b Laboratory of Water Pollution Remediation, School of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou 311121, China
Nitrogen is an essential nutrient substance for aquatic systems and is regarded as a paramount component in the biogeochemical cycle. Increasing anthropogenic activities such as animal husbandry, fertilizer production, landfills, has broken the balance of nitrogen cycle, which consequently results in an undesirable accumulation of various nitrogen compounds (e.g., NH4+, NO2-, NO3-) [1]. Excessive nitrogen discharge causes eutrophication in water bodies, poses a risk for human health and exacerbates the climate change to some extent for the emission of more potent greenhouse gases (N2O) [2-5]. Infact, totalnitrogenof0.3-1.0 mg/Lwasfound tobloom algal in summer, which was far lower than the permissible nitrate concentration in drinking water set by the United States (10 mg NO3--N/L) [6]. The gap between the theoretical standard limits and realistic phenomenawould provide the impetus for development of high effective nitrogen eliminationmethods tomeetmore stringent standard requirement.
Currently, several strategies have been developed to deal with nitrogen-laden wastewater including physico-chemical technology and biological approach. The former methods, such as magnetic anion exchange resin [7], electrodialysis, and reverse osmosis, perform well but the operating cost is high. Besides, in conventional biological nitrogen removal process, intensive aeration during nitrification stage and organic carbon supplement in denitrification procedure are widely employed to achieve a good treatment performance. The relatively higher external organic matter supplements, accompanied by the elevation of cost as well as the secondary organic pollution risk, limited its application in low C/N ratio wastewater such as groundwater and drinking water, where ideally suited for sulfur-driven autotrophic denitrification (SDAD) [8]. SDAD is a process that utilizes the reduced sulfur compounds (e.g., sulfur, sulfide, thiosulfate) as electron donors to reduce nitrate or nitrite into nitrogen gas with no organic carbon addition and lower sludge yields, which gradually enters the public's vision. However, the practical application of this technology remains challenged due to the vulnerable to the varying operation conditions of SDAD bacteria, the generation of sulfate and the alkalinity variation. Besides, the comprehensive assessment of the cost and environmental benefits is still lacking, and these two major factors should be considered in engineering application in advance. Up to now, there are lots of experimental investigations on this technology, while the involved microorganism, influencing factors and application challenges as well as the cost and environmental effect analysis of SDAD process remained to be further clarified.
Thus, this review aims to present a detailed summary of stateof-art studies on the SDAD process, by focusing on: (1) Involved bacteria, (2) crucial factors related to efficiency, (3) application scenarios, and (4) challenges and future prospect. This work may provide a comprehending understanding of SDAD process and subsequently promote its engineering application.
2. Sulfur geochemical cycle and its application in nitrogen removalSulfur, the 14th most abundant element in Earth's crust, plays a vital role in the Earth's geochemical cycle, with its versatile forms such as gypsum (CaSO4), pyrite (FeS2), elemental sulfur (S0) in sediments and sulfate (SO42—) in seawater [9, 10]. With the increasing anthropogenic activities both on industry and agriculture, the balanced biogeochemical cycle of sulfur has been broken, resulting in overflowing odor nuisance, acid rain, sewage corrosion, etc. Hence, the sulfur-related environmental pollution remediation became an urgent issue. To date, there are several technologies including physicochemical and biological approaches applied in remediation of the sulfur-contaminated media. Compared with physicochemical technologies, biological approaches are given as a priority for the treatment of sulfur-laden wastes due to lower operation cost, lower sludge yields, and more potential resource recovery (such as elemental sulfur) [11].
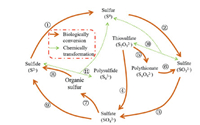
2.1. Microbe mediated sulfur cycleMultiphase nature and diverse intermediate valence states (from —2 to +6) complicate the sulfur cycle, which can be driven chemically and biologically [12]. Since microorganism played an important role in the geochemical cycle of sulfur, nitrogen and carbon, the review herein elucidated the microbe-mediated biological sulfur conversions and transformations. A simplified schematic of the microbial sulfur cycle was presented in Fig. 1. Reduced sulfur compounds such as sulfide, sulfur and thiosulfate served as electron donors for sulfur oxidizing bacteria (SOB) and were oxidized to sulfate via the pathway of steps 1-2-3, steps 2-3 and step 4, respectively. Polysulfide and thiosulfate can be chemically generated via step 11 and step 10, respectively, which further enhances the bioavailability of the sulfur compounds. Also, the thiosulfate in turn can be decomposed into elemental sulfur and sulfite (step 10), or be initially converted to polythionate and subsequently oxidized to sulfate via steps 5-6-3. On the reductive side, sulfate can be either directly converted to sulfide (step 9) by sulfate reducing bacteria (SRB), or assimilated to organic sulfur compounds (e.g., amino sulfur) and ultimately degraded to sulfide by other anaerobic microorganisms (steps 7 and 8) [13, 14].
|
Download:
|
| Fig. 1. Simplified schematic of the microbial sulfur cycle. | |
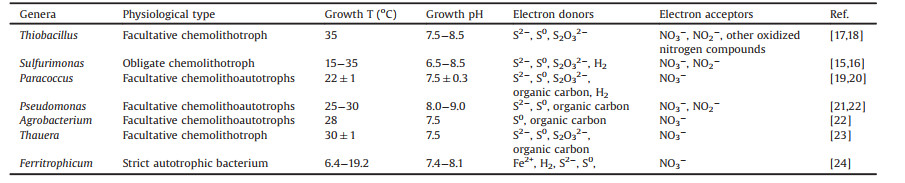
The key microbes involved in the aforementioned sulfur cycle include two groups: SRB and SOB. And the SOB comprised a wide spectrum of chemolithotrophs and phototrophs (e.g., purple sulfur bacteria (PSB) and green sulfur bacteria (GSB)) according to their differences in the energy source and growth conditions. Among them, several SOB are capable of utilizing the reduced sulfur compounds as electron donors to drive nitrate or nitrite reduction, which are termed as SDAD bacteria [11, 15-24] (Table 1). Majority of known SDAD bacteria belong to the phylum Proteobacteria [15]. At the genus level, Sulfurimonas denitrificans (ε-proteobacteria), Thiobacillus denitrificans (β-proteobacteria), were the two most commonly reported SDAD bacteria [15]. For example, Sulfurimonas hongkongensis AST-10 T, isolated from the coastal sediment, was a rod-shaped and Gram-negative chemolithoautotroph bacterium which can use thiosulfate, sulfide or hydrogen as the sole electron donor to drive denitrification process under anoxic/anaerobic conditions [16]. The SDAD bacteria are widely distributed in natural habitats and artificial environments such as marine and terrestrial ecosystem [25], sharing comparatively wide pH and temperature adaptability. Commonly, SDAD bacteria are neutrophilic and mesophilic.
|
|
Table 1 Characteristics of common bacteria in SDAD process. |
Functional enzymes play key roles during bacterial oxidation and reduction process. As the accepted and simplified pathway of sulfide oxidation: S2— to S0 and S0 to SO42—, the involved enzymes were sulfur-oxidizing (Sox) enzymes, sulfide-quinone oxidoreductase (SQR), and flavocytochrome c (FCC) [14, 26, 27]. And the former two were key enzymes for promoting oxidation of S2— to S0, while the latter was also responsible for sulfur oxidation, especially the over oxidization from elemental sulfur or thiosulfate to sulfate [26].
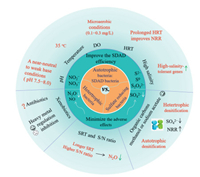
3. Crucial factors affecting SDAD efficiencyAs the autotrophic (mainly refers to the SDAD bacteria), heterotrophic and sulfate-reducing bacteria coexisted in SDAD system, the influencing factors such as operating parameters (e.g., temperature, dissolved oxygen (DO), etc.), carbon sources, and xenobiotics fundamentally affected competitive utilization of substrate by various involved functional bacteria so as to improve the SDAD efficiency and minimize the adverse effects such as intermediate nitrogen compounds and sulfate (Fig. 2).
|
Download:
|
| Fig. 2. Factors affecting efficiency of SDAD. HRT, SRT, and NRR refer to hydraulic retention time, sludge retention time and nitrogen removal rate, respectively. | |
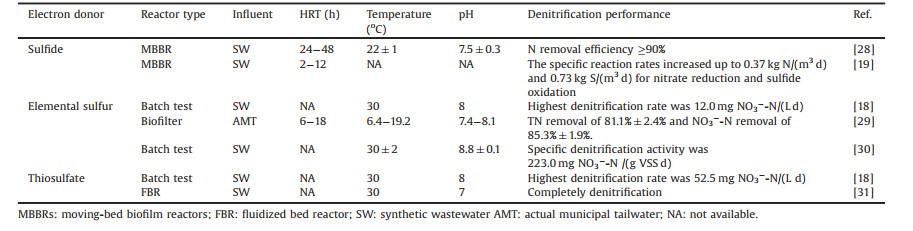
Various substrates (mainly sulfide, elemental sulfur, thiosulfate) have been utilized as electron donors in the SDAD process in order to obtain optimal nitrogen removal efficiency (Table 2 [18, 19, 28-31]).
|
|
Table 2 SDAD with different electron donors. |
Hydrogen sulfide, a well-known odorous component, can serve as an electron donor for simultaneous sulfur and nitrogen removal. Two step reactions were proposed during the biological oxidation of hydrogen sulfide: HS—/S2— to S0 and S0 to SO42— (Eqs. 1 and 2) [19, 28]. Unlike the other reduced sulfur compounds (e.g., sulfur and thiosulfate) involving in denitrification, sulfide-driven autotrophic denitrification commonly results in an alkalinity increase rather than consumption. Additionally, the complete oxidation of sulfide produces less SO42— compared to S0 and S2O32—, which leads to a 5.58 g SO42— generation per gram NO3--N [32]. Thus, it is highly favored for applying sulfide-driven autotrophic denitrification to remedy acidic wastewaters. Chemically-synthesized elemental sulfur (S0chem) has been represented as a feasible electron donor for autotrophic denitri-fication in the treatment of industrial and municipal wastewater, groundwater and drinking water. Despite S0chem was a cheap, non-toxic, chemically stable and readily available electron source [33, 34], the low water solubility (5 μg/L at 20 ℃) induced poor bioavailability limits its larger scale applications [35]. To fix the above drawbacks, electrochemical means was commonly adopted to enhance the conversion of sulfur or its intermediates. Applying a negative potential facilitated the transfer of newly generated negative charged sulfur species (Sx2—) [36]. In addition, biogenic elemental sulfur (S0bio) with an orthorhomic crystalline structure made it more reactive and bioavailable due to its hydrophilic surface and high specific surface area. Capua et al. [18] evaluated the feasibility of using S0bio to drive nitrate removal through batch-scale experiment and results showed that its nitrate removal rate was 1.7 times faster than that of the S0chem. Ucar et al. [35] further proved its feasibility in a membrane bioreactor but found supplying S0bio would result in a higher trans-membrane pressure due to the smaller particle size and colloidal properties. In addition, S0bio induced a lower NO2--N accumulation.
However, elemental sulfur-driven autotrophic denitrification would commonly result in the alkalinity consumption and sulfate formation (removing 1.0 g NO3--N will generate 7.54 g SO42— [36]) according to the reaction Eq. 3 [37, 38]. The above problem may be alleviated by integrating the hetertrophic denitrification or introducing the sulfur-limestone. In addition, nitrite accumulation was observed during sulfur-driven autotrophic denitrification due to the difference affinity of S0 to nitrate or nitrite [18, 39]. The above issue can be fixed either by regulating the operational factors such as sulfur to nitrogen (S/N) ratio, temperature and hydraulic retention time (HRT) or coupling it with anaerobic ammonia oxidation (anammox) process [29, 39].
Thiosulfate is an alternative electron donor for autotrophic denitrification. Capua et al. [18] found thiosulfate could achieve a considerable denitrification rate (52.5 mg NO3--N/(Ld) with S/N ratio of 1.8), which was 10 times higher than that achieved with S0. Similar results were observed by Cardoso in 2006, where they showed that the nitrate removal rate was 4.58 and 9.45 fold higher than the rates with S2— and S0, respectively [40]. More importantly, thiosulfate dosage as high as 2.2 g/L was not found inhibitory to the nitrate removal in pure culture [18]. Similar with the elemental sulfur, using thiosulfate as electron donor would also lead to alkalinity consumption and sulfate formation (shown as in Eq. 4). Moreover, 1 g of NO3--N reduced to N2 accompanied by 12.15 g of SO42— production, which was 1.61 and 2.2 times higher than the SO42— produced using S0 and H2S as electron donors, respectively [32].
3.2. Organic carbonOrganic supplements could facilitate the SDAD process. Qiu et al. [41] firstly demonstrated organic addition accelerated the SDAD rate at batch tests due to the increased sulfur bioavailability by formation of dissolved zero-valent sulfur (e.g., polysulfide) and subsequently suggested an optimal C/N ratio of 0.25—0.5 for the secondary effluent treatment in 272 days laboratory-scale experiments.
Actually, it was unrealistic to undergo absolutely autotrophic denitrification in reactors since endogenous carbon sources such as soluble microbial products and cell lysis products were inevitable [41]. It was revealed that the contribution of SDAD to total nitrogen (TN) removal was 90% on average with no external carbon addition and heterotrophic denitrifying bacteria were commonly found in the SDAD bioreactor based on the sequencing results [35, 41]. Hence, mixotrophic denitrification with a mixed electron donors (reduced sulfur compounds and organic matters) was established. Compared with solo autotrophic denitrification, mixotrophic process possessed higher denitrification rate (0.45 g NO3--N/(Ld) vs. 0.3 g NO3--N/ (Ld)) [42], less sulfate generation (heterotrophic denitrification consumed a proportion of nitrate without the sulfate formation according to Eq. 5) and alkalinity compensation. Different types of external carbon source simultaneously affected the heterotrophic and sulfur-driven autotrophic denitrification. Lee et al. [43] indicated that the dosage of methanol or sodium acetate as supplemental carbon at 60% and 44% of HDNRfraction values (refers to the fraction of NO3--N eliminated by heterotrophic denitrification) achieved complete denitrification without alkalinity addition. In comparison, using glucose and molasses as carbon source required at least 70% of HDNRfraction values for complete denitrification.
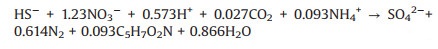
|
(1) |

|
(2) |

|
(3) |
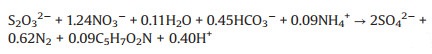
|
(4) |
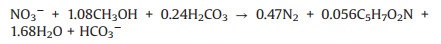
|
(5) |
Currently, the mixotrophic denitrification gained more popularity in treating nitrate and sulfur polluted secondary effluent [20, 41], drinking water [42, 44] and groundwater [45] at lab-, pilotand even full-scale. It worth noting that professor Ren's group has isolated Pseudomonas sp. C27 in 2013 [46], and conducted a series of C-N-S related studies under sulfide stress [47], mixotrophic growth condition [21, 48], micro-aeration condition [49] by applying proteomics analysis technology, which contributed to the thorough comprehension of the mechanism and pathway of bioreaction. However, further studies aimed to mixed microorganism and their synergistic interactions as well as regulating strategies should be considered with the method of omics technology prior to practical application.
3.3. Xenobiotics: antibiotics or/and heavy metalAntibiotics, widely detected in wastewaters, leads to the resistant bacteria occurrence, antibiotics resistance gene transference and ultimately disastrous harm to human health. Hence, it is urgent to interpret the mechanism of antibiotics on the biological nitrogen removal including the SDAD.
However, up to date, the influence of antibiotics on SDAD system was scarcely reported. It was observed that denitrification was more vulnerably affected than that of nitrification and carbon oxidization under sulfamethoxazole and tetracycline hydrochloride stress [50]. Extracellular polymeric substances (EPS) play an important role in resisting the adverse effects of antibiotics. Liu et al. [51] found tetracycline up to 250 mg/Ldid not inhibit the removal rates of nitrate, owing to the large secretion of protein (PN: one of the main components of EPS) in denitrifying sulfide removal process. Moreover, the functional group in EPS could provide adsorption sites for antibiotics such as ciprofloxacin (CIP) and thus protected the microbes against its toxicity [52]. Biodegradation was another pathway for antibiotics dissipation. In SRB sludge system, nearly 28.0% of CIP was biodegraded via desethylation reaction in piperazinyl ring and hydroxylation reaction catalyzed by cytochrome P450 enzymes. And these biodegradation intermediates coupled with the changes in antibiotics resistance genes would facilitate understanding the CIP biodegradation mechanism, which will promote the application of sulfur-mediated biological process in CIP-containing wastewater treatment [53]. Multiple antibiotics appeared synergistic effects on denitrification inhibition and N2O release [54]. Interestingly, ultralow antibiotics dosage resulted in the hormesis effect [54]. As for SDAD, it may be prone to be inhibited by single or multiple antibiotics, but may be regulated to achieve optimal performances by feasible antibiotics dosage within the concentration set by hormesis effect. Moreover, attention should be paid on the horizontal antibiotics resistant genes transference under longterm antibiotics stress.
In addition to antibiotics, other xenobiotics such as heavy metal also affect the performance of SDAD. Chen et al. [3] revealed that the inhibition of denitrification activities became more severe with the increasing concentration of vanadium(V) due to the increases of lactate dehydrogenase release and reactive oxygen species production. Capua et al. [55] showed complete nitrate and nitrite removal was achieved in a fluidized-bed reactor under the giving addition as high as 100 and 200 mg Ni/L of NiEDTA2— and NiCl2, respectively. Besides, some multivalent metals, particularly toxic in a certain valence state, could be converted to be nontoxic metals during SDAD process. Sahinkaya et al. [56] found the reduction efficiency at 1 mg/L Cr(Ⅵ) declined from 95% to 32% when the effluent nitrate concentration increased from 5 to 30 mg NO3--N/L, while 5 mg/L Cr(Ⅵ) had significant inhibition on autotrophic denitrifiers. In-depth understanding the effects of the abovementioned heavy metals on SDAD is still required for its practical application.
3.4. Other influencing factorsOperating parameters such as pH, temperature, dissolved oxygen, HRT and salinity also affected the nitrogen and sulfur removal efficiency of SDAD process. Autotrophic denitrification was strongly influenced by pH and temperature. As shown in Table 2, a near-neutral to weak base conditions and medium temperature would contribute to the optimal performance. Fajardo et al. [57] conducted experiments at 35 ℃ (tested range: 15 ℃, 25 ℃, 35 ℃) and pH 7.5 and 8.0 showed the highest denitrification efficiency. Also, Sposob et al. [58] demonstrated that the sulfide removal was dropped by 9% when temperature declined from 25 ℃ to 10 ℃ and the nitrate was completely removed in studied temperature range, which was due to the microbial community shift induced by temperature. As the micro-aerobic condition could stimulate the activity of sulfur oxidase and accordingly promote the sulfur and nitrogen removal performance. Wang et al. [59] reported the sulfide and nitrate removal efficiency was achieved at 100% and 90%, respectively, under DO concentration of 0.1-0.3 mg/L. Li et al. [29] prolonged the hydraulic retention time from 6 h to 18 h to offset the low temperature inhibition (from 16.3 to 19.2 ℃ to 6.4-9.8 ℃) and contrarily increased nitrogen removal efficiency. In addition, a complete sulfur-based autotrophic denitrification was achieved at a salinity of 15 g/L when treating graphite production-derived wastewater, which ascribed to the high expression of various high-salinity-tolerant genes in Thiobacillus [60]. Thus, it was upmost and cost-effective to optimize these influencing parameters to achieve the best sulfur and nitrogen removal performance.
Besides, operating parameters should be regulated to minimize the adverse effects of by-products during the SDAD process. For example, dissolved nitrous oxide (N2O) were observed and accumulated at low temperature due to the relatively low N2O reduction rate during the SDAD process, but was lower than that of heterotrophic denitrification [61, 62]. The N2O reduction rate was inhibited by free nitrous acid (FNA) or the co-inhibition of FNA and sulfide [58, 63]. Longer sludge retention time or higher initial S/N ratio was suggested to solve aforementioned problem, but the latter would add operational cost [62, 63].
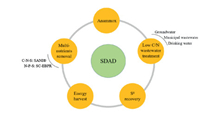
4. Application scenarios of SDAD processThe SDAD process has been widely applied for the low C/N ratio nitrate-polluted wastewater treatment, and its ability of elemental sulfur reclamation and energy harvest has arouse research interest. Besides, it has extended its application fields by integrating other biological nitrogen removal strategies such as anammox and sulfate reduction autotrophic denitrification and nitrification integrated (SANI®) process (Fig. 3).
|
Download:
|
| Fig. 3. Application scenarios of SDAD process. | |
SDAD is promising in treating low C/N ratio wastewaters such as groundwater, secondary effluent of municipal wastewater and even drinking water.
SDAD-based nitrate groundwater remediation had long been studied. Researchers initially introduced sulfur and limestone as an electron donor and a acidity buffer, respectively [64], and then gradually turned to more cost-effective and environmental friendly substrates such as CaCO3-type kitchen wastes to remedy the nitrate-polluted groundwater [65]. Besides, mixotrophic denitrification and bioelectrochemical reaction were recently conducted for application apart from the merely SDAD and bioreaction [44, 66], which no doubt indicated the recognition and consensus of the application of SDAD in nitrate remediation of groundwater. However, the previous studies were mainly at laboratory-scale, rather than in-situ remediation. Due to the complex groundwater environment, the ideal remediation environment of the laboratory was not always suitable for field remediation. Accordingly, researches regarding the field remediation are still required in the future.
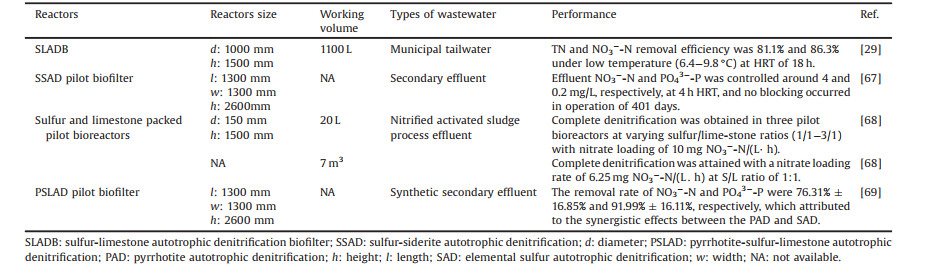
Pilot-scale SDAD had been applied for treating municipal tailwater and second effluent of WWTP (Table 3 [29, 67-69]). Li et al. [29] achieved reliable treatment performance of sulfurlimestone autotrophic denitrification biofilter under low temperature (6.4—9.8 ℃) for municipal tailwater with TN and NO3--N removal efficiency of 81.1% and 86.3%, respectively, at HRT of 18 h. Wang et al. [67] demonstrated that 28 mg NO3--N/L was completely removed at 12 h HRT with optimal volume ratio of sulfur and siderite of 1:3. And no blocking of pilot-scale biofilter was observed during 401 days operation. SDAD can easily be integrated with current mainstream wastewater treatment systems to treat carbon-deficient wastewaters, either as a pre- or post- treatment unit [14].
|
|
Table 3 SDAD in pilot study or engineering application. |
Membrane bioreactor was commonly applied to start up SDAD to treat drinking water. It was found that a complete denitrification could be achieved with no significant membrane fouling at fluxes ≤ 20 L/(m2 h). However, the generated sulfate was close to the theoretical values [70]. And the sulfate permitted value in drinking water is 250 mg/L in China, so it needs for close attention. Mixotrophic denitrification process was considered to be the feasible choice for advantages of elimination of excess sulfate production and alkalinity requirement [42, 43].
Theoretically, it could be considered to adopt SDAD or other coupling system when various forms of sulfur and nitrogen coexist with not extremely acidic or alkaline environment.
4.2. S0 reclamation and energy harvestIt was accepted that there were two step reactions for the sulfide autotrophic denitrification: S2— to S0 and S0 to SO42—. As the oxidation rate of S2— to S0 was 3.31 times faster than that of S0 to SO42—, the S0 accumulation would be observed under certain condition [71]. The regulation of S/N was an effective strategy. Chen et al. [72] examined S/N molar ratio of 5/2 received the higher S0 recovery than the 5/5 or 5/8. Huang et al. [73] revealed that the S2—-S/NO3--N ratio higher or lower than 5/6 failed to reach the optimum S0 reclamation. Because the low S/N (5/8 and 5/9) implied the sufficient supply of nitrate resulting the further oxidization of S0 to SO42—, which was confirmed by the highly expressed nirK and Sox genes. Under extremely high S/N (5/2), oxidized nitrogen supplement became the limiting step, as revealed by the low expressed nirK gene. Yuan et al. [71] found S2—-S/NO3--N ratio (molar ratio) of 0.9:1 would harvest a desired S0, while the generated S0 was subsequently oxidized to SO42— when S2—-S/NO3--N ratio of 0.5:1. Restated, a comparatively higher S/N ratio flavored for S0 formation. However, the regulation of S/N ratio to control the fate of sulfide oxidation was species-specific. For example, Thiobacillus denitrificans could achieve partial sulfide oxidation, conversely, sulfate is the only possible oxidation product for Thiomicrospira CVO [25].
In addition, reactor height and influent loading affected the S0 recovery. The S0 recovery efficiency was improved from 7.4%-78.8% when working height of UASB reactor was shortened from 60 cm to 30 cm, corresponding to the remarkably modification of bacterial community [74]. An influent loading regulated by acetate/nitrate/ sulfide: 1.9/1.6/0.7 kg/(d m3) obtained highest S0 recovery, since the too low or too high influent loading represents insufficient oxidation or over-oxidation of sulfide [26].
S0 accumulated and stored in the cytoplasm when nitrate was scarce, and could be further oxidized to sulfates under a sufficient nitrate supply. The yield of S0 in reactor was indirectly calculated according to the principle of mass conservation, which was possibly due to the lack of directly measuring method and separating or recovering skills. If the S0 was over produced and not timely discharged from the system, it would elevate the mass transfer resistance and ultimately slow down the denitrification efficiency. In order to solve this problem, Chen et al. [72] tested the feasibility of using Zn(Ⅱ) or other cations as adsorbates to recover S0 via electrostatic interactions. S0 is micro-sized colloids with negatively charged surface, providing an emerging way to separation and reclamation of S0. Chen et al. [75] suggested 2.42 mg polyaluminum chloride (PAC) per mg S dosage can achieve optimum S0 flocculation rate of 97.5% at pH 4.74 under 129 rpm, with the aid of the response surface methodology. Although it adopted a novel approach for S0 recovery, it was a tough issue to be applied in situ since the sludge would be flocculated as well. And if used off situ, it increased the operation cost due to the extra land needed. Thus it is urgent to pursuit a high efficient, economic and elegant situ S0 recovery technology.
From energy harvest point of view, microbial fuel cells (MFCs), a new approach as energy-positive wastewater treatment, are gaining increasing popularity because of their potential to tackle environmental pollution and energy shortages confronting in the world [76, 77]. And it has been widely used to treat organic, sulfurous and nitrogenous contaminated wastewater. Specifically, in sulfide-driven microbial fuel cells, sulfide represents a superior electron donor due to its very low redox potential (S/H2S: —0.28 V versus standard hydrogen electrode at pH 7.0) [78] and its ability of transferring eight electrons per sulfur atom by both abiotic and biotic process [79]. The produced electrons were then transferred through external circuit to the cathode where nitrate or other oxidized pollutants were subsequently reduced, concomitantly generating electricity during redox reaction [80, 81]. For example, the SDAD-MFCs obtained a columbic efficiency of 53.0% ± 2.2% at the optimal feeding total organic carbon to sulfide (TOC/S) mass ratio of 4.69 and cathodic feeding DO of 4.2 mg/L in a loop microbial fuel cell system [82], and a sum coulomb production of 554.8 C/d was achieved with desirable feeding S/N molar ratio at 3 in a coupled nitrifying and denitrifying sulfide removal MFC system [83].
Apart from energy harvest, operational parameters affecting the sulfur or TN removal efficiency such as cathode potential [81, 84], temperature [77, 78] and current intensities [85] should be taken into consideration. Wang et al. [81] stated a cathode potential of —139 ± 37 mV favored the optimal sulfur and gaseous nitrogen behavior with formation percent of 32.4% ± 1.9% and 92.5% ± 0.3%, respectively, in potentiostatic three-chamber MFC which could simulate the electron driving force situation occurring in a MFC by its uncontrolled electrode. However, Wu et al. [84] indicated the maximum TN removal efficiency and nitrification efficiency were achieved 56.9% ± 3.2% and 99% respectively with a cathode potential of —1.0 V in electrochemically assisted SANI® process. Restated, the different effects of cathode potential may be due to the different MFCs configuration. Besides, the optimal temperature for maximum TN removal efficiency and power density located in 30 ℃ despite of various MFCs configuration [77, 78, 83], at which the key functional microorganism worked the best. Wang et al. [85] found a 200 mA of current gained the best TN removal efficiency (84.6%) and lowest nitrite accumulation in the combined bioelectrochemical and SDAD system where the hydrogen autotrophic denitrification was the domain process. Controversially, the effluent sulfate concentration gradually increased with the rising current from 50 mA to 300 mA, which was in inconsistence with the relative contribution of hydrogen and sulfur-based autotrophic denitrification to nitrate removal [85]. Thus, further researches concerning the sulfate control are needed.
The SDAD-MFCs provided a novel approach for simultaneously removing organics, sulfide and ammonium coupled with electricity generation from wastewater. However, the main challenges hampering the further and broad application is the electrode poisoning or fouling (elemental sulfur deposition) [11], which deserves further study.
4.3. Coupling with nutrients removal for municipal wastewater treatmentThe sulfur cycle intimately interacts with the carbon, nitrogen and phosphorous cycles [86]. And the removal of sulfur pollutants was also bounded to be related to carbon, nitrogen and phosphorus removal process, so as to form a multi-elemental pollutants removal strategy. Among these removal approaches, two typical processes-SANI® process and sulfur conversion-associated enhanced biological phosphorus removal (SC-EBPR) process-are considered as promising approaches to achieve C-N-S and N-P-S removal, respectively.
In the near decades, Professor Chen's group focused on saline seawater decarbonization and denitrification, established SANI®- process, and successfully put this process into full-scale application (800—1000 m3/d) in Hong Kong wastewater treatment system [87]. SANI® process consists three biological reactions. Sulfate is reduced to sulfide, an electron donor for subsequent autotrophic denitrification, meanwhile the organic carbon is oxidized to carbon dioxide in the first section; in the second reaction, SOB use the sulfide as an electron donor and nitrate or nitrite as electron acceptors to achieve completely sulfide autotrophic denitrification, forming nitrogen gas and regenerating sulfate; in the last reactor, ammonia is oxidized to nitrate by the autotrophic nitrifiers [87]. Interestingly, the sulfate-sulfide seemed in a closed cycle, which provides an idea for the control of sulfate in SDAD approach.
Compared with conventional activated sludge plants in Hong Kong, the SANI® system had prominent advantages: 30%-40% less land occupation, 60%-70% less biological sludge production and elegant effluent quality [87]. And the SANI® system can extend its application in treating freshwater sewages in land and cold areas [88, 89].
In N-P-S simultaneous removal system, limestone was first introduced as a source of inorganic carbon and alkalinity [29, 35] and positively promotes the reaction. Besides, the released Ca2+ can remove the phosphate by forming calcium phosphate precipitate. But, phosphate removal was no more than 60% owing to the inappropriate pH for the precipitation [90, 91]. Thus, iron salts or minerals both naturally and artificially made such as siderite, pyrrhotite were alternatives to limestone not only because the optimal pHforironphosphate precipitate, but also the produced Fe2+ could drive a Fe-based autotrophic denitrification. Wang et al. [67] demonstrated stable NO3- and PO43— removal were achieved at sulfur/siderite of 1/3 in treating secondary effluent within 401 days. But, the used sideritein this studywas notused as an electron donor. Besides, Yang et al. [90] demonstrated the nanostructured pyrrhotite had higher N and P removal rate at shorter HRT due to a much higher specific surface areas compared with natural pyrrhotite particles when treating real secondary effluent. The recycled phosphate salts precipitate contained P2O5 so high that it could be used as fertilizer [91]. The above sulfur conversion-associated enhanced biological phosphorus removal process broadened the application of SDAD and the novel resource recycle skills.
4.4. Coupling with other biological nitrogen removal processThe co-existence of various nitrogen (e.g., ammonia, nitrite and nitrate) and sulfur in actual wastewater and similar niches of the two functional bacteria favored the coupling of SDAD and anammox. The coupling system can simultaneously remove the nitrate, nitrite, ammonium, and reduced sulfur compounds in wastewater. It was revealed that the anammox and SDAD contributed to about 90% and 10% of the total nitrogen removal, respectively [92, 93]. However, the competition of co-substrate (e.g., nitrite) between the sulfur oxidizing bacteria and anammox bacteria [93] as well as the inhibition of low levels of sulfide [94] on anammox activityhampered thewidespread use of this coupling system. Particularly, the constant supply of nitrite was of upmost importance because SDAD could convert the nitrate generated by anammox process into nitrogen gas or intermediate: nitrite which was in turn the available substrate for Anammox bacteria. Hence, some researchers proposed the integrated anammox with sulfur driven partial denitrification with nitrite as the main product [95] and separately coupling strategies [96] to fix the aforementioned bottlenecks. And it obtained over 95%-98.1% [97, 98] and 99.15% ± 0.68% [96] of the total nitrogen removal efficiency based on above two strategies under certain operating condition, respectively. Nonetheless, the high partial denitrification performance required precise operation conditions [2] and the separately coupling system occupied larger land area. Thus, Professor Jin's group tried integrating completely SDAD with anammox in a single reactor and successfully started up the coupling system with the combination of the anammox and methanogenic granules [99] or merely anammox sludge [2]. It was worth mentioning that the anammox sludge could be used as seeding sludge, providing excellent SDAD performance, while the SDAD sludge could reversely start anammox process quickly [100]. This provides a novel strategy for anammox consortia preservation [101]. Apart from the startup of the coupling system, several studies aimed how sulfur to nitrate ratio as well as other factors affected the total nitrogen removal efficiency, which contributed to better understand the mechanism of the coupling systems.
5. Challenges and future needs for feasible application 5.1. The effluent sulfate problemExcessive intake of sulfate would cause diarrhea, dehydration, and gastrointestinal disorders to human being. Currently, the allowance limit of sulfate for drinking water is 250 mg/L in China, which is equivalent to permit concentration of 20.15 mg NO3--N/L if take the thiosulfate as an electron donor without consideration of the background value of sulfate in water. In addition, there is temporarily no national mandatory discharge standard like TN and NH4+. If not effectively controlled, the sulfate would be conversely reduced into sulfide by SRB, especially in the reduced environment such as sediments and soils [37]. Hence, the sulfate problem cannot be neglected and the mitigation strategies should be made in advance. On the one hand, reducing the sulfate generation is a priority by introducingthe mixotrophic denitrification, precise control strategy (S2— to S0) and novel S0 reclamation technology at source. On the other hand, the sulfate in effluent could be decreased to the permit values set by national or specific industry by combing with end-of-pipe treatments such as reverse osmosis or precipitation which may require high operating cost. Unlike reverse osmosis, forward osmosis, an osmotic-pressure driven membrane process, emerged as a relatively cost-effective and promising method for water and nutrient recovery. Yao et al. [102] successfully achieved seawater pretreatment with a nano-filtration-like forward osmosis membrane, which provide new insights to the sulfate control during SDAD process.
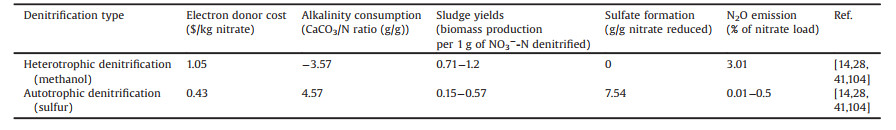
5.2. Comparison of cost and environmental effectsReactor performance is not the unique parameter to be considered in the choice of a SDAD system [103]. Operation costs such as electron donor, alkalinity compensation and sludge disposal as well as the environmental effects needs to be considered. As was shown in Table 4 [14, 28, 41, 104], autotrophic denitrification was a cost-effective and environmental friendly process compared with heterotrophic denitrification, but the sulfate formation was a tricky issue.
|
|
Table 4 Comparison of cost and environmental effects for autotrophic denitrification versus heterotrophic denitrification. |
SDAD had recently been intensively studied in low C/N ratio wastewater treatment. However, how to organically integrate the SDAD with currently conventional wastewater treatment process and achieve full-scale application merit further exploration. As the sulfate generation during the SDAD process, this issue should be solved within system from the elemental cycle system point of view. In other words, it needs to strive to identify cleaner production opportunities at source or in the production process to control or remove sulfates. At last, the coupling of SDAD and emerging processes such as MFCs need further developed in order to pursuit energy-positive and environmental friendly process.
Declaration of competing interestNo conflict of interest exits in the submission of this manuscript, and the manuscript is approved by all authors for publication in Chinese Chemical Letters. This manuscript has not been published in part or fully and is not under consideration for publication or submission elsewhere.
AcknowledgmentThe authors would like to acknowledge the financial support from the National Natural Science Foundation of China (No. 51878231).
| [1] |
K.Y. Show, D.J. Lee, X.L. Pan, Biotechnol. Adv. 31 (2013) 409-420. DOI:10.1016/j.biotechadv.2012.12.006 |
| [2] |
L.Z.J. Xu, W.J. Xia, M.J. Yu, et al., Sci. Total Environ. 682 (2019) 374-381. DOI:10.1016/j.scitotenv.2019.05.147 |
| [3] |
D. Chen, Z.X. Xiao, H.Y. Wang, K. Yang, Bioresour. Technol. 264 (2018) 319-326. DOI:10.1016/j.biortech.2018.05.093 |
| [4] |
Z.Z. Zhang, Y.F. Cheng, B.Q. Zhu, et al., Sci. Total Environ. 653 (2019) 342-350. DOI:10.1016/j.scitotenv.2018.10.401 |
| [5] |
L. Li, Y. Ling, H.Y. Wang, et al., Chin. Chem. Lett. 31 (2020) 28-38. DOI:10.1016/j.cclet.2019.06.035 |
| [6] |
K.I. Suga, N. Fukui, M. Tatani, IFAC Proc. 31 (1998) 553-559. |
| [7] |
Y. Zhou, C.D. Shuang, Q. Zhou, et al., Chin. Chem. Lett. 23 (2012) 813-816. DOI:10.1016/j.cclet.2012.05.010 |
| [8] |
M. Mora, A.D. Dorado, X. Gamisans, et al., Chem. Eng. J. 262 (2015) 235-241. DOI:10.1016/j.cej.2014.09.101 |
| [9] |
R.J. Charlson, T.L. Anderson, R.E. McDuff, Int. Geophysics 72 (2000) 343-359. |
| [10] |
G. Guo, G.A. Ekama, Y.Y. Wang, et al., Bioresour. Technol. 285 (2019) 121303. DOI:10.1016/j.biortech.2019.03.142 |
| [11] |
S. Lin, H.R. Mackey, T.W. Hao, et al., Water Res. 143 (2018) 399-415. DOI:10.1016/j.watres.2018.06.051 |
| [12] |
T.W. Hao, P.Y. Xiang, H.R. Mackey, et al., Water Res. 65 (2014) 1-21. DOI:10.1016/j.watres.2014.06.043 |
| [13] |
K. Tang, V. Baskaran, M. Nemati, Biochem. Eng. J. 44 (2009) 73-94. DOI:10.1016/j.bej.2008.12.011 |
| [14] |
Y.X. Cui, B.K. Biswal, G. Guo, et al., Appl. Microbiol. Biotechnol. 103 (2019) 6023-6039. DOI:10.1007/s00253-019-09935-4 |
| [15] |
M. Zhang, T. Zhang, M.F. Shao, H.H.P. Fang, Chemosphere 76 (2009) 677-682. DOI:10.1016/j.chemosphere.2009.03.066 |
| [16] |
L. Cai, M.F. Shao, T. Zhang, Stand. Genomic Sci. 9 (2014) 1302-1310. DOI:10.4056/sigs.4948668 |
| [17] |
Y.W. Zhang, D.Y. Wei, L. Morrison, et al., Sci. Total Environ. 662 (2019) 287-296. DOI:10.1016/j.scitotenv.2019.01.230 |
| [18] |
F.D. Capua, S.H. Ahoranta, S. Papirio, et al., Process Biochem. 51 (2016) 1576-1584. DOI:10.1016/j.procbio.2016.06.010 |
| [19] |
Y.X. Cui, B.K. Biswal, M.C.M. Loosdrecht, et al., Water Res. 166 (2019) 115038. DOI:10.1016/j.watres.2019.115038 |
| [20] |
S.S. Sun, J. Liu, M.P. Zhang, S.B. He, Bioresour. Technol. 300 (2020) 122651. DOI:10.1016/j.biortech.2019.122651 |
| [21] |
H.L. Guo, C. Chen, D.J. Lee, et al., Bioresour. Technol. 171 (2014) 120-126. DOI:10.1016/j.biortech.2014.08.035 |
| [22] |
D. Tian, H.K. Shon, D.J. Chun, et al., Biotechnol. Lett. 25 (2003) 1605-1608. DOI:10.1023/A:1025678914181 |
| [23] |
L.L. Zhang, C. Zhang, C.Z. Hu, et al., Water Res. 85 (2015) 422-431. DOI:10.1016/j.watres.2015.08.055 |
| [24] |
D. Hafenbradl, M. Keller, R. Dirmeier, et al., Arch.Microbiol. 166 (1996) 308-314. DOI:10.1007/s002030050388 |
| [25] |
M.F. Shao, T. Zhang, H.H.P. Fang, Appl. Microbiol. Biotechnol. 88 (2010) 1027-1042. DOI:10.1007/s00253-010-2847-1 |
| [26] |
C. Huang, Q. Liu, C. Chen, et al., J. Hazard. Mater. 324 (2017) 48-53. DOI:10.1016/j.jhazmat.2016.03.024 |
| [27] |
H. Lu, H.Q. Huang, W.M. Yang, et al., Water Res. 133 (2018) 165-172. DOI:10.1016/j.watres.2018.01.022 |
| [28] |
Y.X. Cui, G. Guo, B.K. Biswal, G.H. Chen, D. Wu, Int. Biodeterior. Biodegrad. 140 (2019) 90-98. DOI:10.1016/j.ibiod.2019.03.016 |
| [29] |
Y.Y. Li, Y.L. Wang, D.J. Wan, et al., Bioresour. Technol. 300 (2020) 122682. DOI:10.1016/j.biortech.2019.122682 |
| [30] |
A. Kostrytsia, S. Papirio, L. Morrison, et al., Bioresour. Technol. 270 (2018) 359-367. DOI:10.1016/j.biortech.2018.09.044 |
| [31] |
G. Zou, S. Papirio, A.M. Lakaniemi, et al., Chem. Eng. J. 284 (2016) 1287-1294. DOI:10.1016/j.cej.2015.09.074 |
| [32] |
F.D. Capua, F. Pirozzi, P.N.L. Lens, G. Esposito, Chem. Eng. J. 362 (2019) 922-937. DOI:10.1016/j.cej.2019.01.069 |
| [33] |
H.S. Moon, S.W. Chang, K. Nam, J. Choe, J.Y. Kim, Environ. Pollut. 144 (2006) 802-807. DOI:10.1016/j.envpol.2006.02.020 |
| [34] |
E. Sahinkaya, A. Yurtsever, Ö. Aktas, D. Ucar, Z.W. Wang, Chem. Eng. J. 268 (2015) 180-186. DOI:10.1016/j.cej.2015.01.045 |
| [35] |
D. Ucar, T. Yilmaz, F.D. Capua, et al., Bioresour. Technol. 299 (2020) 122574. DOI:10.1016/j.biortech.2019.122574 |
| [36] |
W. Hao, B. Miao, P.P. Liu, X. Huang, P. Liang, J. Clean. Prod. 228 (2019) 94-100. DOI:10.1016/j.jclepro.2019.04.221 |
| [37] |
T. Tian, H.Q. Yu, Bioresour. Technol. 299 (2020) 122686. DOI:10.1016/j.biortech.2019.122686 |
| [38] |
M. Mora, A. Guisasola, X. Gamisans, D. Gabriel, et al., Chemosphere 113 (2014) 1-8. DOI:10.1016/j.chemosphere.2014.03.083 |
| [39] |
F.M. Chen, X. Li, C.W. Gu, Y. Huang, Y. Yuan, Bioresour. Technol. 266 (2018) 211-219. DOI:10.1016/j.biortech.2018.06.062 |
| [40] |
R.B. Cardoso, R. S. Alvarez, P. Rowlette, et al., Biotechnol. Bioeng. 95 (2006) 1148-1157. DOI:10.1002/bit.21084 |
| [41] |
Y.Y. Qiu, L. Zhang, X.T. Mu, et al., Water Res. 169 (2020) 115084. DOI:10.1016/j.watres.2019.115084 |
| [42] |
E. Sahinkaya, N. Dursun, Chemosphere 89 (2012) 144-149. DOI:10.1016/j.chemosphere.2012.05.029 |
| [43] |
D.U. Lee, I.S. Lee, Y.D. Choi, J.H. Bae, Proc. Biochem. 36 (2001) 1215-1224. DOI:10.1016/S0032-9592(01)00163-7 |
| [44] |
E. Sahinkaya, N. Dursun, A. Kilic, et al., Water Res. 45 (2011) 6661-6667. DOI:10.1016/j.watres.2011.09.056 |
| [45] |
Y.W. Liu, H.H. Ngo, W.S. Guo, et al., Chem. Eng. Sci. 172 (2017) 414-422. DOI:10.1016/j.ces.2017.07.005 |
| [46] |
C. Chen, K.L. Ho, F.C. Liu, et al., Bioresour. Technol. 145 (2013) 351-356. DOI:10.1016/j.biortech.2012.12.027 |
| [47] |
H.L. Guo, C. Chen, D.J. Lee, A.J. Wang, N.Q. Ren, Enzyme Microb. Technol. 53 (2013) 6-12. DOI:10.1016/j.enzmictec.2013.04.002 |
| [48] |
H.L. Guo, C. Chen, D.J. Lee, Bioresour. Technol. 293 (2019) 122169. DOI:10.1016/j.biortech.2019.122169 |
| [49] |
H.L. Guo, C. Chen, D.J. Lee, A.J. Wang, N.Q. Ren, Enzyme Microb. Technol. 56 (2014) 20-27. DOI:10.1016/j.enzmictec.2013.12.013 |
| [50] |
Y.J. Zhu, Y.Y. Wang, X.X. Jiang, et al., Chem. Eng. J. 325 (2017) 300-309. DOI:10.1016/j.cej.2017.05.073 |
| [51] |
C.S. Liu, J. Xu, D.J. Lee, D.Y. Yu, L.H. Liu, Bioresour. Technol. 205 (2016) 254-257. DOI:10.1016/j.biortech.2016.01.026 |
| [52] |
H.Q. Zhang, S.L. Song, Y.Y. Jia, D. Wu, H. Lu, Water Res. 164 (2019) 114965. |
| [53] |
Y.Y. Jia, S.K. Khanal, H.Y. Shu, et al., Water Res. 136 (2018) 64-74. DOI:10.1016/j.watres.2018.02.057 |
| [54] |
G.Y. Yin, L.J. Hou, M. Liu, et al., Chemosphere 171 (2017) 118-125. DOI:10.1016/j.chemosphere.2016.12.068 |
| [55] |
F.D. Capua, I. Milone, A.M. Lakaniemi, et al., Bioresour. Technol. 238 (2017) 534-541. DOI:10.1016/j.biortech.2017.04.082 |
| [56] |
E. Sahinkaya, A. Yurtsever, D. Ucar, J. Hazard. Mater. 324 (2017) 15-21. DOI:10.1016/j.jhazmat.2016.02.032 |
| [57] |
C. Fajardo, M. Mora, I. Fernández, et al., Chemosphere 97 (2014) 10-15. DOI:10.1016/j.chemosphere.2013.10.028 |
| [58] |
M. Sposob, A.C. Kwiatkowska, R. Bakke, et al., Process Biochem. 69 (2018) 161-168. DOI:10.1016/j.procbio.2018.03.006 |
| [59] |
X.W. Wang, Y. Zhang, J.T. Zhou, et al., Bioresour. Technol. 182 (2015) 75-81. DOI:10.1016/j.biortech.2015.01.123 |
| [60] |
X.C. Xu, R. Zhang, H.B. Jiang, et al., Bioresour. Technol. 306 (2020) 123117. DOI:10.1016/j.biortech.2020.123117 |
| [61] |
Z. Wang, X. Fei, S.B. He, J.C. Huang, W.L. Zhou, Sci. Total Environ. 579 (2017) 1706-1714. DOI:10.1016/j.scitotenv.2016.11.194 |
| [62] |
Y.W. Liu, L. Peng, H.H. Ngo, et al., Environ. Sci. Technol. 50 (2016) 9407-9415. DOI:10.1021/acs.est.6b02202 |
| [63] |
L. Lan, J.Q. Zhao, S. Wang, et al., Bioresour. Technol. Rep. 7 (2019) 100190. DOI:10.1016/j.biteb.2019.100190 |
| [64] |
S.A. Reyes, B.C. Ricardo, S. Margarita, et al., Water Res. 41 (2007) 1253-1262. DOI:10.1016/j.watres.2006.12.039 |
| [65] |
J. Liang, N. Chen, S. Tong, Y.J. Liu, C.P. Feng, Chemosphere 212 (2018) 954-963. DOI:10.1016/j.chemosphere.2018.08.161 |
| [66] |
D.J. Wang, H.J. Liu, J.H. Qu, et al., Bioresour. Technol. 100 (2009) 142-148. DOI:10.1016/j.biortech.2008.05.042 |
| [67] |
W. Wang, D.Y. Wei, F.C. Li, Y.W. Zhang, R.H. Li, Water Res. 160 (2019) 52-59. DOI:10.1016/j.watres.2019.05.054 |
| [68] |
E. Sahinkaya, A. Kilic, B. Duygulu, Water Res. 60 (2014) 210-217. DOI:10.1016/j.watres.2014.04.052 |
| [69] |
R.H. Li, D.Y. Wei, W. Wang, Y.G. Zhang, Bioresour. Technol. 308 (2020) 123302. DOI:10.1016/j.biortech.2020.123302 |
| [70] |
E. Sahinkaya, A. Yurtsever, Ö. Aktas, D. Ucar, Z.W. Wang, Chem. Eng. J. 268 (2015) 180-186. DOI:10.1016/j.cej.2015.01.045 |
| [71] |
Y. Yuan, A.Q. Bian, F. Chen, et al., Chemosphere 234 (2019) 568-578. DOI:10.1016/j.chemosphere.2019.06.109 |
| [72] |
C. Chen, X. Zhou, A.J. Wang, et al., Bioresour. Technol. 121 (2012) 441-444. DOI:10.1016/j.biortech.2012.06.117 |
| [73] |
C. Huang, Z.L. Li, F. Chen, et al., Bioresour. Technol. 197 (2015) 227-234. DOI:10.1016/j.biortech.2015.08.019 |
| [74] |
C. Huang, Z.L. Li, F. Chen, et al., Bioresour. Technol. 200 (2016) 1019-1023. DOI:10.1016/j.biortech.2015.09.109 |
| [75] |
F. Chen, Y. Yuan, C. Chen, et al., J. Environ. Sci. 42 (2016) 227-235. DOI:10.1016/j.jes.2015.07.007 |
| [76] |
C.Y. Lee, K.L. Ho, D.J. Lee, A. Su, J.S. Chang, Int. J. Hydrogen Energy 37 (2012) 15827-15832. DOI:10.1016/j.ijhydene.2012.01.092 |
| [77] |
L.X. Zhong, S.H. Zhang, Y. Wei, R.B. Bao, Biochem. Eng. J. 124 (2017) 6-12. DOI:10.1016/j.bej.2017.04.005 |
| [78] |
S.H. Zhang, R.B. Bao, J.J. Lu, W.J. Sang, Sep. Purif. Technol. 195 (2018) 314-321. DOI:10.1016/j.seppur.2017.12.027 |
| [79] |
Y.M. Gong, A. Ebrahim, A.M. Feist, et al., Environ. Sci. Technol. 47 (2013) 568-573. DOI:10.1021/es303837j |
| [80] |
F.Y. Liang, H. Deng, F. Zhao, Chin. J. Anal. Chem. 41 (2013) 1133-1139. DOI:10.1016/S1872-2040(13)60669-6 |
| [81] |
K. Wang, Sh. Zhang, Z. Chen, R.B. Bao, Chem. Eng. J. 339 (2018) 442-449. DOI:10.1016/j.cej.2018.01.114 |
| [82] |
Y.L. Guo, X. Wei, S.H. Zhang, Bioresour. Technol. 305 (2020) 123082. DOI:10.1016/j.biortech.2020.123082 |
| [83] |
Z. Chen, S.H. Zhang, L.X. Zhong, Bioresour. Technol. 291 (2019) 121888. DOI:10.1016/j.biortech.2019.121888 |
| [84] |
G.M. Wu, Z.J. Li, Y. Huang, et al., Chem. Eng. J. 381 (2020) 122707. DOI:10.1016/j.cej.2019.122707 |
| [85] |
H.Y. Wang, W.L. Lyu, X.L. Hu, et al., Sci. Total Environ. 694 (2019) 133775. DOI:10.1016/j.scitotenv.2019.133775 |
| [86] |
D.E. Canfifield, PNAS 110 (2013) 8443-8446. DOI:10.1073/pnas.1306450110 |
| [87] |
D. Wu, G.A. Ekama, H.K. Chui, et al., Water Res. 100 (2016) 496-507. DOI:10.1016/j.watres.2016.05.052 |
| [88] |
F. Jiang, L. Zhang, G.L. Peng, et al., Water Res. 47 (2013) 5773-5782. DOI:10.1016/j.watres.2013.06.051 |
| [89] |
J. Qian, H. Lu, F. Jiang, G.A. Ekama, G.H. Chen, Chem. Eng. J. 262 (2015) 109-118. DOI:10.1016/j.cej.2014.09.066 |
| [90] |
Y. Yang, T.H. Chen, L. Morrison, et al., Chem. Eng. J. 328 (2017) 511-518. DOI:10.1016/j.cej.2017.07.061 |
| [91] |
C. Vohla, H.J. Bavor, F. Chazarenc, et al., Ecol. Eng. 37 (2011) 70-89. DOI:10.1016/j.ecoleng.2009.08.003 |
| [92] |
Y.F. Deng, G.A. Ekama, Y.X. Cui, et al., Water Res. 163 (2019) 114854. DOI:10.1016/j.watres.2019.114854 |
| [93] |
Y.J. Qin, C.L. Wu, B.Q. Chen, et al., Bioresour. Technol. 294 (2019) 122130. DOI:10.1016/j.biortech.2019.122130 |
| [94] |
R.C. Jin, G.F. Yang, J.J. Yu, P. Zheng, Chem. Eng. J. 197 (2012) 67-79. DOI:10.1016/j.cej.2012.05.014 |
| [95] |
Z.Z. Zhang, Y. Zhang, Y.G. Chen, Bioresour. Technol. 298 (2020) 122444. DOI:10.1016/j.biortech.2019.122444 |
| [96] |
T. Wang, J.B. Guo, Y.Y. Song, et al., Sci. Total Environ. 696 (2019) 133929. DOI:10.1016/j.scitotenv.2019.133929 |
| [97] |
F.M. Chen, X. Li, Y. Yuan, Y. Huang, J. Environ. Sci. 81 (2019) 214-224. DOI:10.1016/j.jes.2019.01.010 |
| [98] |
X.B. Sun, L.F. Du, Y.Q. Hou, et al., Bioresour. Technol. 264 (2018) 253-260. DOI:10.1016/j.biortech.2018.02.081 |
| [99] |
Q. Guo, H.Y. Hu, Z.J. Shi, et al., Chem. Eng. J. 297 (2016) 207-216. DOI:10.1016/j.cej.2016.03.138 |
| [100] |
Z.J. Shi, L.Z.J. Xu, D. Wu, et al., Chemosphere 217 (2019) 279-288. DOI:10.1016/j.chemosphere.2018.11.035 |
| [101] |
Z.J. Shi, L.Z.J. Xu, B.C. Huang, et al., Sci. Total Environ. 723 (2020) 13809. |
| [102] |
Z.K. Yao, L.E. Peng, H. Guo, et al., Desalination 470 (2019) 114115. DOI:10.1016/j.desal.2019.114115 |
| [103] |
F.D. Capua, S. Papirio, P.N.L. Lens, et al., Chem. Eng. J. 280 (2015) 643-657. DOI:10.1016/j.cej.2015.05.131 |
| [104] |
W.M. Yang, H. Lu, S.K. Khanal, et al., Water Res. 104 (2016) 507-519. DOI:10.1016/j.watres.2016.08.049 |
 2020, Vol. 31
2020, Vol. 31