b State Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, China
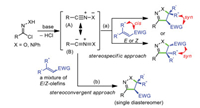
1, 3-Dipolar cycloaddition reaction (1, 3-DCR) provides a very powerful platform for rapid installation of five membered heterocyclic frameworks in a straightforward and atom-economic manner [1-6]. Despite impressive achievements, the application of nitrile oxides and nitrile imines as dipoles represents a challenging task and has been studied only to a limited extend. These classes of dipoles are generated in situ from the dehydrochlorination of hydroximoyl or hydrazonyl halides when exposing to base, and they are unstable with a high propensity to undergo self-cycloaddition [7-14]. Not only that, the mesomeric structures of nitrile oxides and nitrile imines viz. heteropropargyl (A) and heteroallenyl (B) anions (octet structures) also pose the question of regioselectivity (Scheme 1) [14-19]. Since the 1, 3-DCR of nitrile oxides and nitrile imines with olefinic dipolarophiles is stereospecific in nature, the geometrical information of the olefins is retained in the corresponding stereogenic centers of the cycloadducts (Scheme 1a) [20-28]. As a consequence, E/Z isomers usually generate diastereomeric products. The control over the diastereoselectivity is achieved by using isomerically pure olefins, which requires tedious and inefficient purification steps due to the moderate E/Z-selectivity. As such, it is obvious that the development of stereoselective 1, 3-DCR with isomeric mixture of E- and Z-olefinic dipolarophiles is a much more challenging task and highly desirable. In this context, stereoconvergent method has emerged as a promising solution to mitigate these problems, and both E and Z isomers are transformed into a single diastereomer (Scheme 1b) [29]. It provides a wonderful opportunity to overcome the conventional stereospecific reactivity of olefins, and is ensued from conceptually distinct mechanistic pathways involving olefin isomerization and (dynamic) kinetic resolution in most reported examples [30-41].
|
Download:
|
| Scheme 1. 1, 3-DCR of nitrile oxides or nitrile imines with E/Z-olefins. | |
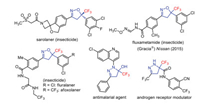
On the other hand, fluorine has found widespread applications as pharmacological modulator in drug development. Strategic introduction of a CF3 group into organic frameworks significantly enhances the metabolic stability, binding affinity, bioavailability, and lipophilicity of molecules [42-48]. For example, as depicted in Fig. 1, trifluoromethylated isoxazolines and pyrazolines are key motifs of various agrochemicals and biologically active molecules, making them highly attractive synthetic targets [49-63]. Therefore, considerable efforts have been devoted towards this direction [64-71]; however, installation of isoxazoline and pyrazoline frameworks bearing a trifluoromethylated quaternary stereogenic center with an adjacent stereogenic center in a stereoconvergent fashion remains as a formidable challenge. In fact, there is no precedent in the literature for such kind of transformation.
|
Download:
|
| Fig. 1. Selected examples of biologically active trifluoromethylated isoxazolines and pyrazolines. | |
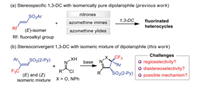
With this background and in continuation to our interest in the synthesis of fluorinated heterocycles using β-fluoroalkyl-α, β-unsaturated arylsulphones (Scheme 2a) [72-74], herein we report the first stereoconvergent 1, 3-DCR of nitrile oxides and nitrile imines with E/Z isomeric mixture of β-CF3-β, β-disubstituted α, β-unsaturated arylsulphones. Accordingly, isoxazolines and pyrazolines bearing a trifluoromethylated quaternary stereogenic center adjacent to a tertiary stereogenic center are obtained as a single diastereomer with excellent regioselectivity under mild reaction conditions (Scheme 2b). The exact mechanistic pathway for this kind of transformation still remains elusive; however, control experiments and theoretical DFT calculations have been carried out to justify the experimental outcome.
|
Download:
|
| Scheme 2. 1, 3-DCR of nitrile oxides and nitrile imines. | |
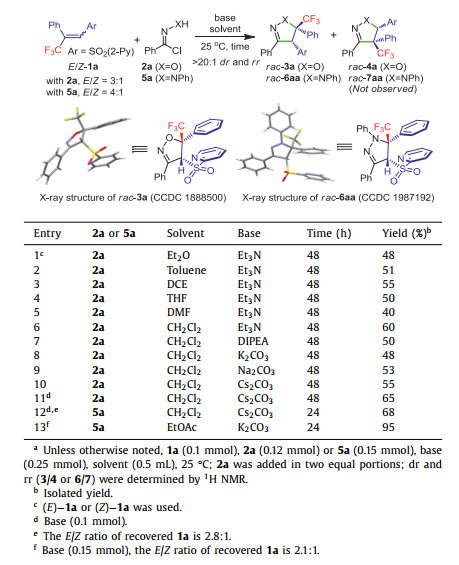
Not only sterically crowded β-CF3-β, β-disubstituted α, β-unsaturated arylsulphones are -less active dipolarophiles, but also it is difficult to separate their E/Z isomers. When isomerically pure (E)−1a or (Z)−1a was separately applied into two batches of 1, 3-DCR with N-hydroxybenzimidoyl chloride 2a (nitrile oxide precursor) in the presence of Et3N (2.5 equiv.), the same diastereomer 3a was unexpectedly delivered in around 48% yield after 48 h with > 20:1 dr and rr (Table 1, entry 1). Much to our satisfaction, the 1, 3-DCR proceeded in a stereoconvergent manner. Based on the single crystal X-ray analysis, the relative configuration of 3a (CCDC: 1888500) was established, and it was found that the two adjacent Ph and SO2(2-Py) moieties adopt syn-orientation. For convenience, 3:1 or 4:1 (E/Z)-isomeric mixture of 1a after column chromatography was used in the following identifying conditions and nitrile oxide scope. The solvent effect study was carried out; in all of selected solvents the reaction proceeded in a stereoconvergent manner without affecting the diastereo- and regioselectivity, and the desired product was obtained in slightly lower yields (40%–55%, entries 2-5) compared to CH2Cl2 (60%, entry 6). Other bases viz. DIPEA, K2CO3, Na2CO3 and Cs2CO3 in CH2Cl2 could not improve the yield (entries 7–10); however, when the amount of Cs2CO3 was reduced to 1.0 equiv, the yield was increased to 65% (entry 11), which was established as the optimal reaction condition for the 1, 3-DCR of nitrile oxides. The 1, 3-DCR of hydrazonyl halide 5a (nitrile imine precursor) and 1a (E/Z= 4:1) was also stereoconvergent, and the corresponding product 6aa was obtained in 68% yield with > 20:1 dr and rr (entry 12). The relative configuration of 6aa was established by single crystal X-ray analysis (CCDC: 1987192), where the two adjacent Ph and SO2(2-Py) moieties also adopt syn-orientation. The efficiency of the reaction was further improved by changing the solvent and base to EtOAc and K2CO3 (1.5 equiv.), respectively (95% yield, > 20:1 dr and rr, entry 13).
|
|
Table 1 Optimization of the reaction conditions.a |
After establishing the optimized reaction conditions, the scope and generality of the stereoconvergent 1, 3-DCR were evaluated. Following the reaction conditions described in entry 11 of Table 1, a variety of hydroximoyl chlorides were used to react with 3:1 E/Z-isomeric mixture of α, β-unsaturated 2-pyridylsulphone 1a (Scheme 3). It was observed that the hydroximoyl chlorides bearing electron-donating and electron-neutral (F and Cl) groups at the para-position of the aryl moiety (R) had no significant influence on the course of the reaction, and the desired products 3a-3f were obtained in 65%–78% yields with excellent diastereo- and regioselectivity (> 20:1 dr). The presence of an electron-withdrawing group (NO2) at the para-position made the reaction less efficient with requiring higher temperature (60 ℃), and the product 3g was obtained in only 38% yield. Hydroximoyl chloride with an electron-donating Me group at the meta-position of the aryl moiety performed well to give the product 3h in 68% yield. However, the presence of F and NO2 groups at the same position required higher temperature (60 ℃), and the respective products 3i and 3j were obtained in 32% and 35% yields without affecting the diastereoelectivity. A similar reactivity trend was observed using ortho-substituted arylhydroximoyl chlorides with Me, F and CF3 groups, providing the corresponding products 3k, 3l and 3m in 48%–78% yields. Hydroximoyl chlorides bearing 2-naphthyl, (E)-PhCH=CH and cyclohexyl groups reacted smoothly with 1a with providing the respective products 3n, 3o and 3p in 55%–82% yields with > 20:1 dr. The variation at the aryl group of substrates 1 (1b: Ar = 4-Me-Ph; 1c: Ar = 4-Br-Ph) provided the corresponding CF3-isoxazolines 3q and 3r in 65% and 76% yields.

|
Download:
|
| Scheme 3. Scope of nitrileoxides to obtain trifluoromethylated isoxazolines. Reaction conditions: 1a (0.1 mmol), 2 (0.12 mmol), Cs2CO3 (1.0 equiv., 32.6 mg), and solvent (0.5 mL); 2 was added in two equal portions. a In ClCH2CH2Cl at 60 ℃. b 2.0 equiv. of 2 and Cs2CO3 were used. | |
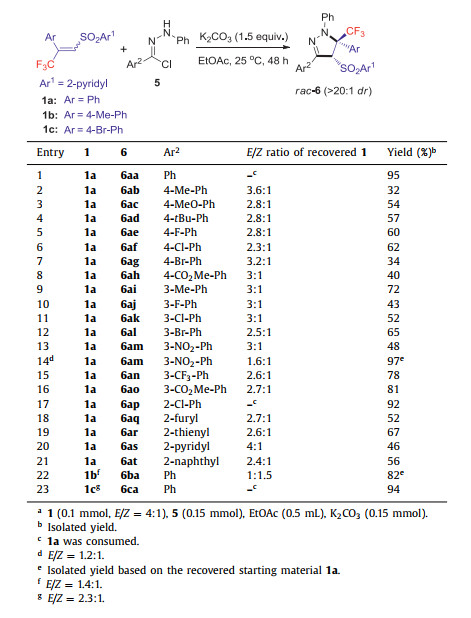
Subsequently, a variety of hydrazonyl chlorides 5 reacted with E/Z-isomeric mixture (E/Z = 4:1) of α, β-unsaturated 2-pyridylsulphone 1 following the reaction conditions described in entry 13 of Table 1, and the results were summarized in Table 2. Aromatic hydrazonyl chlorides with varied substitutions at the ortho-, meta- and para-positions of the aryl moiety (Ar2) participated well in the reaction, affording the CF3-pyrazolines 6aa–6ap as a single diastereomer in moderate to excellent isolated yields (32%–92%, entries 2‒17). The excellent isolated yield of cycloadduct 6am based on the recovered starting material 1a in entry 14 indicates that the low yield of 6am results from the unstability of in-situ generated nitrile imine in entry 13. Heteroaromatic hydrazonyl chloride also participated well in the reaction to deliver the products 6aq–6as (46%–67% yields, entries 18‒20). The synthesis was also successful with hydrazonyl chloride bearing a 2-naphthyl group, delivering the single diastereomeric product 6t in 56% yield (entry 21). CF3-pyrazolines 6ba and 6ca were obtained from 1b and 1c with complete stereoconvergence in 82% and 94% yields, respectively (entries 22 and 23). It should be noted that the low yields usually result from unstable nitrile imines. From the E/Z ratio of the recovered 1, it was apparent that both the isomers participated in the reaction in a stereoconvergent manner even though they were present in different concentrations.
|
|
Table 2 Scope of nitrile imines to obtain trifluoromethylated pyrazolines.a |
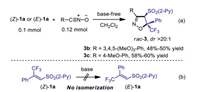
The true mechanism of such kind of stereoconvergent cycloaddition reactions is still not clear, and there have been hot debates about the concerted or stepwise nature of the mechanism based on theoretical studies. According to the kinetic and stereochemical results, Huisgen in 1963 proposed a concerted process but could not rule out an asynchronous mechanism for some specific cases [75-77]. In 1968, Firestone suggested an alternative stepwise mechanism involving a syn-diradical intermediate [78-80]. Following-up mechanism studies focused on the discovery of theoretical models related to a stepwise reaction mechanism [81-89]. Since the 1, 3-DCR of nitrile oxides across trans-alkenes is stereospecific with retaining the trans orientation of two adjacent substituents in cycloadducts, a concerted mechanism is usually admitted. However, to the best of our knowledge, there is still a lack of experimental evidence to put forward the opposite stepwise mechanism. In the present reaction system, the same diastereomer was delivered by injecting isomerically pure (E)−1 or (Z)−1 in different batches (entry 1, Table 1). The probability of initial concerted cycloaddition and subsequent epimerization at the arylsulphonyl carbon center under basic conditions [90] was unlikely in that the 1, 3-DCR with isolated stable nitrile oxides under base-free conditions also produced a single diastereomer of the products 3b and 3c (Scheme 4a). When 1.2 equiv. of D2O was added in the reaction system, no deuteration at the tertiary hydrogen of cycloadduct 6aa was observed (see Supporting information for details). Furthermore, density-functional theory (DFT) calculations have been performed, which showed that the energy barrier during the concerted cycloaddition reaction of (E)−1a and 2a (ΔG⧧ = 21.4 kcal/mol) is too high for a spontaneous process at room temperature; the favored diastereomers (3a and 6aa) exhibit reasonable lower free energies (Scheme 4). On the other hand, the isomerization between vinylsulfone (Z)−1a and (E)−1a under basic conditions was not observed (Scheme 4b).
|
Download:
|
| Scheme 4. Control experiments. | |
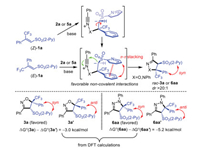
On account of the above results, the present stereoconvergent 1, 3-DCR may involve a stepwise rather than concerted reaction mechanism. As depicted in Scheme 5, firstly, the oxo-Michael addition of phenyl nitrile oxide, or aza-Michael addition of phenyl nitrile imines across alkene (E)−1a or (Z)−1a gives a single putative zwitterion intermediate, which may be oriented and stabilized by the non-covalent interaction between the adjacent two aromatic rings. Since the configuration of (Z)−1a is not retained after the reaction, stereoisomerism resulting from the rotation around the C‒C bond in the intermediate occurs before the next cyclization. Subsequently, the intramolecular nucleophilic attack of carbon anion on the C≡N triple bond proceeds to install thermodynamically more stable isoxazolines or pyrazolines (ΔG0 (3a) ‒ ΔG0 (3a') = ‒3.0 kcal/mol; ΔG0 (6aa) ‒ ΔG0 (6aa') = ‒5.2 kcal/mol) in a highly diastereoselective manner.
|
Download:
|
| Scheme 5. Plausible reaction pathway. | |
In summary, we have successfully achieved the first stereoconvergent 1, 3-dipolar cycloaddition of nitrile oxides and nitrile imines with regard to the configuration of β, β-disubstituted α, β-unsaturated arylsulphone dipolarophiles (E or Z) to get highly valuable trifluoromethylated isoxazolines and pyrazolines as a single diastereomer. During this process two vicinal stereogenic tertiary and trifluoromethylated quaternary centers were readily installed in a single operation, where the aryl and SO2(2-Py) moieties from olefinic dipolarophiles always adopt syn-orientation. Based on the control experiments and theoretical DFT calculations a stepwise mechanism is proposed to rationalize the stereoconvergent outcome. On top of that, the method is featured with mild reaction conditions, broad substrate scope (39 examples), moderate to excellent yields (32%‒95%), and excellent regio- and diastereoselectivity (> 20:1 rr and dr).
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsY.Y. Huang gratefully acknowledges the financial support for this investigation from the National Natural Science Foundation of China (Nos. 21772151, 22072111), and the Fundamental Research Funds for Central Universities (No. WUT: 2021IVA121).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.006.
| [1] |
M. Breugst, H.U. Reissig, Angew. Chem. Int. Ed. 59 (2020) 12293-12307. DOI:10.1002/anie.202003115 |
| [2] |
T. Hashimoto, K. Maruoka, Chem. Rev. 115 (2015) 5366-5412. DOI:10.1021/cr5007182 |
| [3] |
R. Narayan, M. Potowski, Z.J. Jia, A.P. Antonchick, H. Waldmann, Acc. Chem. Res. 47 (2014) 1296-1310. DOI:10.1021/ar400286b |
| [4] |
L.M. Stanley, M.P. Sibi, Chem. Rev. 108 (2008) 2887-2902. DOI:10.1021/cr078371m |
| [5] |
G. Molteni, A. Silvani, Eur. J. Org. Chem. 2021 (2021) 1653-1675. DOI:10.1002/ejoc.202100121 |
| [6] |
L. Wei, X. Chang, C.J. Wang, Acc. Chem. Res. 53 (2020) 1084-1100. DOI:10.1021/acs.accounts.0c00113 |
| [7] |
L.I. Belenkii, Nitrile Oxides, Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, Second Edition, in: H. Feure (Ed.), John Wiley & Sons, Inc., 2008.
|
| [8] |
S. Roscales, J. Plumet, Org. Biomol. Chem. 16 (2018) 8446-8461. DOI:10.1039/C8OB02072H |
| [9] |
J.H. Liao, L. Ouyang, Q. Jin, J. Zhang, R.S. Luo, Org. Biomol. Chem. 18 (2020) 4709-4716. DOI:10.1039/D0OB00963F |
| [10] |
G.P. Savage, Curr. Org. Chem. 14 (2010) 1478-1499. DOI:10.2174/138527210791616812 |
| [11] |
J. Plumet, ChemPlusChem 85 (2020) 2252-2271. DOI:10.1002/cplu.202000448 |
| [12] |
R. Huisgen, M. Seidel, G. Wallbillich, H. Knupfer, Tetrahedron 17 (1962) 3-29. DOI:10.1016/S0040-4020(01)99001-5 |
| [13] |
C. Spiteri, S. Keeling, J.E. Moses, Org. Lett. 12 (2010) 3368-3371. DOI:10.1021/ol101150t |
| [14] |
M.P. Sibi, L.M. Stanley, C.P. Jasperse, J. Am. Chem. Soc. 127 (2005) 8276-8277. DOI:10.1021/ja051650b |
| [15] |
B. Braida, C. Walter, B. Engels, P.C. Hiberty, J. Am. Chem. Soc. 132 (2010) 7631-7637. DOI:10.1021/ja100512d |
| [16] |
D.H. Ess, K.N. Houk, J. Am. Chem. Soc. 130 (2008) 10187-10198. DOI:10.1021/ja800009z |
| [17] |
D.H. Ess, K.N. Houk, J. Am. Chem. Soc. 129 (2007) 10646-10647. DOI:10.1021/ja0734086 |
| [18] |
P. Caramella, P. Grinanger, Nitrile oxides and imines, in: A. Padwa (Ed.), 1, 3-Dipolar Cycloaddition Chemistry, 1, John Wiley & Sons, New York, 1984, pp. 291-392.
|
| [19] |
S. Kanemasa, M. Nishiuchi, A. Kamimura, K. Hori, J. Am. Chem. Soc. 116 (1994) 2324-2339. DOI:10.1021/ja00085a012 |
| [20] |
H. Suga, Y. Hashimoto, Y. Toda, et al., Angew. Chem. Int. Ed. 56 (2017) 11936-11939. DOI:10.1002/anie.201705662 |
| [21] |
M. Schneider, M.J.R. Richter, E.M. Carreira, J. Am. Chem. Soc. 142 (2020) 17802-17809. DOI:10.1021/jacs.0c09520 |
| [22] |
M.A. Weidner-Wells, S.A. Fraga-Spano, I.J. Turchi, J. Org. Chem. 63 (1998) 6319-6328. DOI:10.1021/jo9807621 |
| [23] |
H. Suga, Y. Adachi, K. Fujimoto, et al., J. Org. Chem. 74 (2009) 1099-1113. DOI:10.1021/jo802392c |
| [24] |
X.J. Lian, S.S. Guo, G. Wang, et al., J. Org. Chem. 79 (2014) 7703-7710. DOI:10.1021/jo5012625 |
| [25] |
L. Tu, L.M. Gao, X.M. Wang, et al., J. Org. Chem. 86 (2021) 559-573. DOI:10.1021/acs.joc.0c02244 |
| [26] |
Y.P. Su, Y.A. Zhao, B.B. Chang, et al., J. Org. Chem. 84 (2019) 6719-6728. DOI:10.1021/acs.joc.9b00434 |
| [27] |
A. Singh, A.L. Loomer, G.P. Roth, Org. Lett. 14 (2012) 5266-5269. DOI:10.1021/ol302425h |
| [28] |
G. Wang, X.H. Liu, T.Y. Huang, et al., Org. Lett. 15 (2013) 76-79. DOI:10.1021/ol303097j |
| [29] |
V. Bhat, E.R. Welin, X.L. Guo, B.M. Stoltz, Chem. Rev. 117 (2017) 4528-4561. DOI:10.1021/acs.chemrev.6b00731 |
| [30] |
G.M. Ho, H. Sommer, I. Marek, Org. Lett. 21 (2019) 2913-2917. DOI:10.1021/acs.orglett.9b00946 |
| [31] |
Y.J. Wang, X.Q. Huang, J.S. Hui, L.T. Vo, H.M. Zhao, ACS Catal. 10 (2020) 9431-9437. DOI:10.1021/acscatal.0c02489 |
| [32] |
del Hoyo A.M., A.G. Herraiz, M.G. Suero, Angew. Chem. Int. Ed. 56 (2017) 1610-1613. DOI:10.1002/anie.201610924 |
| [33] |
K. Yang, F. Zhang, T.C. Fang, G. Zhang, Q.L. Song, Angew. Chem. Int. Ed. 58 (2019) 13421-13426. DOI:10.1002/anie.201906057 |
| [34] |
T. Misaki, T. Tatsumi, T. Okamoto, et al., Chem. Eur. J. 24 (2018) 9778-9782. DOI:10.1002/chem.201802271 |
| [35] |
A.N. Baumann, A. Music, J. Dechent, et al., Chem. Eur. J. 26 (2020) 8382-8387. DOI:10.1002/chem.202001394 |
| [36] |
B. Pezzati, M.F. Chellat, J.J. Murphy, et al., Org. Lett. 15 (2013) 2950-2953. DOI:10.1021/ol401042b |
| [37] |
J. Wencel-Delord, F. Colobert, Synthesis (Mass) 48 (2016) 2981-2996. DOI:10.1055/s-0035-1562512 |
| [38] |
H.L. Sang, S.J. Yu, S.Z. Ge, Chem. Sci. 9 (2018) 973-978. DOI:10.1039/C7SC04002D |
| [39] |
R. Zemmouri, M. Kajjout, Y. Castanet, S. Eddarir, C. Rolando, J. Org. Chem. 76 (2011) 7691-7698. DOI:10.1021/jo200798h |
| [40] |
J.K. Park, H.H. Lackey, B.A. Ondrusek, D.T. McQuade, J. Am. Chem. Soc. 133 (2011) 2410-2413. DOI:10.1021/ja1112518 |
| [41] |
Z. Li, H. Yu, H.L. Liu, et al., Chem. Eur. J. 20 (2014) 1731-1736. DOI:10.1002/chem.201303625 |
| [42] |
X.H. He, Y.L. Ji, C. Peng, B. Han, Adv. Synth. Catal. 361 (2019) 1923-1957. DOI:10.1002/adsc.201801647 |
| [43] |
X.Y. Yang, T. Wu, R.J. Phipps, F.D. Toste, Chem. Rev. 115 (2015) 826-870. DOI:10.1021/cr500277b |
| [44] |
T. Furuya, A.S. Kamlet, T. Ritter, Nature 473 (2011) 470-477. DOI:10.1038/nature10108 |
| [45] |
R. Smits, C.D. Cadicamo, K. Burger, B. Koksch, Chem. Soc. Rev. 37 (2008) 1727-1739. DOI:10.1039/b800310f |
| [46] |
H.Z. Gui, Y. Wei, M. Shi, Chem. Asian J. 15 (2020) 1225-1233. DOI:10.1002/asia.202000054 |
| [47] |
Y.Y. Huang, X. Yang, Z. Chen, F. Verpoort, N. Shibata, Chem. Eur. J. 21 (2015) 8664-8684. DOI:10.1002/chem.201500361 |
| [48] |
J. Nie, H.C. Guo, D. Cahard, J.A. Ma, Chem. Rev. 111 (2011) 455-529. DOI:10.1021/cr100166a |
| [49] |
T. Mita, Y. Kudo, T. Mizukoshi, et al., WO 2004018410, 2004.
|
| [50] |
Y. Ozoe, M. Asahi, F. Ozoe, K. Nakahira, T. Mita, Biochem. Bioph. Res. Co. 391 (2010) 744-749. DOI:10.1016/j.bbrc.2009.11.131 |
| [51] |
M. Yaosaka, T. Utsunomiya, Y. Moriyama, T. Matsumoto, K. Matoba, WO 2009001942, 2009.
|
| [52] |
K. Matoba, WO 2009063910, 2009.
|
| [53] |
G.P. Lahm, W.L. Shoop, M. Xu, WO 2007079162, 2007.
|
| [54] |
J.K. Long, T.P. Selby, M. Xu, WO 2009045999, 2009.
|
| [55] |
T. Mita, T. Kikuchi, T. Mizukoshi, M. Yaosaka, M. Komoda, WO 2005085216, 2005.
|
| [56] |
W. Zambach, P. Renold, WO 2009049846, 2009.
|
| [57] |
P. Renold, W. Zambach, P. Maienfisch, M. Muehlebach, WO 2009080250, 2009.
|
| [58] |
T. Murata, Y. Yoneta, J. Mihara, et al., WO 2009112275, 2009.
|
| [59] |
P. García-Reynaga, C.Q. Zhao, R. Sarpong, J.E. Casida, Chem. Res. Toxicol. 26 (2013) 514-516. DOI:10.1021/tx400055p |
| [60] |
W. Cunico, C.A. Cechinel, H.G. Bonacorso, et al., Bioorg. Med. Chem. Lett. 16 (2006) 649-653. DOI:10.1016/j.bmcl.2005.10.033 |
| [61] |
X.Q. Zhang, X.J. Li, G.F. Allan, et al., J. Med. Chem. 50 (2007) 3857-3869. DOI:10.1021/jm0613976 |
| [62] |
R. Pajkert, H. Koroniak, P. Kafarski, G.V. Röschenthaler, Org. Biomol. Chem. 19 (2021) 4871-4876. DOI:10.1039/D1OB00685A |
| [63] |
Y. Furukawa, E. Ikeda, M. Komoda, et al., WO 2007026965, 2007.
|
| [64] |
H. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Angew. Chem. Int. Ed. 50 (2011) 7803-7806. DOI:10.1002/anie.201102442 |
| [65] |
V. Kumar, K. Kaur, J. Fluorine Chem. 180 (2015) 55-97. DOI:10.1016/j.jfluchem.2015.09.004 |
| [66] |
K. Matoba, H. Kawai, T. Furukawa, et al., Angew. Chem. Int. Ed. 49 (2010) 5762-5766. DOI:10.1002/anie.201002065 |
| [67] |
H. Kawai, Y. Sugita, E. Tokunaga, et al., ChemistryOpen 3 (2014) 14-18. DOI:10.1002/open.201300044 |
| [68] |
C.M. Li, X.X. Zhang, J.J. He, S.X. Xu, S. Cao, Chin. J. Chem. 39 (2021) 301-306. DOI:10.1002/cjoc.202000480 |
| [69] |
E.Y. Slobodyanyuk, O.S. Artamonov, O.V. Shishkin, P.K. Mykhailiuk, Eur. J. Org. Chem. 2014 (2014) 2487-2495. DOI:10.1002/ejoc.201301852 |
| [70] |
Z.Y. Wang, Y.Z. Yang, F. Gao, et al., Org. Lett. 20 (2018) 934-937. DOI:10.1021/acs.orglett.7b03811 |
| [71] |
F.G. Zhang, Y. Wei, Y.P. Yi, J. Nie, J.A. Ma, Org. Lett. 16 (2014) 3122-3125. DOI:10.1021/ol501249h |
| [72] |
X. Yang, F. Cheng, Y.D. Kou, et al., Angew. Chem. Int. Ed. 56 (2017) 1510-1514. DOI:10.1002/anie.201610605 |
| [73] |
Y.D. Kou, Z.N. Zhao, X. Yang, et al., Asian J. Org. Chem. 7 (2018) 1830-1834. DOI:10.1002/ajoc.201800435 |
| [74] |
F. Cheng, S.J. Kalita, Z.N. Zhao, et al., Angew. Chem. Int. Ed. 58 (2019) 16637-16643. DOI:10.1002/anie.201908227 |
| [75] |
R. Huisgen, Angew. Chem. Int. Ed. 2 (1963) 565-598. DOI:10.1002/anie.196305651 |
| [76] |
R. Huisgen, Angew. Chem. Int. Ed. 2 (1963) 633-645. DOI:10.1002/anie.196306331 |
| [77] |
R. Huisgen, Angew. Chem. Int. Ed. 7 (1968) 321-328. DOI:10.1002/anie.196803211 |
| [78] |
R.A. Firestone, J. Org. Chem. 33 (1968) 2285-2290. DOI:10.1021/jo01270a023 |
| [79] |
R. Huisgen, J. Org. Chem. 33 (1968) 2291-2297. DOI:10.1021/jo01270a024 |
| [80] |
R. Sustmann, W. Sicking, R. Huisgen, J. Am. Chem. Soc. 125 (2003) 14425-14434. DOI:10.1021/ja0377551 |
| [81] |
S.A. Siadati, Helv. Chim. Acta 99 (2016) 273-280. DOI:10.1002/hlca.201500165 |
| [82] |
R. Huisgen, G. Mloston, E. Langhals, J. Org. Chem. 51 (1986) 4085-4087. DOI:10.1021/jo00371a039 |
| [83] |
R. Huisgen, G. Mloston, E. Langhals, J. Am. Chem. Soc. 108 (1986) 6401-6402. DOI:10.1021/ja00280a053 |
| [84] |
G. Mloston, E. Langhals, R. Huisgen, Tetrahedron Lett. 30 (1989) 5373-5376. DOI:10.1016/S0040-4039(01)93790-6 |
| [85] |
R. Huisgen, J. Penelle, G. Mloston, A.B. Padias, H.K. Hall, J. Am. Chem. Soc. 114 (1992) 266-274. DOI:10.1021/ja00027a035 |
| [86] |
G. Mloston, R. Huisgen, H. Giera, Tetrahedron 58 (2002) 4185-4193. DOI:10.1016/S0040-4020(02)00384-8 |
| [87] |
R. Huisgen, H. Giera, K. Polborn, Tetrahedron 61 (2005) 6143-6153. DOI:10.1016/j.tet.2005.02.062 |
| [88] |
L. Xu, C.E. Doubleday, K.N. Houk, Angew. Chem. Int. Ed. 48 (2009) 2746-2748. DOI:10.1002/anie.200805906 |
| [89] |
Z.X. Yu, K.N. Houk, J. Am. Chem. Soc. 125 (2003) 13825-13830. DOI:10.1021/ja0376487 |
| [90] |
P. Caramella, E. Albini, T. Bandiera, et al., Tetrahedron 39 (1983) 689-699. DOI:10.1016/S0040-4020(01)91846-0 |
 2022, Vol. 33
2022, Vol. 33