The escalating consumption of fossil fuels has driven the development of methods for energy production that aim at enhancing sustainability and reducing negative impacts on the environment [1, 2]. NH3 synthesis is a highly crucial process to chemical production and agriculture. Meanwhile NH3 supplies a green energy carrier and potential transportation fuel [3-6]. Thus far, the conventional Haber–Bosch process remains the only industrial process for large-scale NH3 production. This process was conducted under extreme reaction conditions (350–550 ℃ and 150–350 atm) and used high-purity N2 and H2 as the feed [5, 7], consuming 1%–2% of the worldwide annual energy and contributing to more than 1% of global greenhouse gas emission [8]. It is thus urgently required to develop environment-friendly and less energy-demanding methodologies for NH3 synthesis. As an eco-friendly alternative, electrochemical N2 reduction that uses protons in water as the hydrogen source under mild conditions not only allows for the possibility of artificial N2 fixation, but also can be powered by renewable electric energy. Nevertheless, the development of electrochemical nitrogen reduction reaction (NRR) is significantly restricted by the intrinsic chemical inertness of N2 molecules [9-11]. Considerable attention has been paid to identify electrocatalysts for NRR with high activity, stability, and excellent selectivity [12, 13].
Two-dimensional (2D) materials have attracted broad scientific interest in the past decade for their excellent physical, electronic and chemical properties [14-17]. They have emerged as an interesting candidate in the electrochemical NRR, such as molybdenum disulfide, graphene, and metal-organic frameworks (MOFs) [18-20]. It is worth noting that a new member joined the 2D material family in 2011, namely 2D transition metal carbides, nitrides, and carbonitrides, known as MXene, and has developed very rapidly in the last eight years [21-23]. MXenes were expected to be promising candidates for electrocatalytic NRR applications due to their exceptional properties, including large surface area, rich composition and surface chemistry, adjustable structure, and excellent stability [24-26]. The investigations on MXene in the field of electrochemical NRR are rapidly proceeding, therefore, it is necessary to make a comprehensive summary of applications for MXene-based materials in this field. Herein, we firstly discussed the recent progress concerning the electrochemical NRR using MXene and MXene hybrids as catalysts, and then discussed the fabrication and surface modification of MXene, which might pave a new way to design MXene-based catalysts for conversion of N2 into NH3. Finally, the future research and challenge on MXenes for electrochemical NRR applications were discussed.
2. MXene-based catalysts for electrochemical NRRDue to the intrinsically strong chemical bonds of N2, a mass of energy are required to surmount the kinetic limitation of NH3 synthesis. Therein, well-designed catalysts will provide a lowerenergy pathway for the reaction to occur, allowing the reaction to proceed under relatively mild conditions. Recently, many works have been devoted to exploring MXene catalysts for the electrochemical NRR. To sum up, two types of electrocatalysts based on their chemical composition have been reported, including pure MXene electrocatalysts and MXene-based hybrid ones. In the subsequent sections, we will detail recently reported MXene-based electrocatalysts for NRR.
2.1. Pure MXene catalysts for electrochemical NRRRecently, density functional theory (DFT) calculations on M3C2 transition metal carbides MXene demonstrated that they could be promising catalysts for N2 capture and reduction [27]. Among the M3C2 carbides (M = Ti, Zr, Hf, V, Nb, Ta, Cr and Mo), V3C2 and Nb3C2 exhibit the most outstanding features for NRR with the maximum over-potentials of 0.64 eV and 0.90 eV, respectively (vs. standard hydrogen electrodes) (Fig. 1A). Compared with Nb3C2 (0.85 eV), V3C2 requires a lower activation energy barrier (0.64 eV) in the first step of N2 hydrogenation (from N2 to N-NH*), and the reaction profile is smooth and has a lower energy barrier for next hydrogenation process. Moreover, Gao et al. employed the DFT to predict the feasibility of Mo2TiC2 electrocatalyst for NH3 synthesis [28]. The pathway becomes thermodynamically possible at the potential of 0.26 V. In addition, free energy for the hydrogen evolution reaction (HER) [29] on Mo2TiC2 indicates that HER has a much higher over-potential than NRR, meaning that N2 will more easily occupy the Mo active sites. In other words, HER can be well suppressed. This indicates that the catalyst can get a higher Faradic efficiency (FE).
|
Download:
|
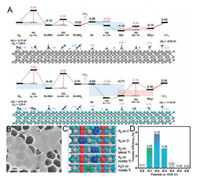
| Fig. 1. (A) DFT + D3 calculation of N2 conversion into NH3 catalysed by V3C2 (top) and Nb3C2 (bottom) MXenes. Adapted with permission [27]. Copyright 2016, Royal Society of Chemistry. (B) SEM image of Ti3C2 nanosheets. (C) Snapshots of N2 on various atomic sites of MXene. (D) FEs of Ti3C2 on FeOOH nanosheet. Adapted with permission [30]. Copyright 2018, Elsevier Ltd. | |
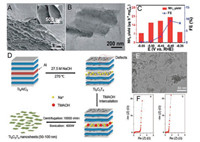
Wang and co-workers conducted an experimental study of Ti3C2 MXene nanosheets as catalysts for electrochemical NRR [30]. They used a mixture of HCl and LiF as an etchant to synthesize Ti3C2 MXene solution, and then they applied the ultrasonic method to obtained MXene flakes with a different scale. Scanning electron microscopy (SEM) image indicates that the exfoliated MXene flakes are very thin (Fig. 1B). DFT calculations were applied to explore the active sites on MXene for N2 adsorption. As shown in Fig. 1C, the middle Ti atoms exhibit the highest N2 adsorption (–1.34 eV) compared to other sites (e.g., C, O and lateral Ti). The Ti3C2 nanosheets supported by stainless steel mesh (SSM) could achieve the FE of 4.62% and NH3 yield rate of 4.72 μg h-1 cm-2 at –0.1 V vs. reversible hydrogen electrode (RHE). Through tailoring Ti3C2 MXene nanosheets to a smaller size and vertically arranging on the FeOOH nanosheet, the MXene/FeOOH showed poor HER activity while achieved a higher FE of 5.78% for NRR (Fig. 1D). Sun and co-workers etched the Ti3C2Tx (T = F, OH) from the MAX (Ti3AlC2) phase using the mixture of HCl and LiF, and then reached the 2D Ti3C2Tx nanosheets by intercalation with dimethylsulfoxide (DMSO) [31]. As shown in Fig. 2A, the obtained 2D Ti3C2Tx nanosheets have a loosely layered structure. The TEM image (Fig. 2B), clearly indicated the flake-structure of the Ti3C2Tx nanosheets, which showed excellent catalytic activity for NRR, achieving an NH3 yield of 20.4 μg h-1 mgcat.-1 with a FE of 9.3% in acids buffer (Fig. 2C). Research showed that the F terminals limited the applications of MXene in electrocatalysis because F is inactive, destroying the conductivity [21]. Wang and co-workers have demonstrated that Ti atomson the edgeswere much favourablefor the NRR. However, the MXene nanosheets prepared in previous works are too large to expose more Ti atoms. Zhang and co-workers applied an alkali-assisted route to obtain fluorine-free Ti3C2Tx (T=O, OH) (NaOH-Ti3C2Tx) nanosheets with small size (50–100nm) in their recent work [32]. Fig. 2D is the synthetic route of NaOH-Ti3C2Tx nanosheets. TEM image of NaOH-Ti3C2Tx nanosheets delaminate with TMAOH indicates that the lateral size of the nanosheets is 50–100nm (Fig. 2E) Electrochemical impedance spectroscopy (EIS) measurement (Fig. 2F) proves that NaOH-Ti3C2Tx nanosheets have better conductivity than Ti3C2Tx nanosheets treated with HF (HF-Ti3C2Tx). The NH3 yield of the NaOH-Ti3C2Tx can reach a value of 36.9 μg h-1 mgcat.-1, ~1.59 times higher than the maximum value of HF-Ti3C2Tx(23.2 μg h-1 mgcat.-1).
|
Download:
|
| Fig. 2. (A) SEM and (B) TEM images of Ti3C2Tx nanosheets. (C) NH3 yields and FEs of Ti3C2Tx nanosheets at a different potential. Adapted with permission [31]. Copyright 2018, Royal Society of Chemistry. (D) Scheme for the generation of NaOH-Ti3C2Tx nanosheets. (E) TEM image of NaOH-Ti3C2Tx nanosheets delaminated with TMAOH. (F) EIS data of (a) HF-Ti3C2Tx and (b) NaOH-Ti3C2Tx. Adapted with permission [32]. Copyright 2019, Royal Society of Chemistry. | |
2.2. MXene hybrids catalysts for electrochemical NRR 2.2.1. Noble metal-MXene catalysts for electrochemical NRR
The above researches show that MXenes are promising NRR electrocatalyst. However, the pure one itself is still challenging to achieve the requirement of high catalytic efficiency. Subsequently, MXene hybrids can be designed for converting N2 to NH3. By avoiding the aggregation, the layered structure of MXene could enhance the chemical properties of the nanosized particles such as accelerating the electron transfer. The surface of MXene materials after Al-removing has a large number of functional groups (such as -OH, –F). Although the electrical conductivity and mechanical strength of MXenes are somewhat affected by these functional groups, these functional groups provide direction exchange sites and play a role of efficient reductant to some oxides [33]. The nanoscale metal particles formed on MXene surface create a great chance to improve its conductivity, increasing the performance in the applications of electrocatalysis. Alternatively, noble metal nanostructures and nanocomposites have shown remarkable electrochemical NRR activity [34-36].
In nature, the web woven by spider provides an obvious platform for the interaction between predators and prey, inspired by this, Li and co-workers designed an ideal system for efficient electrochemical NRR—a "web" (Ti3C2 nanosheets) for effective capture of N2 and "predators" (Au nanoparticles) for N2 conversion [37]. They used the mixture of LiF and HCl as an etchant to prepare Ti3C2 nanosheets, OH on the surfaces of Ti3C2 could provide anchoring sites for Au nanoparticles. Then the nano-sized Au particles could be loaded onto the surface of Ti3C2 nanosheets (Au/Ti3C2) by ultrasound reduction approach.
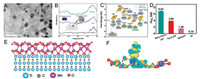
TEM image (Fig. 3A) evidences that Au nanoparticles less than about 10 nm in diameter are distributed on the surface of Ti3C2 uniformly. Moreover, Li and co-workers found that certain Au with high chemical valence was preserved in the ultrasonic reduction process. Temperature programmed desorption of N2 (N2-TPD) dates show that the peak attributed to chemisorbed N2 (at about 280 ℃) of Au/Ti3C2 significantly increased relative to the Ti3C2. It indicates that Au with high valence promotes the chemical adsorption of N2. Furthermore, it can be seen from the optimized geometry shown in the inset of Fig. 3B, the length of the N≡N bond extends from 1.154 Å to 1.365 Å after chemical adsorption of N2 on the surface, indicating that Au promotes the dissociation of N≡N bond. The DFT calculation (Fig. 3C) shows that the high energy adsorbed by N2 at the interface between the Au and Ti3C2 weakens the N≡N, and reduces the activation energy barrier. In the condition of 0.94% Au loading, Au/Ti3C2 shows an excellent averageNH3 yield of 30.06 μg h-1 mgcat.-1 with a high FE of 18.34%.
|
Download:
|
| Fig. 3. (A) TEM image of Au/Ti3C2. (B) N2-TPD dates of Ti3C2 (blue), Au@Ti3C2 (black) and Au/Ti3C2 (green). Inset: The adsorption geometry of N2 on Ti3C2 and the change of N—N bond length after adsorption on Ti3C2. (Ti: light grey, C: dark grey, and N: blue). The blue and red isosurface denote the electron loss and accumulation, respectively. (C) Free energy profile for N2 conversion into NH3 pathways on Au (111), Ti3C2, and Au/Ti3C2 samples. Adapted with permission [37]. Copyright 2019, American Chemical Society. (D) Amount of NH3 with different electrodes after 2 h electrolysis. (E) Optimized structure of the MnO2-Ti3C2Tx heterostructure and (F) deformation charge density plot of the N2-adsorbed configuration on MnO2-Ti3C2Tx heterostructure surface. Adapted with permission [39]. Copyright 2019, Royal Society of Chemistry. | |
2.2.2. Transition metal oxide-MXene catalysts for electrochemical NRR
The wide applications of precious metal-based MXene hybrid materials for electrocatalysis are impeded to some extent by their high price. Non-noble metal-based materials, particularly transition metal (TM) oxide materials, have been widely applied as NRR electrocatalysts due to their earth-abundant reserves, and low toxicity as well as availability of d-orbital electrons for p-back donation, which can activateN2. However, their low conductivity is detrimental to electrochemical properties, which is expected to be enhanced by the supporting platforms of MXene [38]. Accordingly, MnO2-decorated Ti3C2Tx (MnO2-Ti3C2Tx) could function as an electrocatalyst for efficient N2-to-NH3 conversion at ambient conditions [39]. Such MnO2-Ti3C2Tx catalysts were obtained by direct chemical synthesis. Ti3C2Tx improved the electrical conductivity of MnO2, meanwhile prevented the aggregation during electrochemical processes. As shown in Fig. 3E, adsorption between N2 and the MnO2-Ti3C2Tx heterostructure suggests that the unsaturated surface of Mn atoms could serve as active sites to adsorb and activate the N2 molecules. This electrocatalyst achieved a high NH3 yield rate (34.12 μg h-1 mgcat.-1) and FE (11.39%), respectively. Also, MnO2 and Ti3C2Tx have NRR activity as well (Fig. 3D). It is worth noting that MnO2-Ti3C2Tx/CP (6.82 μg) exhibits greatly enhanced electrocatalytic NRR activity, ~4.7 times higher than MnO2/CP (1.46 μg) and ~1.8 times higher thanTi3C2Tx/ CP (3.86 μg), meaning that MnO2 and Ti3C2Tx promote electrocatalysis synergistically.
To further improve the FE of electrochemical N2-to-NH3 conversion, we used the marginal titanium atoms with thermodynamically metastable on the surface of Ti3C2Tx MXene as nucleating site to successfully construct a heterojunction structure of TiO2 nanoparticles distributed on the Ti3C2Tx surface (TiO2/ Ti3C2Tx). Meanwhile, a mass of oxygen vacancies were introduced into the material to promote the performance of the NRR electrocatalysis. The TiO2/Ti3C2Tx was synthesized via a one-step ethanol-thermal strategy. DFT calculation showed N2 can adsorb on the surfaces of different catalysts in the form of end-on mode, while Ti-edge atoms and oxygen vacancies could act as active sites to activate and polarize N2 molecules. The free energy scheme indicated the lowest energy barrier of TiO2/Ti3C2Tx could compare to other samples. Accordingly, this electrocatalyst exhibited an NH3 yield of 32.17 μg h-1 mgcat.-1 at –0.55V vs. RHE with a FE of 16.07% at –0.45V vs. RHE [40].
2.2.3. MXene-based single atom catalysts for electrochemical NRRRecently, single atom catalysts (SACs) showed superb performance in NRR electrocatalysis systems [41]. The highly unsaturated metal atomically dispersed on a matrix exhibits excellent activity, and the strong interaction between the single atom and the matrix can efficiently anchor the individual atoms thus preventing their aggregation. Catalytic properties can be improved by coupling the active metal with suitable support. Chen and co-workers carried out DFT computations to investigate the NRR performance of the defective Mo2TiC2O2 MXene nanosheets embedded with a series of TM atoms (including 3d, 4d and 5d except for Tc, Hg and lanthanide) (Mo2TiC2O2-TMSA) [42]. The barrier of possible potential-determination step—the highest energy barrier (ΔGmax) determines the thermodynamic possibility of the electrocatalytic process. As shown in Fig. 4A, the ΔGmax of candidates containing Zr, Mo, Hf, Ta, W, Re and Os are all quite low, indicating all of them exhibited excellent NRR performance. Among them, Zr-containing nanosheet (Mo2TiC2O2-ZrSA) has the lowest DG max of 0.15 eV. From Fig. 4B, we can see these elements' limiting potentials for HER (UL(HER)) and NRR (UL(NRR)) potential, when UL(NRR) < UL(HER), the electrocatalysts are selective for NRR. Similarly, Zr has the lowest limiting potential.

|
Download:
|
| Fig. 4. (A) Detailed information of candidates. (B) Calculated limiting potentials for HER (UL(HER)) and NRR (UL(NRR)) on the surfaces of seven candidates. The dashed line represents UL(HER) = UL(NRR). When UL(NRR) < UL(HER), the electrocatalysts are selective for NRR. Adapted with permission [42]. Copyright 2019, Wiley-VCH. | |
3. The fabrication of MXenes
MXenes are widely applied in many fields, but the applications on electrochemical NRR are fewer than expected. This should be related to their complex preparation process. The removal of the interlayered A atoms of the MAX will lead to the formation of dangling bonds on the surface M atoms. Multifarious etching processes will result in different terminal group bonding to the M atoms. Therefore, the surface characteristics of MXene are closely related to the preparation method. Moreover, the etching conditions varied from the strength of the "A"-containing bonds. Suitable etching conditions are critical to achieving highyields and purity. Since the first experimental realization of Ti3C2Tx in 2011 [21], many efforts have been devoted to seeking novel strategies to prepare a wide variety of MXene. Gogotsi and co-workers proposed that the Ti3C2Tx can be easily separated from the MAX (Ti3AlC2) phase by etching the Al atoms selectively in hydrofluoric acid (HF) at room temperature [21]. Until now, on the methods of synthesizing MXene materials, as the most widely used and universal method, the HF etching method still play the important role. However, HF is highly toxic and corrosive. There is thus an urgent need to explore effective, gentler, and harmless methods to synthesize MXene.
3.1. Modified acid etching methodTo avoid the application of HF directly for extracting A atoms from MAX, firstly, the researchers used a mixture of HCl and fluoride salt (LiF, NaF, KF, CaF2 and FeF3) as an etchant in place of HF, called "in situ HF method" [43]. During the etching, the in situ formed HF selectively remove the A atoms from MAX. Besides, due to the intercalation of metal ions and water molecule into the interlayered space, the interlayered interaction of the obtained MXene layers became weaker. The nature of the mixture of HCl and fluoride salt compared with HF is much milder, so the MXene flakes did not have surface defects. Furthermore, instead of the HF etchant, ammonium bifluoride (NH4HF2) was also demonstrated as a new kind of etchant [44]. The intercalation of NH3 and NH4+ species resulted in a 25% larger c-lattice parameter of the obtained MXene film than HF etching.
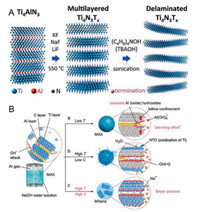
3.2. Molten fluoride salt etching methodRecently, most attention was still paid on the preparation of carbides and carbonitrides MXene, while nitrides MXene (Tin+1NnTx) were rarely studied. This should because the Al-N bond in Tin+1AlNn is stronger than Al-C bond in Tin+1AlCn, accordingly, it is more difficult to etch Al in Tin+1AlNn [45]. More importantly, the nitrides have low cohesive energy, and the nitrogen-containing MXenes are less stable than the corresponding carbon-containing analogs. Therefore, Tin+1NnTx would dissolve in the HF solution. To meet these challenges, Gogotsi and co-workers prepared nitrides MXene (Ti4N3Tx) by heating the mixture of Ti4AlN3 and ternary eutectic molten fluoride salt (LiF, NaF and KF) at 550 ℃ under Ar protection, as shown in Fig. 5A [46]. However, compared to other HF-etched titanium carbide MXenes, the delaminated Ti4N3Tx showed inferior crystallinity [47]. This indicates that the moltenfluoride salt etching method still has some problems in the preparation of nitride-based MXenes.
|
Download:
|
| Fig. 5. (A) Schematic diagram of the preparation process of Ti4N3Tx from Ti4AlN3 by molten salt treatment. Adapted with permission [46]. Copyright 2016, Royal Society of Chemistry. (B) Schematic of the reaction of the alkali-assisted hydrothermal method using NaOH solution. (a) Al (oxide) hydroxides prevent the Al extraction process at low temperatures. (b) Some Al (oxide) hydroxides dissolve in NaOH (low NaOH concentrations at high temperatures). In addition, higher water content causes the MXene to oxidize and yields NTOs. (c) Based on the Bayer process, high temperatures and high NaOH concentrations will dissolve Al (oxide) hydroxides in NaOH effectively. Adapted with permission [48]. Copyright 2018, Wiley-VCH. | |
3.3. Alkaline etching method
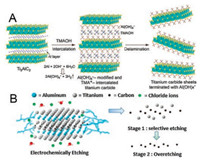
The modified acid etching method avoids using HF directly, but there is still HF release in the solution. Inspired by the Bayer process widely used in the refining of bauxite, Zhang and co-workers proposed an alkali-assisted hydrothermal method using NaOH solution as an etchant to prepare a typical Ti3C2Tx (T= OH, –O) (Fig. 5B) [48]. They used high temperature (270 ℃) condition to promote the conversion of Al(OH)3 and AlO(OH) to dissolvable Al (OH)4-, and the applied of highly alkaline concentrate (27.5 mol/L) with low water containment to prevent oxidation of Ti species. This Bayer process-based method is free of fluorine and could reach multilayer Ti3C2Tx with a purity of ~92 wt%. This process avoids the release of HF, but it is necessary to heat concentrated NaOH solution under hydrothermal conditions of high temperature and pressure, this is also dangerous. To reduce the danger more effectively, Geng and co-workers proposed a milder method of organic-base-driven intercalation and delamination of Ti3AlC2, which used tetramethylammonium hydroxide (TMAOH) as an etchant (Fig. 6A) [49]. Commercially, TMAOH is widelyapplied toetching Al. The Al(OH)4 formed in the process of the Ti3AlC2 delamination can easily form a bond with the surface Ti atom, while the TMA+ cation inserted the interlamellar space, thereby promoting delamination.
|
Download:
|
| Fig. 6. (A) Schematic diagram of the insertion and delamination process. The organic base (TMAOH) reacts with the Al atomic layer in the gallery to promote key processes, including disrupt of the Ti-Al bonds by the way of Al hydrolysis, and the insertion of bulky TMA+ into thegallery, ina single step. The disruptionof Ti-Al metallic bonds and bulky-ion insertion contributes the succeeding disassembly of the precursor layered crystals into their elementary layers.Adapted with permission [49]. Copyright 2016, Wiley-VCH. (B) The electrochemical etching mechanism of Ti2AlC with diluted HCl. Adapted with permission [52]. Copyright 2019, American Chemical Society. | |
3.4. Electrochemical etching method
The electrochemical etching route has been proven to be an effective etching strategy for selectively extracting nanolayer materials from MAX precursors. However, there are still some unsatisfactory factors in the electrochemical etching method, such as a long etching period, less favourable for production scale, and toxicity of the intercalants [50, 51]. Recently, Hao and co-workers developed a general strategy based on a thermally assisted electrochemical etching process to synthesize MXene (Ti2CTx, Cr2CTx, and V2CTx) [52]. The electrochemical etching route with diluted HCl is applied to prepare Ti2CTx. As shown in Fig. 6B, the etching process was divided into two phases: the voltage applied in stage 1 firstly etched Al atoms to form the layered carbide (Ti-Al bond is weaker than the Ti-C bond); in stage 2, both Al and Ti atoms were etched until only a single layer of carbon atoms remained. In addition, etching of Ti2AlC can be accelerated by gentle heating. Moreover, the corresponding MXene can be prepared by applying different etching voltages (at 0.3 V vs. RHE for Ti2AlC and 0.4–0.7 V for V2AlC and 0.6–1.0 V for Cr2AlC).
4. Surface modification of MXeneSurface modification of MXene can alter the surface chemistry of MXene by adjusting its interlayer distance, ion diffusion, and hydrophilicity/hydrophobicity [53]. Active sites that facilitate nitrogen adsorption and activation can also be introduced during the modification of MXene, such as Au/Ti3C2, MnO2/Ti3C2Tx, and TiO2/Ti3C2Tx [37, 39, 40]. Among them, Au and MnO2 acted as the active sites for adsorption and activation of N2 in Au/Ti3C2 and MnO2-Ti3C2Tx catalysts, respectively, while the oxygen vacancies in TiO2/ Ti3C2Tx were considered to be their active sites. In addition, these active sites and MXene could synergistically promote the performance of NRR. Therefore, classifications for MXene-based nanocomposites are important to facilitate the study of NRR catalysts.
4.1. Inorganic and polymer modification of MXeneSeveral recent reports have comprehensively reviewed the current surface modification of MXene [54, 55]. In brief, a series of MXene-based inorganic nanostructure have been investigated, which include noble metal (Au [34], Ag [56], Pt [57], Ru [58], etc.), transition metal oxide-MXene materials (TiO2 [40]), MnO2 [39], Fe2O3 [59], Nb2O5 [60], etc.), metal hydroxide [61], and chalcogenides [62]. Moreover, the incorporation of polymers into conductive MXene substrates provides a unique set of physicochemical properties such as variable band gaps, mechanical stiffness, controlled charge transport. MXene-polymer composites are fascinating materials for electrochemical applications [63, 64]. In addition to inorganic nanostructures and polymers, other materials such as carbon nanotubes, graphene, biomaterials, and MOF have also been hybridized with MXene for applying in the field of electrochemistry [55].
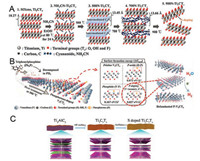
4.2. Heteroatom doping on MXeneTo further promote the electrochemical performance of MXene, the introduction of heteroatom doping such as non-metallic atoms with low electronegativity into MXene can be a fashionable strategy. Heteroatom doping on MXene can effectively increase the intrinsic activity and improve the distribution of atomic charge density and/or spin density, contributing to the enhancement of the electrochemical properties of the doped materials [53, 55, 65]. An and co-workers used cyanamide as the interlayer intercalation agent and nitrogen source, applying the step-by-step strategy to synthesize a highly N-doped Ti2CTx (Fig. 7A) [65]. Cyanamid forms p-C3N4 on the surface of Ti2CTx nanosheets by condensation reaction at 500–700 ℃, which promotes delamination of Ti2CTx, followed by heat treatment of p-C3N4 and Ti2CTx complexes at 900 ℃, nitrogen species can be doped into the inner carbon layer and/or defect sites of the Ti2CTx nanosheets (N doping amount 15.48%). The synergistic effect of 2D structure and heteroatoms doping capacitor electrode with high capacitance makes such highly N-doped Ti2CTx an excellent electrochemical. Wang and coworkers employed one-step thermal annealing to synthesize Ndoped Ti3C2Tx (N-Ti3C2Tx) [67]. They used the electrostatic selfassembly between Ti3C2Tx with negatively charge and melamine with positively charge as precursors. This nitrogen doping strategy gives N-Ti3C2Tx a porous structure, a high surface area and strong physical and chemical adsorption capacity.
|
Download:
|
| Fig. 7. (A) Schematic illustration of the synthesis of 900N-Ti2CTx. Adapted with permission[65]. Copyright 2018, Wiley-VCH. (B) Synthesis procedures of the P-doped V2CTx nanosheets by heat treatment with triphenyl phosphineand and their possible chemical compositions which can be determined by calculated surface formation energy. Adapted with permission [53]. Copyright 2018, Wiley-VCH. (C) Synthesis procedures of the preparation of S-doped T3C2Tx. Adapted with permission [66]. Copyright 2017, Royal Society of Chemistry. | |
Recently, phosphorous doped V2CTx MXene was obtained by a simple heat treatment approach with triphenylphosphine as a phosphorous source (Fig. 7B) [53]. This approach can control chemical composition according to the phosphorylation temperature to optimize the chemical structure. DFT calculations proved that P—C bonding reveals the lowest surface formation energy and Gibbs free energy compared to others such as P-oxide and P–V (phosphide). In addition, Xu and co-workers not only introduced P but also O into the Mo2CTx MXenes (P-Mo2CTx) through a simple phosphating strategy with red phosphorus as the P source [68]. The enhanced electrochemical performance of P-Mo2CTx can be attributed to the following factors: first, the expanded interlayer distance and the introduced P and O elements could increase the active sites; second, the introduction of P can improve the conductivity of P-Mo2CTx. Besides, the N and S doping strategy was also effective to increase interlayer spacing and enhance the conductivity of MXene (Fig. 7C) [66].
5. Summary and outlookMXene-based materials have many attractive properties such as high surface area, tunable electronic structure and high electronic conductivity all make a promising material for electrocatalysis applications. In order to further explore their application, it is necessary to better understand the MXene materials. In this concept article, we have summarized the applications of MXenebased nanomaterials as emerging catalysts for electrochemical NRR, including pure MXene and MXene hybrids. Efforts have shown that MXene-based nanomaterials have achieved great performances in this field, indicating that these are very promising materials as electrocatalysts for NRR. Moreover, we have outlined the fabrication of MXenes and the surface modification of MXenes.
It can be predicted that MXene-based hybrid will yield unusually brilliant results in electrocatalytic nitrogen fixation shortly. However, researches on MXene-based catalysts are mainly focused on Ti-based MXene at a nascent stage. Works of other metal-based MXene (including V, Nb, Cr, Mo, etc.) in electrocatalysis of N2-to-NH3 conversion have rarely been reported. When it is used as electrode materials, the two-dimensional MXene materials easily aggregate and stack. Although the reaction activity and transmission rate of electrolyte ions can be enhanced via being inserted with inorganic metal ions, organic molecules, polymer molecules and CNTs between layers, ion transfer rate will exponentially decrease with increasing the thickness of the electrode material. As for the preparation of MXenes, most of the preparation methods of MXenes at present are top-down ones using harmful reagent (e.g., HF) and complicated conditions, so greener and more efficient preparation methods such as electrochemical ones should be promoted. Although considerable progress has been made in NRR by MXene, it is still far from the industrialization. But it can be promoted from the following aspects. (1) We need to develop a green, non-toxic and efficient method for large-scale preparation of MXenes. (2) Several MXenes with high catalytic activity and good stability should be selected for nitrogen reduction, and the reagents for modifying the MXene should be cost-effective. (3) Finally, more diverse MXenes categories should be investigated through the combination of theoretical prediction and experimental research. Moreover, identifying the catalytic nature and elucidating the catalytic mechanism in MXene-based catalysts remain a challenge. Therefore, considering the great potential of MXene-based catalysts in electronic hydrogenation of N2 for NH3, various MXenes should be investigated through the combination of theoretical prediction and experimental research.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21874079 and 20575071), Natural Science Foundation for Outstanding Young Scientists of Shandong Province (No. ZR2018JL011), Key R & D Project of Shandong Province (No. GG201809230180), Qingdao Science & Technology Planning Project (No. 17-6-3-15-gx), Science & Technology Fund Planning Project of Shandong Colleges and Universities (No. J16LA13 & J18KA112) and Taishan Scholars Program of Shandong Province (No. tsqn201909088).
| [1] |
S. Chu, A. Majumdar, Nature 488 (2012) 294-303. DOI:10.1038/nature11475 |
| [2] |
J. Liang, D. Chen, X. Yao, et al., Small 15 (2019) 1903398. |
| [3] |
V. Rosca, M. Duca, M.T. de Groot, M.T. Koper, Chem. Rev. 109 (2009) 2209-2244. DOI:10.1021/cr8003696 |
| [4] |
R. Service, Science 345 (2014) 610. DOI:10.1126/science.345.6197.610 |
| [5] |
J.G. Chen, R.M. Crooks, L.C. Seefeldt, et al., Science 360 (2018) eaar6611.
|
| [6] |
X. Guo, H. Du, F. Qu, J. Li, J. Mater. Chem. A 7 (2019) 3531-3543. DOI:10.1039/C8TA11201K |
| [7] |
B.H. Suryanto, H.L. Du, D. Wang, et al., Nat. Catal. 2 (2019) 290-296. DOI:10.1038/s41929-019-0252-4 |
| [8] |
V. Smil, Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production, MIT press, 2004.
|
| [9] |
J. Yang, Y. Guo, W. Lu, R. Jiang, J. Wang, Adv. Mater. 30 (2018) 1802227. DOI:10.1002/adma.201802227 |
| [10] |
H.P. Jia, E.A. Quadrelli, Chem. Soc. Rev. 43 (2014) 547-564. DOI:10.1039/C3CS60206K |
| [11] |
G. Zhang, Q. Ji, K. Zhang, et al., Nano Energy 59 (2019) 10-16. DOI:10.1016/j.nanoen.2019.02.028 |
| [12] |
V. Kyriakou, I. Garagounis, E. Vasileiou, A. Vourros, M. Stoukides, Catal. Today 286 (2017) 2-13. DOI:10.1016/j.cattod.2016.06.014 |
| [13] |
C. Guo, J. Ran, A. Vasileff, S.Z. Qiao, Energy Environ. Sci. 11 (2018) 45-56. DOI:10.1039/C7EE02220D |
| [14] |
K. Novoselov, A. Mishchenko, A. Carvalho, A.C. Neto, Science 353 (2016) aac9439.
|
| [15] |
Q. Fu, X. Bao, Chem. Soc. Rev. 46 (2017) 1842-1874. DOI:10.1039/C6CS00424E |
| [16] |
L. Cong, H. Xie, J. Li, Adv. Energy Mater. 7 (2017) 1601906. DOI:10.1002/aenm.201601906 |
| [17] |
M. Sun, H. Liu, J. Qu, J. Li, Adv. Energy Mater. 6 (2016) 1600087. DOI:10.1002/aenm.201600087 |
| [18] |
B.H.R. Surrnto, D.B. Wang, L.M. Azofra, et al., ACS Energy Lett. 4 (2019) 430-435. DOI:10.1021/acsenergylett.8b02257 |
| [19] |
J. Deng, C. Liu, Chem 4 (2018) 1773-1774. DOI:10.1016/j.chempr.2018.07.014 |
| [20] |
S.J. Luo, X.M. Li, B.H. Zhang, Z.L. Luo, M. Luo, ACS Appl. Mater. Interfaces 11 (2019) 26891-26897. DOI:10.1021/acsami.9b07100 |
| [21] |
M. Naguib, M. Kurtoglu, V. Presser, et al., Adv. Mater. 23 (2011) 4248-4253. DOI:10.1002/adma.201102306 |
| [22] |
P. Zhang, D.J. Wang, Q.Z. Zhu, et al., Nano-micor Lett. 11 (2019) 81. DOI:10.1007/s40820-019-0312-y |
| [23] |
Y.T. Liu, P. Zhang, N. Sun, et al., Adv. Mater. 30 (2018) 1707334. DOI:10.1002/adma.201707334 |
| [24] |
Y. Zhong, X. Xia, F. Shi, et al., Adv. Sci. 3 (2016) 1500286. DOI:10.1002/advs.201500286 |
| [25] |
Z. Li, Y. Wu, Small 15 (2019) 1804736. DOI:10.1002/smll.201804736 |
| [26] |
Q. Zhao, Q.Z. Zhu, J.W. Miao, et al., Small 15 (2019) 1904293. DOI:10.1002/smll.201904293 |
| [27] |
L.M. Azofra, N. Li, D.R. MacFarlane, C. Sun, Energy Environ. Sci. 9 (2016) 2545-2549. DOI:10.1039/C6EE01800A |
| [28] |
Y. Gao, Y. Cao, H. Zhuo, et al., Catal. Today 339 (2018) 120-126. |
| [29] |
H. Du, R. Kong, X. Guo, F. Qu, J. Li, Nanoscale 10 (2018) 21617-21624. DOI:10.1039/C8NR07891B |
| [30] |
Y. Luo, G.F. Chen, L. Ding, et al., Joule 3 (2019) 279-289. DOI:10.1016/j.joule.2018.09.011 |
| [31] |
J.X. Zhao, L. Zhang, X.Y. Xie, et al., J. Mater. Chem. A 6 (2018) 24031-24035. DOI:10.1039/C8TA09840A |
| [32] |
T. Li, X. Yan, L. Huang, et al., J. Mater. Chem. A 7 (2019) 14462-14465. DOI:10.1039/C9TA03254A |
| [33] |
Y. Ying, Y. Liu, X. Wang, et al., ACS Appl. Mater. Interfaces 7 (2015) 1795-1803. DOI:10.1021/am5074722 |
| [34] |
Y. Yang, S.Q. Wang, H. Wen, et al., Angew. Chem. Int. Ed. 131 (2019) 15506-15510. DOI:10.1002/ange.201909770 |
| [35] |
Z.H. Xue, S.N. Zhang, Y.X. Lin, et al., J. Am. Chem. Soc. 141 (2019) 14976-14980. DOI:10.1021/jacs.9b07963 |
| [36] |
H.K. Lee, C.S.L. Koh, Y.H. Lee, et al., Sci. Adv. 4 (2018) eaar3208.
|
| [37] |
D. Liu, G. Zhang, Q. Ji, Y. Zhang, J. Li, ACS Appl. Mater. Interfaces 11 (2019) 25758-25769. DOI:10.1021/acsami.9b02511 |
| [38] |
N. Sun, Q.Z. Zhu, B. Anasori, et al., Adv. Funct. Mater. 29 (2019) 1906282. DOI:10.1002/adfm.201906282 |
| [39] |
W. Kong, F. Gong, Q. Zhou, et al., J. Mater. Chem. A 7 (2019) 18823-18827. DOI:10.1039/C9TA04902A |
| [40] |
Y.F. Fang, Z.C. Liu, J.R. Han, et al., Adv. Energy Mater. 9 (2019) 1803406. DOI:10.1002/aenm.201803406 |
| [41] |
X. Liu, Y. Jiao, Y. Zheng, M. Jaroniec, S.Z. Qiao, J. Am. Chem. Soc. 141 (2019) 9664-9672. DOI:10.1021/jacs.9b03811 |
| [42] |
L. Li, X.Y. Wang, H.R. Guo, et al., Small Methods 3 (2019) 1900337.
|
| [43] |
X. Wang, C. Garnero, G. Rochard, et al., J. Mater. Chem. A 5 (2017) 22012-22023. DOI:10.1039/C7TA01082F |
| [44] |
A. Feng, Y. Yu, F. Jiang, et al., Ceram. Int. 43 (2017) 6322-6328. DOI:10.1016/j.ceramint.2017.02.039 |
| [45] |
M. Naguib, V.N. Mochalin, M.W. Barsoum, Y. Gogotsi, Adv. Mater. 26 (2014) 992-1005. DOI:10.1002/adma.201304138 |
| [46] |
P. Urbankowski, B. Anasori, T. Makaryan, et al., Nanoscale 8 (2016) 11385-11391.
|
| [47] |
M. Alhabeb, K. Maleski, B. Anasori, et al., Chem. Mater. 29 (2017) 7633-7644. DOI:10.1021/acs.chemmater.7b02847 |
| [48] |
T. Li, L. Yao, Q. Liu, et al., Angew. Chem. Int. Ed. 57 (2018) 6115-6119. DOI:10.1002/anie.201800887 |
| [49] |
J. Xuan, Z. Wang, Y. Chen, et al., Angew. Chem. Int. Ed. 55 (2016) 14569-14574. DOI:10.1002/anie.201606643 |
| [50] |
W. Sun, S.A. Shah, Y. Chen, et al., J. Mater. Chem. A 5 (2017) 21663-21668. DOI:10.1039/C7TA05574A |
| [51] |
S. Yang, P. Zhang, F. Wang, et al., Angew. Chem. Int. Ed. 57 (2018) 15491-15495. DOI:10.1002/anie.201809662 |
| [52] |
S.Y. Pang, Y.T. Wong, S. Yuan, et al., J. Am. Chem. Soc. 141 (2019) 9610-9616. DOI:10.1021/jacs.9b02578 |
| [53] |
Y. Yoon, A.P. Tiwari, M. Choi, et al., Adv. Funct. Mater. 29 (2019) 1903443. DOI:10.1002/adfm.201903443 |
| [54] |
H. Yu, Y. Wang, Y. Jing, et al., Small 15 (2019) 1901503. DOI:10.1002/smll.201901503 |
| [55] |
H. Wang, Y. Wu, X. Yuan, et al., Adv. Mater. 30 (2018) 1704561. DOI:10.1002/adma.201704561 |
| [56] |
G. Zou, Z. Zhang, J. Guo, et al., ACS Appl. Mater. Interfaces 8 (2016) 22280-22286. DOI:10.1021/acsami.6b08089 |
| [57] |
X. Xie, S. Chen, W. Ding, Y. Nie, Z. Wei, Chem. Commun. 49 (2013) 10112-10114. DOI:10.1039/c3cc44428g |
| [58] |
X. Li, C. Zeng, G. Fan, Int. J. Hydrogen Energy 40 (2015) 9217-9224. DOI:10.1016/j.ijhydene.2015.05.168 |
| [59] |
H. Zhang, M. Li, J. Cao, et al., Ceram. Int. 44 (2018) 19958-19962. DOI:10.1016/j.ceramint.2018.07.262 |
| [60] |
C.J. Zhang, S.J. Kim, M. Ghidiu, et al., Adv. Funct. Mater. 26 (2016) 4143-4151. DOI:10.1002/adfm.201600682 |
| [61] |
Y. Wang, H. Dou, J. Wang, et al., J. Power Sources 327 (2016) 221-228. DOI:10.1016/j.jpowsour.2016.07.062 |
| [62] |
J. Ran, G. Gao, F.T. Li, et al., Nat. Commun. 8 (2017) 13907. DOI:10.1038/ncomms13907 |
| [63] |
M. Boota, M. Pasini, F. Galeotti, et al., Chem. Mater. 29 (2017) 2731-2738. DOI:10.1021/acs.chemmater.6b03933 |
| [64] |
M. Zhu, Y. Huang, Q. Deng, et al., Adv. Energy Mater. 6 (2016) 1600969. DOI:10.1002/aenm.201600969 |
| [65] |
Y. Yoon, M. Lee, S.K. Kim, et al., Adv. Energy Mater. 8 (2018) 1703173. DOI:10.1002/aenm.201703173 |
| [66] |
J. Li, D. Yan, S. Hou, et al., J. Mater. Chem. A 6 (2018) 1234-1243. DOI:10.1039/C7TA08261D |
| [67] |
W. Bao, L. Liu, C. Wang, et al., Adv. Energy Mater. 8 (2018) 1702485. DOI:10.1002/aenm.201702485 |
| [68] |
G. Qu, Y. Zhou, T. Wu, et al., ACS Appl. Energy Mater. 1 (2018) 7206-7212. DOI:10.1021/acsaem.8b01642 |
 2020, Vol. 31
2020, Vol. 31 

