxic aqueous suspensions of γ-Al2O3, smectitic clay and amorphous silica using batch experiments complemented with synchrotron X-ray absorption spectroscopic analyses. It was observed that secondary Fe2+ precipitates formed at pH > 7, and precipitation type and precipitation rate were affected by different adsorbents base, pH, and reaction time. Tang et al. [45] determined the effects of simultaneous adsorption of aqueous uranyl onto aluminum oxide over a range of pH and concentration conditions via batch experiments, speciation calculations, X-ray absorption spectroscopy, and XRD, and found that uranyl polymeric species or oxyhydroxide precipitates become more important with increased pH.
Theoretical computational simulation has proved to be a useful tool to study the formation process of metal-phyllosilicate precipitation. Zhang et al. [46] performed first principles molecular dynamics (FPMD) simulations systematically to characterize the chemistry of the incipient clustering processes by taking Pb2+ and Ni2+ as the model cations on clay mineral. The results indicated that Ni2+ can provide available complexing sites (i.e., OH− groups) for the subsequent cations through hydrolysis and the complexation of subsequent cations was thermodynamically favored, resulting in nucleation and precipitation eventually. Zhang et al. [47] also simulated the Ni-phyllosilicate precipitation pathway. These results indicate that the type of metal ion determines whether metal hydroxide surface precipitates form and which type of surface precipitate could form.
2.2.3. Reductive precipitationActinide ions (U-Pu) exist in various oxidation states in aqueous environment. These actinide ions with high oxidation state (e.g., uranyl, neptunyl and plutonyl) are soluble and mobile, especially in the presence of CO32− in a high concentration. Since their accessible reduction products are usually in solid form, the interaction of these actinide ions with reducing minerals (e.g., pyrite, magnetite) couples adsorption and reductive precipitation. The latter is triggered by surface-induced electron transfer processes and affects strongly their mobility (Fig. 1). Similarly, the mobility of anionic Se and Tc can also be attenuated by reductive precipitation after interacting with reducing minerals.
Reductive precipitation study with neptunyl and plutonyl under the environmental conditions was scarce, and U(Ⅵ) had been studied as the representative of highly charged actinide. Pyrite and magnetite are most widely studied reducing minerals. Ma et al. [48] investigated reduction of U(Ⅵ) by magnetite, and indicated that uranyl nitrate or uranyl acetate was mainly reduced to UO2+x oxides (e.g., U4O9, U3O8). Wang et al. [49] compared U(Ⅵ) adsorption on magnetite, ferrihydrite and goethite under anaerobic condition, and reduction of U(Ⅵ) to U(Ⅳ) was found on magnetite. Ma et al. [50] systemically studied U(Ⅵ) reduction by synthetic and natural pyrite. It was found that generation of reducing products (such as U4O9, U3O8, U3O7, and uraninite) mainly resulted from U(Ⅵ) reactions with surface-associated Fe2+ of iron minerals and surface S2− generated from the anoxic grinding process. However, in addition to iron mineral, redox activity of Fe2+ in other mineral structures is low, and U(Ⅵ) was not reduced by biotite surface [51].
Domestic study on reductive precipitation of high oxidation state Tc and Se had an early start [52]. More systematic studies were carried out in the past decade. Kang et al. [53] and Hou et al. [54] investigated reduction of Tc(Ⅶ) with pyrite in groundwater, and observed that Tc(Ⅶ) can be reduced rapidly to Tc(Ⅳ). Wang et al. [55] used Re(Ⅶ) as the analog of Tc(Ⅶ), and studied reduction and re-oxidation of Re by pyrite as a function of pH, O2, presence of humic acid, EDTA and nitrate. It was found that acidic pH and anoxic condition were favored to Re(Ⅶ) reduction because of more abundant electron sources (Fe2+ and S22−). Besides, HA, EDTA and nitrate enhanced the Re remobilization in various degrees. Inconsistent to U(Ⅵ), Se(Ⅳ) can be reduced by Fe2+ in heterogeneous systems containing clay and calcite, as well as Fe2+ bearing minerals (such as magnetite, siderite) [56]. Systematic studies on Se(Ⅳ) reduction by pyrite were performed by Kang et al. [57, 58] and Ma et al. [59]. According to their results, the formation of Se(0) was favorable at acidic condition, whereas the formation of FeSe2 was favorable at nearly neutral to alkaline conditions. Furthermore, the formation of Se(0) is kinetically favorable at the early stage of the reaction, while it will be gradually transformed to FeSe2 after the depletion of aqueous Se(Ⅳ). Formation of Se(0) and FeSe2 on pyrite after interacting with Se(Ⅳ) was also observed by Wang et al. [60] using in-situ scanning electrochemical microscopy (SECM).
2.3. ModelingIn addition to experimental data, adsorption modeling played a vital important role in safety assessment of HLW disposal. Langmuir, Freundlich and other similar isotherm models were established from experimental data under a fixed condition with only varying RN concentration. Thus, these models are insufficient to predict RN adsorption behaviors in the disposal system where chemical condition varied temporally and spatially. Surface complexation models have been developed in order to predict quantitatively and accurately adsorption under varying conditions. In this section, the basic approaches and domestic progress RN complexation modeling with mineral surface and humic substance are summarized.
2.3.1. Basic approachesSurface complexation models treat adsorption processes similar to aqueous speciation schemes. The mass law equation and mole balance formalism are applied after defining surface sites as surface ligands that interact with cationic and anionic RN and form surface complexes. The surface complexation reactions and their thermodynamic constants are determined based on the speciation of RN and then quantitative prediction of RN adsorption on a given sorbent as a function of environmental factors (e.g., pH, ionic strength) is realized.
The construction of surface complexation model starts with the quantitative description of surface site (surface hydroxyl). Generally, it is believed that the hydroxyl groups on the adsorbent surface are amphoteric and their protonation and deprotonation reaction determines the acidity and basicity of the adsorbent surface. Therefore, by determining the adsorbent acid-base properties through potentiometric titration and fitting the titration data in the framework of protonation and deprotonation reaction, the surface site capacity and deprotonation and protonation reaction constants of surface sites can be obtained. Surface charge and potentials affect all surface reactions and many electrostatic models have been proposed to describe the electrostatics at the interface, such as non-electrostatic model (NEM), constant capacitance model (CCM), diffused layer model (DLM), triple layer model (TLM) [4]. Domestic researchers have discussed the applicability of these electrostatic models [23, 61, 62]. For a complex mineral assemblages (e.g., granite), their components are wrapped with each other. The definition of granite surface sites and the electrostatics is therefore a challenge. For such complex mineral assemblage, a modeling strategy of "generalized composition" is proposed [28], in which all surface reactions are assumed to take place on a type of "general" surface sites, and deprotonation and protonation reactions of surface sites are neglected as well as the interface electrostatics. At this time, the site capacity of granite surface is estimated according to empirical formula [28].
2.3.2. Surface complexation modeling for mineralsThe research team from Lanzhou University performed systematic modeling study of Eu(Ⅲ) and U(Ⅵ) adsorption on TiO2, SiO2, γ-alumina and goethite, in which the CCM, NEM and DLM were used to describe surface potential and their applicability were discussed [6-9]. In general, the adsorption of Eu(Ⅲ) can be described by the formation of three inner-sphere surface complexes SOEu2+, SOEuOH+, and SOEu(OH)3−, whereas U(Ⅵ) adsorption data on γ-alumina and goethite were described by the formation of two inner-sphere surface complexes. It should also be noted that the fitting results showed that in the former cases surface complexes formed were SOUO2+ and SOUO2(OH)2−, which were different from SOUO2+ and SOUO2OH in the latter case. The research team from Lanzhou University also studied RN adsorption modeling on different clay minerals, including kaolinite, bentonite and illite. Guo et al. [61] and Liu et al. [62] constructed the models of Eu(Ⅲ) adsorption on Jinchuan bentonite and Gaomiaozi bentonite, respectively. Chen et al. [63] further extended the model to a ternary adsorption system (Eu(Ⅲ)/PO43−/bentonite). By adding binary surface complexation reaction for PO43− and designing ternary surface complexes for enhanced adsorption of both Eu(Ⅲ) and PO43−, Eu(Ⅲ) and PO43− adsorption in ternary system was quantitatively reproduced. Yang et al. [64] studied the adsorption of U(Ⅵ) on bentonite at different temperatures and found that the description of U(Ⅵ) adsorption data at room temperature required an ion exchange reaction and three surface coordination reactions (forming SOUO2+, SO(UO2)3(OH)5 and SO(UO2)3(OH)72−, respectively). The enthalpy changes of the three surface coordination reactions were calculated by van't Hoff equation, and then the prediction of U(Ⅵ) adsorption on bentonite under different temperature was realized. Ma et al. [23] constructed the adsorption model of Am(Ⅲ) and Np(Ⅴ) on Maoming kaolinite. Comparing titration data of Maoming kaolinite with that of kaolinite from other areas in the literature, it was found that acid-base properties of kaolinite from different areas were obviously different, indicating that the existing model parameters on kaolinite were not generic.
Systematic modeling studies of RN on Beishan granite were carried out in the past decade using the idea of generalized composition. The site capacity of Eu(Ⅲ) was one magnitude higher that of Se(Ⅳ), indicating that Eu(Ⅲ) and Se(Ⅳ) may be adsorbed on different sites of granite [29]. According to the adsorption model parameters of Co(Ⅱ) and Ni(Ⅱ) on Beishan granite, Chen et al. [30] established the linear free energy relationship (LFER) between the surface complexation constant and the hydrolysis constant of divalent transition metal cation, by which adsorption of other divalent transition metal cation on granite can be predicted without experimental and modeling process. Based on Eu(Ⅲ) adsorption model on granite, Jin et al. [32] realized the quantitative description of Am(Ⅲ) adsorption on granite in CaCl2 electrolyte by introduction 2 Am(Ⅲ) surface complexation reactions and a cation exchange reaction between Ca2+ and Na+. Jin et al. [32] constructed the adsorption model of U(Ⅵ) on Beishan granite at different temperature. The model contained three surface coordination reactions, forming three surface complexes SOUO2+, SO(UO2)2(OH)2+ and SO(UO2)3(OH)5, respectively. The △H of three surface complexation reactions was calculated by van't Hoff equation. This model with △H can provide satisfactory prediction for literature data of U(Ⅵ) adsorption on granite samples.
Results from spectroscopic studies and theoretical calculations can be used to constrain the "parameters" of surface complexation modeling, which can ensure that established model has certain physical meaning. Liu et al. [65, 66] calculated acid chemistry of gibbsite and 2:1-type dioctahedral phyllosilicates with first principles molecular dynamics (FPMD), and obtained pKas can be used to quantitative define surface site capacity of gibbsite, illite and montmorillonite, respectively. Zhang et al. [67] performed systematic first principles molecular dynamics (FPMD) simulations to investigate the structures, free energies and acidity constants of Ni(Ⅱ) complexes formed on edge surfaces of 2:1 phyllosilicates, and from the theoretical view gave an explanation for the "strong site" and "weak site" defined in 2 site protolysis non electrostatic surface complex and cation exchange (2SPNE SC/CE) model. Zhang et al. [68] also studied the structures, free energies, and acidity constants of UO22+ surface complexes on montmorillonite in order to elucidate the surface complexation mechanisms of uranyl ion (UO22+) on clay mineral edges at the atomic scale, and found that FPMD simulation results can serve as input parameters for an electrostatic thermodynamic surface complexation model (SCM) that adequately reproduced adsorption data from the literature. Gao et al. [69] constructed the adsorption model of Am(Ⅲ) on montmorillonite in combination with DFT calculation. By comparing the theoretical calculation results and modeling parameters, it was concluded that "monodentate surface complex" in the context of SCM only means stoichiometric coefficient of 1, other than structurally monodentate binding. When constructing the adsorption model of Np(Ⅴ) on γ-FeOOH, Yang et al. [70] considered only one surface complexation reaction forming =FeONpO2 based on EXAFS analysis results, and abstained satisfactory fitting results.
2.3.3. NICA-Donnan/Model Ⅵ for humic substanceHumus substance (HS) possesses active functional groups which can strongly coordinate with RN and significantly affect the adsorption and transport behavior of RNs. How to quantitatively describe the effect of HS on RN adsorption is always a challenging subject. By considering HS as a "permeable gel" and "rigid spheres of uniform size", respectively, NICA-Donnan model and Model Ⅵ satisfactorily explain the binding between HS and metal ions. In recent years, Chinese researchers have achieved steady progress in this field.
Weng and her colleagues [71] proposed LCD (ligand charge distribution) model based on CD-MUSIC (charge distribution multi-site ion complexation) model describing the binding of metal ions to mineral surface and NICA-Donnan model describing the HS complexation with metal ions, and successfully explained the adsorption of both cation and anion on goethite in the presence of HS. Ye et al. [72] combined with SHM model (Stockholm humic mode, very similar to Model Ⅵ except for its electrostatic effect) and surface complexation model, quantitatively explained Eu(Ⅲ) adsorption on silica in the presence of FA, suggesting that a discrete-site approach to HS is promising to quantify heavy metal adsorption in metal/HS/mineral ternary system. In combination with EXAFS analysis, Xiong et al. [73] applied NICA-Donnan model to satisfactorily describe Pb(Ⅱ) binding to the FA and HA. Furtherly, they [74] interpreted quantitatively the effects of soil fulvic (JGFA) and humic acid (JGHA) on Pb(Ⅱ) binding to goethite–HS complexes by LCD modeling combined with EXAFS analysis. LCD modeling results indicated that with increased HS loading more Pb(Ⅱ) was bound to adsorbed HS and less to goethite, which are in good agreement with the experimentally observed Pb speciation derived from the EXAFS analysis.
3. TransportRN transport is studied by both laboratory-scale and in-situ experiments. Up to now, RN transport studies in China are performed mainly with laboratory-scale experiments, which can provide necessary technical parameters for in-situ experiments. Through-diffusion, in-diffusion and column methods are commonly used in laboratory-scale transport experiments. In through-diffusion experiment, a rock slice is sandwiched between two liquid storage tanks, and driven by a concentration gradient, RN diffuses through rock slice from storage tank on one side to the storage tank on other side. In-diffusion is that solid sample contacts RN solution on one side, and RN diffuses into the solid phase also with a concentration gradient. Capillary diffusion is a typical in-diffusion method. Column method is generally used to study the penetration process of RN into solid medium at a certain flow rate (usually generated by a pump). In such experiment, RN transport is driven by both concentration gradient and flow rate. RN transport driven by concentration gradient is referred to diffusion or diffusive transport, while RN transport resulting from both concentration gradient and advective velocity gradient is referred to advective–diffusive transport or dispersion [75].
The experimental data from through-diffusion and in-diffusion are usually described by Fick's Law:
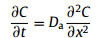
|
(2) |
where C(x, t) is the concentration of the solute in solution at time t (s) and at position x (m) along the diffusion direction; Da (m2/s) is the effective diffusion coefficient.
The experimental data from column experiment are described by one-dimensional convection dispersion equation:
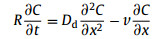
|
(3) |
where Dd (m2/s) is the effective dispersion coefficient; v is the average velocity of pore water, m/s; R is the retardation factor.
The calculated diffusion coefficient and dispersion coefficient are important parameters for the safety assessment of high-level radioactive waste disposal. In this section, experimental studies of RN transport in different media and the development of their numerical models over the past decade were reviewed.
3.1. Slice through-diffusion and capillary in-diffusion experimentThe diffusion of Sr, I, Pu and Se in granite slices has been successively studied by through-diffusion method [76-79]. Dang et al. [76] considered granite as porous medium and calculated the effective diffusion coefficient D according to Fick's Law. Yang et al. [77] studied the influence of granite mineral composition on the Se(Ⅳ) diffusion in granite matrix, and Wang et al. [78, 79] systematically studied the effects of pH, O2, temperature and ionic strength on the Se(Ⅳ) diffusion. On the basis of Fick's Law, additional effects of the decay and sampling dilution on the concentration were considered in their studies, and thus the governing equation was expressed as follows:
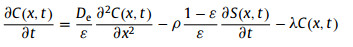
|
(4) |
where C(x, t) (cpm/mL) is the concentration of the solute in the solution at time t (s) and at position x along the diffusion direction; De (m2/s) is the effective diffusion coefficient of the solute in the rock matrix; ε, ρ (kg/dm3) are porosity and dry bulk density of the rock sample, respectively; λ is the decay constant of the solute; S(x, t) (cpm/g) is the concentration of the solute on the rock matrix at time t and position x. The items on the right side of the equation correspond to the net increase of solute caused by solute diffusion, the decrease of solute caused by rock sheet adsorption and the decrease of solute caused by radioactive decay, respectively.
Tian et al. [80] and He et al. [81] used the in-diffusion capability method to study I− and Se(Ⅳ) diffusion in compacted Gaomiaozi bentonite and crushed Beishan granite respectively. Tian et al. [80] regarded the compacted bentonite as a homogeneous isotropic porous medium, calculated D according to Fick's Law and summarized the relationship between ionic strength and Da. The same method was applied by He et al. [81] to calculate the Da of Se in crushed Beishan granite. Unlike I− diffusion in compacted bentonite, Se(Ⅳ) will undergo redox reaction with Fe-bearing components of granite during its diffusion.
3.2. Column experimentColumn experiments included fractured and crushed rock columns. Over the past decade in China, RN transport experiments with fractured rock were scarce, and column experiments were mainly performed with crushed rock column. Yuan et al. [82] studied the transport of I− in the crushed granite column, and used a classic one dimensional advection–dispersion equation including sorption effect and ignoring radioactive decay to describe the experimental data. According to the initial and boundary conditions, an analytical solution was obtained to calculate dispersion coefficient (Dd). Ge et al. [83] studied the U(Ⅵ) transport in water saturated sand and crushed granite columns, respectively, and fitted the experimental data with a two-site kinetic non-equilibrium adsorption model:
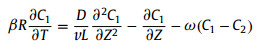
|
(5) |
where subscripts 1 and 2 represent site 1 and site 2, respectively; C1 and C2 represent relative concentrations of solutes at two sites, respectively; T is the dimensionless time of solute transport; L is the length of granite column; β is the percentage of solute instantaneously adsorbed on solid; ω is the mass transfer coefficient of solute between two phases; D is the effective diffusion coefficient; v is the average velocity of pore water; R is the retardation factor; Z is the transport distance.
The quantitative prediction of pollutant transport is also involved in the field of environmental engineering. Therefore, some calculation methods from this field can provide reference for us to quantitatively describe the RN transport [84, 85]. For example, dispersion coefficient (Dd) and the advance or tailing phenomenon in heterogeneous media varied at different scales, and these cannot be well simulated with one-dimensional convection dispersion equation. Thus, the fractional convection dispersion equation was introduced, where the general situation of solute non Fick phenomenon and pollutant transport were considered [86].
3.3. Transport in real environmental conditionThe real environmental conditions are much more complicated than fractured columns and porous media. Liu and his colleagues [87-90] have carried out systematic studies and established various mathematical models for complicated transport conditions. When the groundwater flows through a fracture which may be slowly closed under stress, Liu and his colleagues established a model with time-dependent aperture to describe pollutant transport, and developed a program to study solute transport behavior in such fracture [87]. Further, in the case of an arbitrary number of rock units and an arbitrary-length decay chain, a model considering convection, molecular diffusion to multi-component rock matrix and radioactive decay was developed, and its analytical solutions were obtained [88]. For the scale effect on Dd (i.e., Dd observed in the field experiment increased with distance), a single channel model was established considering convection, diffusion, adsorption and radioactive decay chain on the channel surface and the geological layer of rock matrix, and the transport process of decaying solute in the fracture was well described with the idea of decoupling [89]. Facing the solute transport in a single channel in porous media, Besides, Liu et al. discussed the dispersion effect from path separation based on a single channel model, and obtained the solution of the model [90]. Liu and his colleagues also explored the comprehensive method for visualization of solute transport in fractured rocks [91].
4. Radioactive colloid"Colloid" refers to colloidal particle composed of inorganic minerals, organic matter and microorganisms in natural waters with sizes ranging from 1 nm to 1000 nm. The presence/formation of colloid can significantly alter the transport behavior of RNs, and thus model prediction considering only ion state deviates from the real situation. With the recognition of important role of colloids, study on radioactive colloids has gradually carried out in China in recent years.
Formation of environmental colloids in groundwater often originates from physical disturbances (e.g., rainfall, water injection) and chemical disturbances (e.g., surface adsorption, solution pH change). Due to the water erosion, bentonite, commonly used buffer backfilling material for HLW disposal, can disperse into aqueous solution and from colloidal particles. The concentration and particle size of colloidal bentonite are affected by the characteristics of clay, hydrodynamic and chemical conditions [92]. Environmental colloids possess strong affinity to RN, and RN can be easily adsorbed by environmental colloids via ion exchange and surface coordination reactions, forming so-called radioactive pseudo-colloid.
In addition to pseudo-colloid, the hydrolysis of some high-valence actinide ions may generate radioactive intrinsic colloids. Wang et al. [93] studied the colloidal behavior of Np with various valences in the presence of humic acid, and found that Np(Ⅴ) had no obvious colloid behavior, while Np(Ⅳ) existed in the form of intrinsic colloid. Np(Ⅳ) can also form pseudo-colloid with humic acid. Liu et al. [94] proposed that Pu ions with different valences formed intrinsic colloid following the order similar to that Pu ions hydrolyzed: Pu4+ > PuO22+ > Pu3+ > PuO2+, where Pu4+ is most likely to form intrinsic colloids. Intrinsic colloid of Pu4+ colloids can be obtained by neutralizing acidic solutions containing Pu4+, or interacting Pu4+ with some reducing microorganisms. Guan et al. [95] found that Am(Ⅲ) mainly existed in the form of ions in unfiltered groundwater, while Am(Ⅲ) mainly formed intrinsic colloid in ultrapure water.
4.1. Colloid stabilityThe release, transport and transformation of colloids are dependent to the stability of colloids. The stability of colloid is affected by many physical and chemical factors, such as temperature, pH, ionic strength, coexisting ions, colloid properties. Xian et al. [96] and Xu et al. [97] studied the stability of colloidal Gaomiaozi bentonite as a function of pH and ionic strength. It was found that the colloid particle size gradually increased with the increase of ionic strength, indicating that the colloid was prone to aggregate at high ionic strength. Besides, the aggregation of Gaomiaozi bentonite colloid was reversible. When the ionic strength and pH became conducive to dispersion, aggregated bentonite colloids would be re-dispersed.
The interaction between different colloids also affects the colloid stability. Gui et al. [98] studied the effects of colloidal kaolin, goethite and HA on the aggregation kinetics of biochar colloids from different sources. It was found that kaolin colloid and HA can enhance the stability of the biochar colloid by increasing the electrostatic repulsion of the system, whereas goethite colloid resulted in the aggregation of biochar colloid because of rapid combination with biochar colloid. Sun et al. [99] found that when the ionic strength is high (> 100 mm), the presence of U(Ⅵ) decreased the stability of biochar colloid. Zhang et al. [100] studied the interaction between gibbsite colloid and bentonite colloid, and found that they accumulated through electrostatic interaction and inhibited the transport of each other.
4.2. Co-transport of RN and environmental colloidsDomestic colloid transport study in recent year mainly focused on the radioactive pseudo-colloid, i.e., co-transport of RN and environmental colloids, and study on intrinsic radioactive colloid was scarce [101]. Systematic co-transport study was performed with RN and colloidal oxygen/hydroxide colloids and clay minerals. Both sand and crushed Beishan granite were selected as solid medium.
Co-transport of RN with colloidal aluminum and ferric hydroxide/oxide was most studied. Lin et al. [102] observed that α-FeOOH colloid of an low colloid concentration (0.2 mg/L) facilitated Pu transport in granite fissures, whereas the mobility of Pu began to decrease when the colloid concentration was greater than 1 mg/L. Ge et al. [83] studied the co-transport of akaganeite colloid (AKC) and U(Ⅵ) in a saturated quartz sand column, and observed an U(Ⅵ) concentration-dependent effect of AKC. The presence of AKC facilitated U(Ⅵ) transport at relatively low U(Ⅵ) concentration, whereas it impeded U(Ⅵ) transport at relatively high U(Ⅵ) concentrations. HA can strongly affect the transport of colloidal aluminum and ferric hydroxide/oxide by forming composite colloid with them. Yao et al. [103] studied the transport of HA-Fe colloid and Se(Ⅴ) in saturated porous media, and found that HA-Fe/Se(Ⅴ) transport was enhanced under low pH and high ionic strength conditions. However, Yang et al. [104] found that the effect of HA on the co-transport of U(Ⅵ) and gibbsite colloids was dependent to HA amount. Gibbsite colloids impeded U(Ⅵ) transport at relatively low HA concentration (5 mg/L), and facilitated U(Ⅵ) transport at relatively high HA concentration (20 mg/L).
The common colloidal clay minerals can also promote the RN transport. Sun et al. [99] studied the co-transport behavior of kaolin colloid and Eu(Ⅲ) in saturated quartz sand column, and found that high pH or low ionic strength is conducive to the co-transport of Kaolin colloid and Eu(Ⅲ). Similar observations were reported by Wei et al. [105] and Xu et al. [106] in their co-transport studies with colloidal illite and bentonite, respectively. Du et al. [107] observed that attapulgite colloid on the transport of U(Ⅵ) in the quartz sand column, and found that U(Ⅵ) adsorption decreased the stability of attapulgite colloid, resulting in U(Ⅵ) retention in the quartz sand column.
The properties of porous media had a certain effect on the transport of colloids and RN, because different surface properties meant different adsorption and deposition sites. Sun et al. [108] prepared quartz sand column with different surface roughness to study the effect of surface roughness on the transport of colloidal montmorillonite and kaolinite, and they observed that surface roughness significantly decreased only the mobility of montmorillonite. Han et al. [109] compared TiO2 nanoparticle transport in quartz sand and iron oxide coated quartz sand, and found that the mobility of TiO2 nanoparticles in coated quartz sand was significantly weaker than that in quartz sand.
5. SummaryStudies related to RN adsorption, diffusion and transport over the past decade in China have been reviewed in this paper. It can be concluded that clear progress has been achieved with respect to experimental, modeling, theoretical calculation and spectroscopic analysis. Comparing with European countries which are advanced in RN migration studies, e.g., France, Germany and Switzerland, China lacks large-scaled field experiments and China's laboratory-scale studies to some extent are not close to the real needs of HLW geological disposal. These are mainly due to that no URL was established before and domestic researchers were not aware enough of realizing HLW geological disposal. Besides, inadequate fund support may also be a reason. Finally, several suggestions have been proposed for China's future RN migration study.
(1) In the context of environmental radiochemistry, high performance materials and their composites are extensively studied to enhance RN removal [110]. However, economy and long-term effectiveness of these adsorption materials as additive of repository barriers are still unknown. Future studies should focus more on the actual needs of radioactive waste disposal in China, and the experimental conditions should be closer to the real disposal situation.
(2) Few experimental studies were performed with long-lived actinide RNs (e.g., Np and Pu), especially under the reducing conditions related to the HLW geological disposal. More studies are required to understand the migration properties of these actinide RNs.
(3) Reduction and enhanced adsorption of RN by various bacteria at/around anticipated HLW repositories have drawn the attention of some domestic scholars [111]. However, more systemic studies are required, because effects of microbes are various, including mineralization, pore clogging and so on.
(4) Although progress has been made in the study of the colloid effects on RN transport in the past few years, study on the formation mechanisms and transport behaviors of intrinsic colloids needs to be further strengthened.
(5) China's first URL for HLW disposal will be under construction soon. The experimental technology and devices for in-situ conditions have to be studied urgently, and preliminary studies on sorption, diffusion and transport related to URL need to be carried out as well.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21806064, U1730245, 21906074, 22176079) and Fundamental Research Funds for the Central Universities (No. lzujbky-2021-sp27). Authors appreciate Supercomputing Center of Lanzhou University for computational resources.
| [1] |
H. Wang, J. Rong, Electric Power 54 (2021) 89-94. |
| [2] |
Y. Wei, Y. Wu, H. Li, Up-to Data On Nuclear Fuel Cycle, Shanghai Jiao Tong University Press, Shanghai, 2016.
|
| [3] |
J. Wang, Geological Disposal of High-level Radioactive Waste in China in the New Century, China Atomic Energy Press, Beijing, 2016.
|
| [4] |
H. Geckeis, J. Luetzenkirchen, R. Polly, Chem. Rev. 113 (2013) 1016-1062. DOI:10.1021/cr300370h |
| [5] |
X. Wang, C. Liu, C. Wang, Sci. Sin. Chim. 50 (2020) 1585-1599. DOI:10.1360/SSC-2020-0138 |
| [6] |
Z. Guo, S. Wang, K. Shi, Radiochim. Acta 97 (2009) 283-289. |
| [7] |
Z. Guo, C. Yan, J. Xu, Colloids Surf. A 336 (2009) 123-129. DOI:10.1016/j.colsurfa.2008.11.032 |
| [8] |
Z.J. Guo, Y. Li, W.S. Wu, Appl. Radiat. Isot. 67 (2009) 996-1000. DOI:10.1016/j.apradiso.2009.02.001 |
| [9] |
Z.J. Guo, H.Y. Su, W.S. Wu, Radiochim. Acta 97 (2009) 133-140. |
| [10] |
X. Wang, K. Shi, Z. Guo, Sci. China Chem. 53 (2010) 2628-2636. DOI:10.1007/s11426-010-4086-9 |
| [11] |
L. Zhang, H. Zhang, Z. Ge, J. Radioanal. Nucl. Chem. 288 (2011) 537-546. DOI:10.1007/s10967-010-0960-3 |
| [12] |
J. Mou, C. Li, G. Wang, J. Radioanal. Nucl. Chem. 292 (2012) 1099-1104. DOI:10.1007/s10967-011-1567-z |
| [13] |
L. Chen, Y. Dong, J. Radioanal. Nucl. Chem. 295 (2013) 2117-2123. DOI:10.1007/s10967-012-2253-5 |
| [14] |
Z. Chen, L. Chen, S. Lu, J. Radioanal. Nucl. Chem. 308 (2016) 505-516. DOI:10.1007/s10967-015-4482-x |
| [15] |
W. Jia, S. Lu, J. Radioanal. Nucl. Chem. 299 (2014) 1417-1426. DOI:10.1007/s10967-013-2865-4 |
| [16] |
S. Yu, H. Mei, X. Chen, J. Mol. Liq. 203 (2015) 39-46. DOI:10.1016/j.molliq.2014.12.041 |
| [17] |
P. Li, Z. Liu, F. Ma, J. Mol. Liq. 206 (2015) 285-292. DOI:10.1016/j.molliq.2015.02.014 |
| [18] |
P. Zong, X. Wu, J. Gou, J. Mol. Liq. 209 (2015) 358-366. DOI:10.1016/j.molliq.2015.05.052 |
| [19] |
T. Yu, W.S. Wu, Q.H. Fan, Chin. Chem. Lett. 23 (2012) 1189-1192. DOI:10.1016/j.cclet.2012.07.011 |
| [20] |
X. Chen, S. Peng, J. Wang, J. Radioanal. Nucl. Chem. 303 (2015) 509-519. DOI:10.1007/s10967-014-3458-6 |
| [21] |
Y. Zhao, Z. Shao, C. Chen, Appl. Clay Sci. 87 (2014) 1-6. DOI:10.1016/j.clay.2013.11.021 |
| [22] |
W. Du, X. Liu, L. Tan, J. Radioanal. Nucl. Chem. 292 (2012) 1173-1179. DOI:10.1007/s10967-011-1573-1 |
| [23] |
F. Ma, Q. Jin, P. Li, Appl. Geochem. 84 (2017) 325-336. DOI:10.1016/j.apgeochem.2017.07.002 |
| [24] |
F. Ma, Q. Jin, P. Gao, At. Energ. Sci. Technol. 51 (2017) 790-797. |
| [25] |
Y. Zhang, X. Fan, X. Su, J. Nucl. Radio. Chem. 27 (2005) 136-143. |
| [26] |
T. Jiang, J. Yao, D. Zhou, J. Nucl. Radio. Chem. 33 (2010) 25-31. |
| [27] |
D. Zhou, H. Long, X. Chen, J. Nucl. Radio. Chem. 36 (2014) 216-221. |
| [28] |
Z. Guo, Z. Chen, W. Wu, Sci. Sin. Chim. (2011) 907-913. |
| [29] |
Z. Guo, Z. Chen, W. Wu, Acta Phys. Chim. Sin. 9 (2011) 2222-2226. |
| [30] |
Z. Chen, R. Zhang, X. Yang, Acta Phys. Chim. Sin. 29 (2013) 2019-2026. DOI:10.3866/PKU.WHXB201306271 |
| [31] |
Q. Jin, G. Wang, M. Ge, Appl. Geochem. 47 (2014) 17-24. DOI:10.1016/j.apgeochem.2014.05.004 |
| [32] |
Q. Jin, L. Su, G. Montavon, Chem. Geol. 433 (2016) 81-91. DOI:10.1016/j.chemgeo.2016.04.001 |
| [33] |
G. Sheng, S. Yang, J. Sheng, Environ. Sci. Technol. 45 (2011) 7718-7726. DOI:10.1021/es202108q |
| [34] |
J. Hu, X. Tan, X. Ren, Dalton Trans. 41 (2012) 10803-10810. DOI:10.1039/c2dt31057k |
| [35] |
C.F. Wu, L. Chen, S.T. Yang, Chemosphere 210 (2018) 392-400. DOI:10.1016/j.chemosphere.2018.07.029 |
| [36] |
X. Wang, S. Yu, Z. Chen, Sci. China Chem. 60 (2017) 107-114. DOI:10.1007/s11426-016-0163-6 |
| [37] |
Z. Niu, T. Ohnuki, E. Simoni, Chem. Eng. J. 351 (2018) 203-209. DOI:10.1016/j.cej.2018.06.034 |
| [38] |
H. Wang, Z.F. Chai, D.Q. Wang, Dalton Trans. 44 (2015) 1646-1654. DOI:10.1039/C4DT02872D |
| [39] |
H. Wang, Z. Chai, D.Q. Wang, Green Energy Environ. 2 (2017) 30-41. DOI:10.1016/j.gee.2016.11.011 |
| [40] |
Q. Wang, X.P. Kong, H. B.-.Zhang, Appl. Surf. Sci. 414 (2017) 405-412. DOI:10.1016/j.apsusc.2017.04.062 |
| [41] |
H.Y. Mei, Y. Liu, X.L. Tan, J. Hazard. Mater. 399 (2020) 1-10. |
| [42] |
X. Tan, X. Ren, J. Li, Rsc Adv. 3 (2013) 19551-19559. DOI:10.1039/c3ra42853b |
| [43] |
H. Xu, M. Zhou, Y. Fang, Minerals 8 (2018) 17. |
| [44] |
Y. Zhu, E.J. Elzinga, Environ. Sci. Technol. 48 (2014) 4937-4945. DOI:10.1021/es500579p |
| [45] |
Y. Tang, R.J. Reeder, Geochim. Cosmochim. Acta 73 (2009) 2727-2743. DOI:10.1016/j.gca.2009.02.003 |
| [46] |
C. Zhang, X. Liu, X. Lu, Geochim. Cosmochim. Acta 248 (2019) 161-171. DOI:10.1016/j.gca.2019.01.010 |
| [47] |
C. Zhang, X. Liu, X. Lu, Environ. Sci. Technol. 53 (2019) 13704-13712. DOI:10.1021/acs.est.9b04393 |
| [48] |
Y. Ma, X. Cheng, M. Kang, Chemosphere 254 (2020) 126855. DOI:10.1016/j.chemosphere.2020.126855 |
| [49] |
Y. Wang, J. Wang, P. Li, Environ. Sci. Innov. 23 (2021) 101615. |
| [50] |
B. Ma, A. Fernandez-Martinez, M. Kang, Environ. Sci. Technol. 54 (2020) 8104-8114. DOI:10.1021/acs.est.0c01854 |
| [51] |
W. Zhou, D. Xian, X. Su, Chemosphere 255 (2020) 126942. DOI:10.1016/j.chemosphere.2020.126942 |
| [52] |
D. Shen, X. Fan, X. Su, J. Nucl. Radio. Chem. 23 (2001) 72-78. |
| [53] |
M. Kang, F. Chen, Y. Yang, J. Nucl. Radio. Chem. 32 (2010) 160-166. |
| [54] |
L. Huo, W. Xie, T. Qian, Chemosphere 174 (2017) 456-465. DOI:10.1016/j.chemosphere.2017.02.018 |
| [55] |
T. Wang, T. Qian, D. Zhao, Sci. Total Environ. 725 (2020) 138423. DOI:10.1016/j.scitotenv.2020.138423 |
| [56] |
M.L. Kang, C.L. Liu, F.R. Chen, Sci. Sin. Chim. 43 (2013) 536-543. DOI:10.1360/032012-316 |
| [57] |
M. Kang, F. Bardelli, L. Charlet, Appl. Geochem. 47 (2014) 130-140. DOI:10.1016/j.apgeochem.2014.05.018 |
| [58] |
M. Kang, F. Bardelli, B. Ma, Radiochim. Acta 104 (2016) 649-656. DOI:10.1515/ract-2015-2496 |
| [59] |
B. Ma, M. Kang, Z. Zheng, J. Hazard. Mater. 276 (2014) 422-432. DOI:10.1016/j.jhazmat.2014.05.066 |
| [60] |
J. Wang, L. Xie, S. Li, J. Phys. Chem. C 125 (2021) 3018-3026. DOI:10.1021/acs.jpcc.0c10333 |
| [61] |
Z. Guo, J. Xu, K. Shi, Colloids Surf. A 339 (2009) 126-133. DOI:10.1016/j.colsurfa.2009.02.007 |
| [62] |
F. Liu, Y. Ye, N. Guo, Sci. Sin. Chim. 43 (2012) 242-252. |
| [63] |
Z. Chen, G. Montavon, Z. Guo, Appl. Clay Sci. 101 (2014) 369-380. DOI:10.1016/j.clay.2014.07.034 |
| [64] |
Z. Yang, L. Huang, Y. Lu, Radiochim. Acta 98 (2010) 785-791. DOI:10.1524/ract.2010.1784 |
| [65] |
X. Liu, J. Cheng, M. Sprik, Geochim. Cosmochim. Acta 120 (2013) 487-495. DOI:10.1016/j.gca.2013.06.043 |
| [66] |
X. Liu, J. Cheng, M. Sprik, Geochim. Cosmochim. Acta 140 (2014) 410-417. DOI:10.1016/j.gca.2014.05.044 |
| [67] |
C. Zhang, X. Liu, X. Lu, Geochim. Cosmochim. Acta 203 (2017) 54-68. DOI:10.1016/j.gca.2017.01.014 |
| [68] |
C. Zhang, X. Liu, R.M. Tinnacher, Environ. Sci. Technol. 52 (2018) 8501-8509. DOI:10.1021/acs.est.8b02504 |
| [69] |
P. Gao, D. Zhang, Q. Jin, Chem. Geol. 581 (2021) 120414. DOI:10.1016/j.chemgeo.2021.120414 |
| [70] |
C. Yang, B.A. Powell, S. Zhang, Radiochim. Acta 103 (2015) 707-717. DOI:10.1515/ract-2015-2405 |
| [71] |
N.D. Bryan, L. Abrahamsen, N. Evans, Appl. Geochem. 27 (2012) 378-389. DOI:10.1016/j.apgeochem.2011.09.008 |
| [72] |
Y. Ye, Z. Chen, G. Montavon, Sci. China Chem. 57 (2014) 1276-1282. DOI:10.1007/s11426-014-5120-0 |
| [73] |
J. Xiong, L.K. Koopal, W. Tan, Environ. Sci. Technol. 47 (2013) 11634-11642. DOI:10.1021/es402123v |
| [74] |
J. Xiong, L. Weng, L.K. Koopal, Environ. Sci. Technol. 52 (2018) 1348-1356. DOI:10.1021/acs.est.7b05412 |
| [75] |
B. Bijeljic, M.J. Blunt, Water Resour. Res. 43 (2007) W12S11. |
| [76] |
H. Dang, X. Hou, W. Liu, J. Nucl. Radio. Chem. 036 (2014) 53-59. |
| [77] |
X. Yang, X. Ge, J. He, Environ. Sci. Technol. 52 (2017) 1320-1329. |
| [78] |
C. Wang, X. Yang, F. Wei, J. Radioanal. Nucl. Chem. 319 (2019) 365-377. DOI:10.1007/s10967-018-6344-9 |
| [79] |
C. Wang, X. Yang, J. He, F. Wei, Radiochim. Acta 107 (2018) 39-54. DOI:10.1515/ract-2018-2969 |
| [80] |
W. Tian, C. Li, X. Liu, J. Radioanal. Nucl. Chem. 295 (2013) 1423-1430. DOI:10.1007/s10967-012-2284-y |
| [81] |
J. He, B. Ma, M. Kang, J. Hazard. Mater. 324 (2016) 564-572. |
| [82] |
G. Yuan, T. Chen, P. Liu, J. Radioanal. Nucl. Chem. 321 (2019) 693-699. DOI:10.1007/s10967-019-06627-1 |
| [83] |
M. Ge, D. Wang, J. Yang, Water Res. 147 (2018) 350-361. DOI:10.1016/j.watres.2018.10.004 |
| [84] |
Q. Cheng, X. Chen, Z. Zhang, J. Hohai Univ. 46 (2018) 486-491. |
| [85] |
L. Zhang, Z. Jiang, S. Zhang, J. Shijiazhuang Tiedao Univer. 33 (2020) 1-7. |
| [86] |
X. Li, G. Li, X. Jia, J. Shandong Univ. Technol. 25 (2011) 52-55. |
| [87] |
Z. Zhao, L. Liu, I. Neretnieks, Int. J. Rock Mech. Min. Sci. 66 (2014) 69-75. DOI:10.1016/j.ijrmms.2013.12.004 |
| [88] |
P. Shahkarami, L. Liu, L. Moreno, J. Hydrol. 520 (2015) 448-460. DOI:10.1016/j.jhydrol.2014.10.060 |
| [89] |
B. Mahmoudzadeh, L. Liu, L. Moreno, J. Hydrol. 536 (2016) 133-146. DOI:10.1016/j.jhydrol.2016.02.046 |
| [90] |
L. Liu, I. Neretnieks, P. Shahkarami, Hydrol. J. 26 (2018) 297-320. |
| [91] |
S. Pirouz, N. Ivars, M. Luis, Hydrol. J. 27 (2018) 101-119. |
| [92] |
Y. Cai, Y. Chen, W. Ye, Chin. J. Geotech. Eng. 42 (2020) 1996-2005. |
| [93] |
B. Wang, D. Liu, J. Yao, At. Energ. Sci. Technol. 44 (2010) 1031-1038. |
| [94] |
Y. Liu, C. Li, D. Wang, Chin. Environ. Sci. Technol. 39 (2016) 66-73. |
| [95] |
Z. Guan, D. Zhou, H. Long, J. Nucl. Radio. Chem. 33 (2011) 84-88. |
| [96] |
D. Xian, W. Zhou, D. Pan, Colloids Surf. A 601 (2020) 125020. DOI:10.1016/j.colsurfa.2020.125020 |
| [97] |
Z. Xu, D. Pan, Y. Sun, Appl. Clay Sci. 161 (2018) 436-443. DOI:10.1016/j.clay.2018.05.002 |
| [98] |
X. Gui, B. Song, M. Chen, Sci. Total Environ. 771 (2021) 145414. DOI:10.1016/j.scitotenv.2021.145414 |
| [99] |
Y. Sun, D. Pan, X. Wei, Environ. Pollut. 266 (2020) 115189. DOI:10.1016/j.envpol.2020.115189 |
| [100] |
Z. Zhang, C. Gao, Y. Sun, Appl. Clay Sci. 205 (2021) 106033. DOI:10.1016/j.clay.2021.106033 |
| [101] |
J. Xie, X. Wang, J. Lu, J. Environ. Radioact. 116 (2013) 76-83. DOI:10.1016/j.jenvrad.2012.09.009 |
| [102] |
J. Lin, H. Dang, J. Xie, J. Contam. Hydrol. 164 (2014) 251-258. DOI:10.1016/j.jconhyd.2014.06.008 |
| [103] |
Y. Yao, N. Mi, C. He, Chemosphere 240 (2020) 124987. DOI:10.1016/j.chemosphere.2019.124987 |
| [104] |
J. Yang, Z. Zhang, Z. Chen, Sci. Total Environ. 688 (2019) 450-461. DOI:10.1016/j.scitotenv.2019.05.395 |
| [105] |
X. Wei, D. Pan, Z. Xu, Sci. Total Environ. 768 (2021) 144174. DOI:10.1016/j.scitotenv.2020.144174 |
| [106] |
Z. Xu, Z. Niu, D. Pan, Sci. Total Environ. 793 (2021) 148545. DOI:10.1016/j.scitotenv.2021.148545 |
| [107] |
L. Du, P. Wang, X. Li, Appl. Geochem. 100 (2019) 363-370. DOI:10.1016/j.apgeochem.2018.11.009 |
| [108] |
H. Sun, X. Yin, Y. Wang, J. Agro-Environ. Sci. 31 (2012) 2361-2366. |
| [109] |
P. Han, X. Wang, L. Cai, Colloids Surf. A 454 (2014) 119-127. DOI:10.1016/j.colsurfa.2014.04.020 |
| [110] |
Z. Wang, L. Zhang, K. Zhang, Chemosphere 287 (2022) 132313. DOI:10.1016/j.chemosphere.2021.132313 |
| [111] |
S. Wang, L. Shi, S. Yu, J. Environ. Radioact. 242 (2022) 106798. DOI:10.1016/j.jenvrad.2021.106798 |
 2022, Vol. 33
2022, Vol. 33 

