b College of Pharmacy, Fujian Medical University, Fuzhou 350108, China
Mesoporous silica materials (MSMs) have cylindrical pores with diameter in the range of 2–50 nm. The huge internal space allows high loading of various molecules, especially for hydrophobic drugs [1-3]. Drug crystallization is inhibited by the mesopores [4], contributing to higher drug dissolution than the raw drugs [5, 6]. But the drug release can also be slow and incomplete under both sink [5, 6] and nonsink [7, 8] conditions because the release is greatly influenced by various factors including pore size, mesoporous structure, surface area and surface functionalization [3, 9].
The solubility and dissolution of hydrophobic drugs can also be promoted by the self-microemulsifying drug delivery system (SMEDDS) [10-12]. SMEDDS is usually composed of oil, emulsifier and co-emulsifier [13, 14]. Nano-sized emulsion droplets in which drugs are dissolved can be formed upon water or gastro-intestinal liquid in a short period [15, 16]. The in vivo drug absorption is improved through various ways such as lymphatic uptake, enhanced mucus diffusion and reduced food effect [17-20]. The SMEDDS has been commercially available for drugs like ciclosporin (Sandimmun Neoral


The mesoporous structure of MSMs provides extensive space for adsorption of SMEDDS [24]. Combination of MSMs and SMEDDS not only guarantees fast and complete drug dissolution, but also prevents the drawbacks of SMEDDS [25]. To explore the influence of the mesopores towards the solidification of SMEDDS, two types of MSMs were prepared and sirolimus (SRL) was used as model drug in this study. Mesoporous silica nanospheres (MSNs) usually have small diameter and pore size while Santa Barbara Amorphous-15 (SBA-15) is large in both particles and mesopores [26]. SBA-15 was named because it was developed in the University of California, Santa Barbara [27]. SBA-15 has hexagonal mesopores with thick walls, which improves the stability of the particles and mesoporous structure [28].
SRL-SMEDDS was prepared using Labrafil M1944CS (oil), Cremophor EL (emulsifier) and Transcutol HP (co-emulsifier) according to our previous study [29]. MSNs and SBA-15 were prepared as reported [27, 30]. The MSMs were measured by the particle sizing system Nicomp 380 (PSS, US). The hydrodynamic size of MSNs and SBA-15 were 195.35 ± 5.82 nm and 2312.19 ± 106.93 nm, respectively.
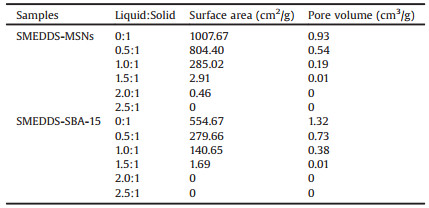
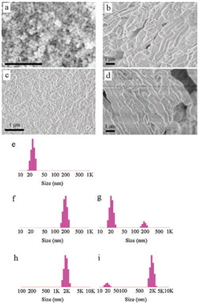
The morphology of the MSMs and SMEDDS-MSMs was observed using scanning electron microscope (SEM, Nova NanoSEM230, Philips, Netherlands) and transmission electron microscope (TEM, JEM2100 F High Resolution TEM, JEOL, Japan). The MSNs were spherical particles with cylindrical pores (Figs. 1a and b). The size of MSNs was around 50–90 nm (Fig. 2a). The SBA-15 were in rod-like shapes with length of about 0.9–1.2 μm and width of about 0.5-0.8 μm (Fig. 2b). The mesopores of SBA-15 were straight and long (Figs. 1c and d). When SMEDDS was added, the boundary between particles of MSNs or SBA-15 became unclear and aggregation of the particles was observed (Figs. 2c and d). When the SMEDDS-MSMs were dispersed in water, the size distribution of SMEDDS-MSMs presented as combination of SMEDDS and MSMs (Figs. 2e–i).

|
Download:
|
| Fig. 1. Morphology of the mesoporous silica materials (MSMs) by transmission electron microscopy (TEM). (a) mesoporous silica nanospheres (MSNs), (b) magnified MSNs, (c) Santa Barbara Amorphous-15 (SBA-15), and (d) magnified SBA-15 | |
|
Download:
|
| Fig. 2. Morphology of the MSMs by scanning electron microscopy (SEM) and hydrodynamic size distribution. SEM images of (a) MSNs, (b) SBA-15, (c) MSNs with self-microemulsifying drug delivery system (SMEDDS-MSNs), and (d) SMEDDSSBA-15. Hydrodynamic size distribution of (e) SMEDDS, (f) MSNs, (g) SMEDDSMSNs, (h) SBA-15 and (i) SMEDDS-SBA-15 | |
The surface area and pore volume were analyzed by nitrogen adsorption-desorption using a surface area and porosity analyzer (JW-BK112, JWGB Sci & Tech Co., China). The MSNs had BET surface area of 1007.67 m2/g, BJH pore volume of 0.93 m3/g (Table 1), and BJH most frequent pore size of 2.70 nm. For SBA-15, the surface area and pore volume were 554.67 m2/g and 1.32 m3/g, respectively. The pore size was 10.91 nm.
|
|
Table 1 The surface area and pore volume of SRL-SMEDDS-MSMs with different ratios of liquid:solid |
The nitrogen adsorption-desorption isotherms were shown in Fig. S1 (Supporting information). With the increase of SMEDDS, the surface area and pore volume of MSNs and SBA-15 rapidly decreased (Table 1). For both of MSNs and SBA-15, the surface area and pore volume were hardly detected with a ratio of 2:1 (liquid: solid). Though the MSNs had smaller surface area than SBA-15, the higher pore volume provided additional space for SMEDDS. Therefore, the MSNs and SBA-15 in this study showed similar capacity for adsorption of SMEDDS. In addition, the SMEDDSMSMs kept excellent flow properties (Fig. S2 in Supporting information). To prevent the influence of excess SMEDDS, the ratio of liquid:solid was fixed at 2:1 for the following study.
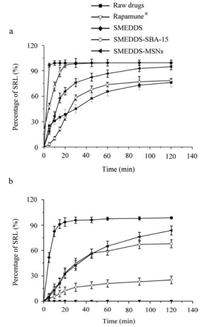
The dissolution of raw SRL powders, SRL-SMEDDS, commercial tablets (Rapamune
|
Download:
|
Fig. 3. In vitro dissolution of raw SRL, Rapamune |
|
For the raw drugs and the commercial tablets, satisfying dissolution was only observed with the assist of surfactant. When SDS was removed from the dissolution media, no dissolution of SRL was found for the raw drugs (Fig. 3b). The commercial tablets showed a remarkably reduced dissolution with only 25.21% ± 4.52% of SRL at 120 min. Thus, a desired in vivo dissolution of the commercial tablets could only be achieved with enough intestinal surfactant like bile salts [31, 32]. In contrast, the SMEDDS maintained a fast and complete dissolution with 95.82% ± 3.67% at 30 min. Compared with the tablets, the SRL-SMEDDS-MSMs showed more favorable dissolution profiles. Additionally, the MSNs kept a higher dissolution at 120 min than the SBA-15 (83.62% ± 5.34% vs. 67.93% ± 4.87%, P < 0.05).
For macropores (> 50 nm) that were much larger than the emulsion droplets, the pore size was the key factor influencing the drug dissolution. Larger pores had faster exchange of media throughout the particles and the self-emulsifying process could also take place inside the pores [33]. Meanwhile, micropores (< 2 nm) much smaller than the emulsion droplets could remarkably delay the diffusion of SMEDDS because of reduced media exchange rate through the tiny pores [24, 29]. In this study, the mesopore diameters of MSNs and SBA-15 were smaller than that of the emulsion droplets (23.4 ± 3.1 nm). However, the dissolution profiles were relatively satisfying. Especially, MSNs with smaller pore diameter had faster and more SRL dissolution as compared with SBA-15.
The distance of the diffusion path might be the rate-limiting step for the dissolution of SRL. The SBA-15 had much larger pore length than MSNs, which restricted the diffusion rate of SRLSMEDDS in the mesopores. SRL was molecularly distributed in the SMEDDS-MSMs (Fig. S3 in Supporting information). However, the drug, oil, emulsifier and co-emulsifier might have different interaction strength with the surface of the mesopores since the silanol group on the surface of MSNs and SBA-15 could interact with amino, hydroxyl and sulfhydryl groups [4, 34]. Therefore, the diffusion of each component could be different, which might have a negative effect towards the formation of emulsion droplets. Moreover, the length of the mesopores would strengthen the influence of the interaction between SMEDDS and MSMs. Consequently, the length of the mesopores played a more important role than the pore diameter in the SRL dissolution. Therefore, the mesopores presented quite different dissolution behavior from macropores and micropores.
The pharmacokinetic study using beagle dogs was carried out to investigate the in vivo absorption of Rapamune®, SRL-SMEDDSMSNs and SRL-SMEDDS-SBA-15. The animal study was approved by the Fuzhou General Hospital Animal Care and Use Committee. Experimental design, blood sample treatment and quantitative analysis were performed based on previous study [29]. The relative bioavailability (Fr) of SRL-SMEDDS-MSNs and SRL-SMEDDS-SBA- 15 to Rapamune® was calculated using Eq. 1.
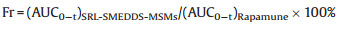
|
(1) |
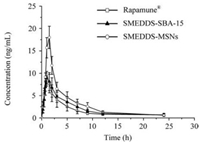
The mean blood concentration-time profiles were shown in Fig. 4 and Table 2. The SRL-SMEDDS-MSNs had significantly higher Cmax (18.97 ±1.35 ng/mL) than the commercial tablets (9.03 ±1.03 ng/mL, P < 0.01) and SRL-SMEDDS-SBA-15 (9.98 ± 0.70 ng/mL, P < 0.01). Compared with the tablets, the MSNs and SBA-15 had increased AUC. Especially, the AUC of MSNs (73.36 ± 9.36 ng h/mL) was much higher than that of tablets (42.24 ± 7.41 ng h/mL, P < 0.01) and SBA-15 (50.93 ± 10.41 ng h/mL, P < 0.05). The relative bioavailability of SRL-SMEDDS-MSNs and SRLSMEDDS-SBA-15 was 174.62% and 120.57%, respectively. The AUC of SBA-15 was increased as compared with the tablets but no significance was found (P > 0.05), which might be caused by the incomplete drug dissolution.
|
Download:
|
Fig. 4. Blood concentration-time profiles of the Rapamune |
|
|
|
Table 2 Pharmacokinetic parameters after oral administration of the Rapamune  |
In conclusion, the SRL-SMEDDS solidified with MSNs and SBA- 15 were successfully developed. The MSNs and SBA-15 with different pore diameter and particle size had similar capability for adsorption of SMEDDS. The SRL-SMEDDS-MSMs showed improved drug dissolution than raw SRL and commercial tablets. The length of mesopores played a more important role than the pore diameter in dissolution and absorption of SRL. The SMEDDS-MSNs presented as superior substrates of hydrophobic drugs for oral drug delivery.
AcknowledgmentsThis work was supported by the Natural Science Foundation of Fujian Province (Nos. 2017J01822 and 2018J01347), Fujian Medical University (No. 2017XQ1202), and Fuzhou General Hospital (No. 2017Q06).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.022.
| [1] |
A. Maleki, H. Kettiger, A. Schoubben, et al., J. Control. Release 262 (2017) 329-347. DOI:10.1016/j.jconrel.2017.07.047 |
| [2] |
P. Kumar, P. Tambe, K.M. Paknikar, V. Gajbhiye, J. Control. Release 287 (2018) 35-57. DOI:10.1016/j.jconrel.2018.08.024 |
| [3] |
Y. Zhou, G. Quan, Q. Wu, et al., Acta Pharm. Sin. B 8 (2018) 165-177. DOI:10.1016/j.apsb.2018.01.007 |
| [4] |
K.K. Qian, R.H. Bogner, J. Pharm. Sci. 101 (2012) 444-463. DOI:10.1002/jps.22779 |
| [5] |
R.J. Ahern, J.P. Hanrahan, J.M. Tobin, et al., Eur. J. Pharm. Sci. 50 (2013) 400-409. DOI:10.1016/j.ejps.2013.08.026 |
| [6] |
T. Limnell, H.A. Santos, E. Makila, et al., J. Pharm. Sci. 100 (2011) 3294-3306. DOI:10.1002/jps.22577 |
| [7] |
M. Van Speybroeck, R. Mols, R. Mellaerts, et al., Eur. J. Pharm. Biopharm. 75 (2010) 354-365. DOI:10.1016/j.ejpb.2010.04.009 |
| [8] |
M. Vialpando, S. Smulders, S. Bone, et al., J. Pharm. Sci. 105 (2016) 2782-2793. DOI:10.1016/j.xphs.2016.03.003 |
| [9] |
S. Pattnaik, K. Pathak, Curr. Pharm. Des. 23 (2017) 467-480. |
| [10] |
M. Kazi, R. Al Amri, F.K. Alanazi, M.D. Hussain, Curr. Drug Deliv. 15 (2018) 918-929. DOI:10.2174/1567201815666180116090910 |
| [11] |
A. Khattab, L. Hassanin, N. Zaki, AAPS PharmSciTech 18 (2017) 1657-1672. DOI:10.1208/s12249-016-0632-x |
| [12] |
W. Wu, Y. Wang, L. Que, Eur. J. Pharm. Biopharm. 63 (2006) 288-294. DOI:10.1016/j.ejpb.2005.12.005 |
| [13] |
S. Dokania, A.K. Joshi, Drug Deliv. 22 (2015) 675-690. DOI:10.3109/10717544.2014.896058 |
| [14] |
V. Pandey, S. Kohli, Crit. Rev. Ther. Drug Carrier Syst. 35 (2018) 99-155. DOI:10.1615/CritRevTherDrugCarrierSyst.v35.i2 |
| [15] |
D.J. Hauss, Adv. Drug Deliv. Rev. 59 (2007) 667-676. DOI:10.1016/j.addr.2007.05.006 |
| [16] |
C.J. Porter, C.W. Pouton, J.F. Cuine, W.N. Charman, Adv. Drug Deliv. Rev. 60 (2008) 673-691. DOI:10.1016/j.addr.2007.10.014 |
| [17] |
F. Li, R. Hu, B. Wang, et al., Acta Pharm. Sin. B 7 (2017) 353-360. DOI:10.1016/j.apsb.2017.02.002 |
| [18] |
Y. Sunazuka, K. Ueda, K. Higashi, et al., Int. J. Pharm. 546 (2018) 263-271. DOI:10.1016/j.ijpharm.2018.05.031 |
| [19] |
X. Xue, M. Cao, L. Ren, et al., AAPS PharmSciTech 19 (2018) 1847-1859. DOI:10.1208/s12249-018-0991-6 |
| [20] |
S. Kamboj, V. Rana, Int. J. Pharm. 501 (2016) 311-325. DOI:10.1016/j.ijpharm.2016.02.008 |
| [21] |
Y. Lei, Y. Lu, J. Qi, et al., Int. J. Nanomed. 6 (2011) 795-805. |
| [22] |
L.A.D. Silva, S.L. Almeida, E.C.P. Alonso, et al., Int. J. Pharm. 541 (2018) 1-10. DOI:10.1016/j.ijpharm.2018.02.020 |
| [23] |
N.T. Tung, C.S. Tran, T.M. Pham, et al., Int. J. Pharm. 537 (2018) 9-21. DOI:10.1016/j.ijpharm.2017.12.027 |
| [24] |
R.B. Chavan, S.R. Modi, A.K. Bansal, Int. J. Pharm. 495 (2015) 374-384. DOI:10.1016/j.ijpharm.2015.09.011 |
| [25] |
K. Midha, M. Nagpal, G. Singh, G. Aggarwal, Curr. Drug Deliv. 14 (2017) 1078-1096. |
| [26] |
R. Narayan, U.Y. Nayak, A.M. Raichur, S. Garg, Pharmaceutics 10 (2018) 118. DOI:10.3390/pharmaceutics10030118 |
| [27] |
D. Zhao, J. Feng, Q. Huo, et al., Science 279 (1998) 548-552. DOI:10.1126/science.279.5350.548 |
| [28] |
P.T. Tanev, T.J. Pinnavaia, Science 267 (1995) 865-867. DOI:10.1126/science.267.5199.865 |
| [29] |
X. Hu, C. Lin, D. Chen, et al., Int. J. Pharm. 438 (2012) 123-133. DOI:10.1016/j.ijpharm.2012.07.055 |
| [30] |
D. Wang, J. Huang, X. Wang, et al., Biomaterials 34 (2013) 7662-7673. DOI:10.1016/j.biomaterials.2013.06.042 |
| [31] |
J.J. Zimmerman, G.M. Ferron, H.K. Lim, V. Parker, J. Clin. Pharmacol. 39 (1999) 1155-1161. |
| [32] |
F. Baxevanis, J. Kuiper, N. Fotaki, Eur. J. Pharm. Biopharm. 107 (2016) 234-248. DOI:10.1016/j.ejpb.2016.07.013 |
| [33] |
S.G. Gumaste, A.T.M. Serajuddin, Eur. J. Pharm. Sci. 110 (2017) 134-147. DOI:10.1016/j.ejps.2017.05.014 |
| [34] |
J. Hu, T.L. Rogers, J. Brown, et al., Pharm. Res. 19 (2002) 1278-1284. DOI:10.1023/A:1020390422785 |
 2018, Vol. 29
2018, Vol. 29