b Advanced Research Institute of Multidisciplinary Science, Beijing Institute of Technology, Beijing 100081, China;
c School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China;
d CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;
e Key Laboratory of Advanced Energy Materials Chemistry(Ministry of Education), Nankai University, Tianjin 300071, China
Energy consumption is ever increased in our modern society [1-3]. Once considering the whole cycling life, the current fossil fuels suffers from the remarkably low efficiency and extremely prolonged conversion duration of organic matters generated by photosynthesis, during which the clean and renewable sources of solar energy is inefficiently converted in to organism. Furthermore, its consumption renders an inevitable environmental pollution due to the N, S and heavy metals [4]. A direct utilization of the clean and renewable power, such as solar, wind, and tide energy, can tackle the root of the problem, with bypassing the extremely long conversion process as well as the inefficient photosynthesis. Nevertheless, these clean sources are unstable, which appear and disappear with the change of time and seasons. Accordingly, emerging energy materials and sustainable energy conversion and storage technologies play a vital role in the utilization of clean power and need to be urgently developed [5].
Among various alternatives, environment-benign and economic-viability electrochemical technologies, such as water splitting [6-9], fuel cells [10-12], and metal-air batteries [13-15] have witnessed ever increasing concerns. Taken rechargeable zinc-air batteries as example, the anode is Zn foil, cathode is O2 of air, and electrolyte is KOH solution, with excellent safety and low operating cost of less than 10 $/kWh [16]. During discharging, Zn is transformed into zinc cations at anode with the forms of zincate ions (Zn(OH)42-) and/or zinc oxide (ZnO, at supersaturated concentrations). At the same time, oxygen reduction reactions (ORR, O2 + 2H2O + 4e- → 4OH-, alkaline) proceed at air electrode. When charging, zinc ions are deposited at Zn anode surface and oxygen evolution reaction (OER, O2 + 2H2O + 4e- → 4OH-, alkaline) occurs on air electrode. The overall reaction is Zn interacts with O2 to generate ZnO, which can be converted into Zn again when battery charging and no pollutions are produced during all the charge and discharge processes. Furthermore, the theoretical energy density is as high as 1086 Wh/kg, 6-fold that of Li-ion batteries [17, 18]. Besides energy storage, clean energy conversion technologies also play a vital role in sustainable energy system. Electrochemically water splitting is one of the most promising approaches with OER as a half reaction [19]. It converts water into high-purity hydrogen, the one as energy carrier with zero carbon and highest gravimetric energy density among all chemical fuels (142 MJ/kg), which is significant for hydrogen economics [20]. However, both OER and ORR involve multistep electron/proton transfer and suffer from sluggish kinetics, thereby resulting in high overpotential and low energy efficiency [21-23]. High-performance electrocatalysts are greatly required to boost OER and ORR process [24-26]. To date, although precious metal catalysts have demonstrated favorable OER activity, such as IrO2 for OER [27] and Pt for ORR [28], their practical applications are greatly prohibited by the high cost, low abundance, and unsatisfied stability. Emerging as outstanding alternatives, precious-metal-free catalysts, such as transition-metal materials [29, 30], nanocarbons [31-33], and their hybrids [34, 35], have witnessed ever growing intentions due to their high activity, economic viability, tunable structure and surface chemistry. Nevertheless, smart design and versatile techniques aiming at comprehensively regulating intrinsic acidity and favorable extrinsic characters are still a challenge and urgently required for the construction of advanced materials for oxygen electrocatalysis.
The reactivity of electrocatalysts is primarily determined by the intrinsic electronic structure, which dominates with the adsorption and desorption of intermediates with a volcano-correlated plot [36]. As gas-involved oxygen electrocatalysis processes, OER and ORR occur on the three-phase boundary of a solid catalyst, liquid electrolyte and gas reactant/products [37]. During the oxygen electrocatalysis, reactants in electrolytes are firstly experienced a transfer to the catalyst surface, followed by electron transfer, and subsequent surface reactions, including adsorption, activation, intermediate generation, product formation, and desorption [38-40]. Consequently, the nanostructures, such as accessibility of active sites, interface resistance, and mass transfer channels, also exert an evident effect on apparent catalytic activity. Moreover, to thoroughly demonstrate the potentials of high intrinsic activity and favorable nanostructure in practical energy devices, a rational design and construction of electrocatalysts to meet the requirements as macroscopic electrodes is further desirable [41-43].
Based on the understanding of OER and ORR electrocatalysis, we herein reviewed recent advances in material design and construction in three respects: (1) electronic structure regulation; (2) nanostructure tailor; and (3) freestanding electrode construction. The principles and discussions presented in this review are expected to shed fresh concept for the fabrication of advanced energy materials for OER and ORR electrocatalysis, which is also instructive for other gas-involved processes, such as hydrogen evolution [44-47], hydrogen oxidation [48], nitrogen and CO2 reduction [49-54].
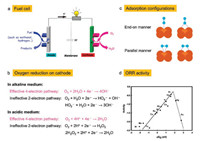
2. Oxygen reduction 2.1. Brief description and reaction mechanismORR is employed as the cathode reaction in various nextgeneration energy conversion technologies, such as metal-air batteries and full cells (Fig. 1a) [55-57]. Nevertheless, ORR suffers from sluggish kinetics induced by the inherent multiple electron and proton transfer and the complex reaction process involving O2 adsorption, O—O bond cleavage, and oxide removal [22, 58, 59]. An excellent catalytic efficiency is primarily demanded for ORR electrocatalysts on the one hand, with the aim to reduce the loadings thereby minimizing the cost of electrocatalysts for practical applications. The catalyst cost accounts for as high as almost 60% in that of a practical fuel cell. On the other hand, the loss in durability and/or performance should be avoided when decreasing the catalyst loadings under the corrosive operating conditions [60].
|
Download:
|
| Fig. 1. (a) Schematic of typical fuel cells. (b) ORR pathways in alkaline and acidic media. Reproduced with permission [55]. Copyright 2016, Royal Society of Chemistry. (c) Different configurations of O2 adsorption on catalyst surface. Reproduced with permission [25]. Copyright 2012, Royal Society of Chemistry. (d) Trends in oxygen reduction activity (defined in the text) plotted as a function of oxygen binding energy. Reproduced with permission [73]. Copyright 2004, American Chemical Society. | |
ORR is a complex reaction process including multiple electron and proton transfer and many oxygen-containing intermediates (such as OH, O, HO2-), which can be briefly divided in to 4-electron and 2-electron reaction routes (Fig. 1b). The direct 4-electron pathway is high-effective and desirable. In contrast, 2-electon process is unfavorable due to its low energy efficiency and caused degradation of battery cathode by HO2- (alkali)/H2O2 (acid). After the first 2-electron oxygen reduction, another 2-electron reduction may further follow, or a disproportionation of produced peroxide [61-63].
The two reaction pathways occur concomitantly and competitively during the ORR process, of which the route-selectivity is significantly affected by adopted catalyst categories, adsorption configurations, and reaction conditions. Compared to the end-on adsorption form with only one oxygen atom cooperated to catalyst surface (Fig. 1c, Eqs. 1-3), the parallel adsorption manner is more favorable for the direct reduction pathway (Eqs. 1, 4, 5). Besides, a high potential more facilitates the 4-electron route, and a low potential is favorable to the peroxide path. Generally, it is accepted that the 4-electron pathway is dominant on the precious metal catalysts while the 2-electron pathway is more likely occur on the carbon based electrocatalysts. There are various complicate pathways on transition-metal materials that mainly depend on intrinsic and extrinsic characters of materials. Additionally, the interactions between hydrated cations and adsorbed species and transport effects also exert effect on the path selectivity [12, 25, 64-66].
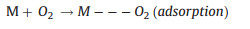
|
(1) |
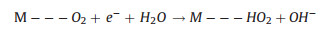
|
(2) |
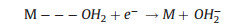
|
(3) |
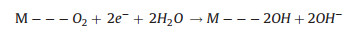
|
(4) |
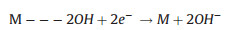
|
(5) |
The ORR process involves the steps of O2 adsorption, hydration, electron/proton transfer, peroxide/oxide formation, and desorption of surface hydroxide, of which the first electron transfer [67-71], oxygen hydration [72], and intermediate desorption [73-75] are generally recognized to be rate-limiting for ORR kinetics. For instance, Nørskov and co-workers investigate the free-energy landscape of ORR through DFT calculations [73]. The adsorbed oxygen and hydroxyl species are too stable to reduction reaction when the overpotential is low. The rate-limiting step is the adsorption of intermediates. Besides, they also calculated the adsorption energy of O* and OH*, and obtained the ORR activity trend on the surface of various transition and noble metal materials (Fig. 1d).
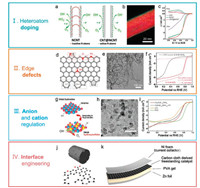
2.2. Electrocatalyst design and development 2.2.1. Electronic structure modulationThe ORR catalytic activity is primarily dominated by the electronic structure of active sites. For carbon based materials, introducing heteroatoms (N, P, S, B, O, etc.) is demonstrated to be a significantly effective strategy for electronic structure regulation [76-82]. The sp2 conjugated carbon electroneutrality is break when incorporating heteroatoms in the matrix, with the formation of ORR active sites. Through incorporating nitrogen into vertically aligned carbon nanotubes, Dai and co-workers describe a pioneering electrocatalyst with high ORR activity and favorable stability [83]. Noteworthily, the bulk dopants contribute scarcely to the ORR electrocatalysis but can induce a significant decrease in the electron conductivity, which is unfavorable to electrocatalysis on the contrary. Zhang and co-workers controllably incorporated nitrogen dopants on the surface of the carbon nanotubes and synthesized a nitrogen-doped nanocable with bifunctional ORR and OER catalytic activity for the first time (Figs. 2a-c) [84]. The nitrogen-containing shell fully exposes the regulated active sites. Moreover, the undestroyed nanotube core renders a great electron highway for ORR electrocatalysis. Consequently, the nanostructured N-doped nanocables presented a prominently superior ORR performance to deeply N-doped and undoped counterparts. Liu, Dai, and co-workers further investigated different N sites for electrocatalysis [85]. The electron-donating quaternary N is responsible for ORR experimentally. Additionally, it is also reported that a mixed doping on carbon matrix may render a greater ORR catalytic performance than mono one [86]. For instance, Qiao and co-workers found that the synergy of doped N and O prominently improved the ORR activity in carbon based catalysts [38].
|
Download:
|
| Fig. 2. (a) Scheme for the full exposure of "active sites" on the surface: NCNTs with bulk doping of nitrogen atoms, while CNT@NCNT coaxial nanocables with surface enriched nitrogen for OER and ORR. (b) The element mapping showing the distribution of N (green), C (red), and O (blue), of CNT@NCNT coaxial nanocables with the surface N/C ration of 0.0238. (c) Rotating ring disk voltammograms recorded for CNT, NCNT, and CNT@NCNT electrode. Reproduced with permission [84]. Copyright 2014, John Wiley and Sons. (d) Different kinds of N-doping and topological defects. (e) TEM image of NGF. (f) LSV curves. Reproduced with permission [87]. Copyright 2016, John Wiley and Sons. (g) Schematic of electronic structure regulation. (h) TEM image of the constructed hydroxysulfide with porous graphene as substrates. (i) LSV curves for ORR electrocatalysis evaluation. Reproduced with permission [111]. Copyright 2017, John Wiley and Sons. (j) Schematic of defect-rich graphene skin on carbon fiber. (k) Schematic of flexible zincair battery as o-CC-H2 with air electrode. Reproduced with permission [118]. Copyright 2018, Elsevier. | |
Tang et al. further elaborately probed all possible active sites of N-doped carbon catalyst via DFT calculations, and found that doping at edges is more effective than that in bulk for ORR electrocatalysis (Fig. 2d) [87]. Intriguingly, it is also revealed that the topological defects, especially the one with adjacent heptagon and pentagon, exhibit greater ORR reactivity than doping ones, which is owning to the generation of a permanent dipole moment and thus regulated electronic structure. Inspired by this concept, a nitrogen-doped graphene mesh (NGM) with abundant pore edges and topological defects are fabricated through directly carbonizing a mixture of sticky rice and melamine with in-situ generated Mg(OH)2 as templets (Fig. 2e). Contributed by the edge effects and topological defects, NGM with only a trace nitrogen of 0.41 at% delivered an evidently higher ORR activity than N-doped graphene with a nitrogen content of as high as 7.48 at% (Fig. 2f). This concept is also confirmed by the related studies on other pure carbon materials and further high-end characterizations, clearly indicating the vital role of topological defects in ORR catalysis [88-92].
Besides carbon catalysts, transition metal materials (oxides [12, 24, 93, 94], chalcogenides [95-98], nitrides [99, 100], phosphides [101-103], metal-N-C [104-107], etc.) are also a family of promising candidates for ORR electrocatalysis. The electronic structure of active metal cations can be effectively regulated by a direct substitution of original metal, which is also referred as cation regulation. For instance, the half-wave overpotential can be greatly reduced by over 150 mV in 0.1 mol/L KOH for ORR when a low-degree replacement of Co by Mn (~2 at%) in a Co based electrocatalyst. This is ascribing to the improved cooperating environment and thus facilitated adsorption performance of intermediates [108].
Notably, metal cations as active sites are intimately interacted with anions. Therefore, the change of anion properties can exert another significant effect on ORR through modulating the electronic structure of active sites. Accordingly, anion regulation has attracted tremendous research interest very recently for optimizing ORR catalytic performance [109, 110]. Specifically, different anions possess distinct polarizability and electronegativity. Therefore, the incorporation of anions with proper characters can effectively improve the cooperated environment of metal active sites with a more balanced local electronic structure for ORR electrocatalysis. As a proof of concept, both sulfur and oxygen were adopted as anions to modulate intrinsic ORR activity of CoNi based catalysts (Figs. 2g-i) [111]. By immersing CoNi hydroxide in concentrated S2- solution at room temperature, the pristine oxygen was replaced by sulfur due to their different solubility equilibrium. Sulfur possesses lower electronegativity and higher polarizability than oxygen. Consequently, the incorporation of sulfur by substituting oxygen induces to more electrons be shared with cations by dispersing electrons into metal 3d orbits. As a result, the cooperated environment and electronic structure of active sites can be effectively modulated through controlling the incorporation of sulfur element. Benefited to the moderative kinetics of proposed room-temperature sulfurization, the incorporated sulfur content is precisely controlled with immersing duration. The as-constructed CoNi hydroxysulfide delivers a prominently higher ORR reactivity than CoNi hydroxides. Notably, anion regulation as a promising strategy that is still at the infant stage in improving energy electrocatalysis process.
2.2.2. Tailored nanostructuresIn addition to intrinsic activity, extrinsic physiochemical characters, such as active site accessibility and electron conductivity, also play a vital role in electrocatalysis. This is very prominent for gas-involved ORR process. Although cation and anion regulation can effectively improve the intrinsic ORR reactivity, the apparent activity is highly hindered by the poor conductivity of transition meal catalysts. The conductive substrate is therefore highly requested. Nanocarbon with excellent conductivity, high surface area, and tunable surface chemistry is widely adopted as substrates to endow electron highway of the hybrid electrocatalysts. With in-situ growth of carbon nanotubes on a CoFeMgAl layered double oxide (LDO), in which Fe and Co are highly separated by Mg and Al, serving as catalytic active sites for both ORR electrocatalysis and carbon nanotube growth, Wang et al. constructed a LDO/CNT hybrids with active point-conductive lineactive point connections [112]. Compared with a physical mixture of active plates and conductive filters, the point-line-point hybrids possess favorable electron pathway and also exhibit a greatly reduced charge transfer resistance and increased electrochemical active surface area. Consequently, the smart design improves the conductivity and leads to a further exposure of active sites by avoiding the LDO plate stacking, thereby rendering a significantly enhanced ORR catalytic performance. Besides, spatially confined growth is demonstrated as an effective strategy to fully expose active sites [113]. Through confining the nucleation and growth of targeted compounds on substrates, nanoscale active clusters with uniform distribution can be achieved, with more exposed active sites and improved accessibility [114]. Utilizing the inherent topological defects of N-doped hierarchical graphene, Zhang and co-workers anchors the meal cations on the pore edges to atomically distribute the Co-Nx-C active sites. As a result, the electrocatalytic performance is greatly enhanced for ORR process [115].
2.2.3. Self-supporting electrodeNotwithstanding these advances in modulating electronic structure and tailoring nanostructure, there is a long way towards ultimate application in practical cells for effective energy conversion. Traditional electrode fabrication using drop-casting method is facile and effective for electrocatalyst evaluation. Nevertheless, it also inevitably brings unfavorable disadvantages for practical energy devices: (1) electrocatalysts peel off from electrodes: the bond between catalyst powder and substrate such as carbon cloth and Ni foam with binders is not robust enough; the electrocatalysts trend to peel off from electrode especially in gas-involved process, leading to an evident degradation; (2) larger resistance due to the insulate binder interface; (3) unevenly distributed powders from the coffee-effect when catalyst ink dropping that renders more dead volume and poor utilization of electrocatalysts; and (4) tedious procedure for electrode fabrication.
To address these issues, binder-free freestanding materials have been developed and attracting great attentions recently [42, 116, 117]. Taking a surface modified carbon cloth (o-CC-H2) fabricated by Zhang and co-workers as an example [118], a porous defect-rich graphene skin was in-situ generated on the carbon fiber skeleton through H2 etching (Fig. 2j). Contributed by the abundant topological defect sites, high electron pathway, porous mass transfer channels, and strongly coupled interface, o-CC-H2 catalyst exhibits an ORR current density three times higher than pristine carbon cloth. More importantly, it can be directly used as air electrode for flexible zinc-air batteries, which exhibits superior catalytic activity and favorable duration when discharging even under bending conditions (Fig. 2k).
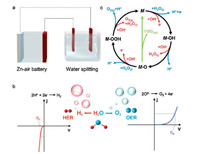
3. Oxygen evolution reaction 3.1. Reaction mechanismOER is the half-reaction of both electrochemically water splitting and secondary metal-air batteries [119-122]. OER plays a vital role in next-generation energy conversion and storage system (Fig. 3a). It is a multi-step electron/proton coupled reaction, and also strongly limited by the intrinsic mass transfer/adsorption of reactants and the desorption of products [123]. In water splitting unit, OER as the anode reaction is a four-electron process. In contrast, the cathode reaction of HER involves only a 2-electron transfer. As a result, the required overpotential to effectively split water is theoretically higher for anode than that of cathode (Fig. 3b). In the case of metal-air batteries, OER occurs on the air electrode when battery charging, which is crucial for energy efficiency. Moreover, OER also greatly impacts the deposition of metal ions and generated performance of O2. A benign cationic deposition performance is of great benefit to metal dendrite control and operation safety. A favorable generation performance of O2 on the catalyst surface greatly facilitates the avoid of catalyst peeling, which is aroused from the bursting crash of O2 bubbles and is also unfavorable for ORR catalyst layers. Therefore, the material design and fabrication is expected more challenge and crucial for OER electrocatalysis aiming at practical energy applications.
|
Download:
|
| Fig. 3. (a) Zinc-air batteries (left) and water splitting devices (right). OER is the half reaction in the anode of water splitting unit and air electrode of metal-air batteries. Reproduced with permission [159]. Copyright 2018, John Wiley and Sons. (b) Polarization curves for HER and OER. (c) The OER mechanism for acid (blue line) and alkaline (red line) medium. The black line indicates that the oxygen evolution involves the formation of a peroxide (M-OOH) intermediate (black line) while another route for direct reaction of two adjacent oxo (M-O) intermediates (green) to produce oxygen is possible as well. Reproduced with permission [29]. Copyright 2017, American Chemical Society. | |
The pathways of OER is outlined as follows [124]:
OER in acid media:
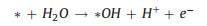
|
(6) |
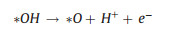
|
(7) |
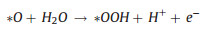
|
(8) |
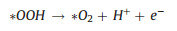
|
(9) |
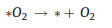
|
(10) |
OER in alkaline media:
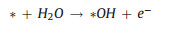
|
(11) |
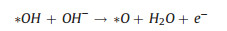
|
(12) |
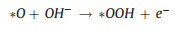
|
(13) |
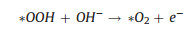
|
(14) |
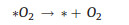
|
(15) |
Notably, it is also possible that the final O2 molecule is formed through direct interactions between *O intermediates (Fig. 3c), rather than the decomposition of *OOH (Eqs. 9 and 14) [29]. Among these elemental reaction steps mentioned above, the adsorption of *O and generation of *OOH are believed to afford higher barrier for OER process [36].
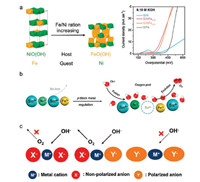
3.2. Material design and development 3.2.1. Electronic structure modulationElaborately designed nanocarbon electrocatalysts have attracted many attentions in ORR electrocatalysis. Nevertheless, their OER performance is generally not satisfied yet [85, 92, 125]. Transition metals (Fe, Co, Ni, Mn, etc.) are demonstrated to be favorable active sits for OER electrocatalysis and attracts tremendous attention recently [126-137]. Aiming at achieving superb water oxidation performance, electronic structure modulation through cation and anion regulation is still the primary strategy for transition-metal materials [138]. Tang et al. fabricated a series of NiFe (oxy)hydroxides to probe the role of Ni substitution by Fe in OER electrocatalysis. With an increase of the Fe content, a phase transfer form Fe doped Ni-based hydroxide host to Ni doped Febased oxyhydroxide host is demonstrated (Fig. 4a) [139]. The substitution of Ni by Fe is revealed effective to improve the OER reactivity. Besides metal substitution, Li et al. further proposed a p-block metal regulation strategy to improve electronic structure and intermediate adsorption behavior through cation regulation (Fig. 4b) [140]. The Sn4+ is facilely dissolved from SnNiFe perovskite during electrochemical activation, rendering higher metal oxidative sites and abundant vacant sites on catalyst surface. Consequently, high energy hanging bands are generated and reactant hydroxyl fusion is greatly enhanced, which significantly improves the OER catalytic performance.
|
Download:
|
| Fig. 4. Cation and anion regulation in electronic structure for superb OER performance. (a) Schematic of phase evolution in NiFe (oxy)hydroxides with the increase of Fe content (left), and LSV curves of all tested samples (right). Reproduced with permission [139]. Copyright 2016, Royal Society of Chemistry. (b) Schematic of p-block metal regulation for superb water oxidation. The dissolution of Sn4+ in SnNiFe perovskite during electrochemical activation renders abundant vacant sites and high energy hanging bands on the catalyst surface, with a greatly enhanced OER activity. Reproduced with permission [140]. Copyright 2017, Nature. (c) Schematic of controllable anion regulation for OER process. A rational degree of anion regulation leads to a more balanced electronic structure for water oxidation. Reproduced with permission [143]. Copyright 2017, John Wiley and Sons. | |
In addition to cation modulation, anion regulation is also an effective strategy to modulate intrinsic reactivity for oxygen electrocatalysis. Furthermore, it is more widely used for OER process compared to ORR [141]. The concept of anion regulation for OER electrocatalysis was firstly proposed by Qiao and co-workers [142]. Benefited from the favorably regulated electronic structure through doped oxygen anions, the constructed CoFePO catalysts exhibited superior OER reactivity. Zhang and Co-workers further probed the role of precise control of anion regulation in OER electrocatalysis (Fig. 4c). A moderately sulfurized O-containing NiFe compounds delivered the highest OER reactivity compared to the samples with evidently lower and higher sulfur content, respectively [143].The dual-ligand anion regulation of S and O with distinct properties renders a more balanced cooperating environment for NiFe active sites, thus greatly enhances the OER reactivity, which clearly indicates the vital role of controllable anion regulation in electrochemically water oxidation [49].
Noteworthily, electrocatalysts widely experience a transformation during the OER process in cations, anions, and associated electronic structures, attributing to the high oxidative potentials. Cations trend to be transferred into higher valence states, such as form Ni(OH)2 to NiOOH [144, 145], which is generally recognized to be beneficial for OER electrocatalysis [146-148]. In the case of anions, incorporated pnictide and chalcogenide (such as P, N, S, Se) undergo redox reactions and trend to be partly or completely removed during OER process, leading to a regulated electronic structure as well as altered extrinsic properties, including nanostructures, hierarchical morphology, and even local/surface phases. For instance, Mabayoje et al. investigated the role of sulfur anions in oxygen electrocatalysis with NiS as catalysts [149]. The sulfur anions were depleted and NiS was converted into nickel oxide when water oxidation, during which a more exposed catalyst surface was in-situ generated, contributing to a full exposure of active sites and thus leading to an enhanced catalytic performance. NiS plays a pre-catalyst for OER electrocatalysis.
Remarkably, a partial removal during OER process was also reported for the incorporated anions. It facilitates to the construction of heterostructures, bringing the merits with decreased interfacial resistance, boosted charge transfer, exposed active sites, and improved mass transfer channels for oxygen electrocatalysis, such as NiPS3@NiOOH [150], Se-(NiCo)Sx/(OH)x [150], and Ni2P/NiOx [151]. More importantly, the residual anions also facilitate the improvement in intrinsic OER activity. Taking NiFe disulfides as examples, the S residues contribute to an optimized electronic structure, facilitating the decrease in the gap of adsorption energy between O* and OH*, therefore leading to an enhanced OER electrocatalytic performance [152]. Benefited from the comprehensive modulation in both intrinsic electronic structure and extrinsic physicochemical properties, the partial removal of incorporated anions is favorable for OER electrocatalysis.
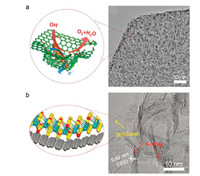
3.2.2. Tailored nanostructuresAlthough superior OER intrinsic activity can be achieved for active sites by cation and anion regulation, favorable extrinsic characters are also of great benefit to electrocatalysis, synergistically boosting the apparent OER reactivity. The utilization of conductive substrate to spatially confine active compounds has been demonstrated as an effective strategy [153, 154]. Tang et al. adopts N-doped mesoporous graphene framework (NGF) as substrates to in-situ grow NiFe LDH through co-precipitation method (Fig. 5a) [155]. NGF is fabricated through CVD method with prepared MgO as templates, rendering uniformly distributed nitrogen and abundant mesoporous on surface. Doped nitrogen is used to anchor the LDH active compounds and the surface mesoporous contribute to a spatially growth of the active components. As a result, NiFe LDH with an average size of 5 nm is uniformly distributed on NGF surface encircled by graphene layer. It brings the merits of exposed active sites from nanosized active compounds, facilitated electron path contributed by conductive substrates, reduced resistance form the strong coupled interfaces, and enhanced mass transfer due to the structural hierarchy. Taken together, NiFe/NGF possess superior active sites of nano-sized NiFe LDH and excellent extrinsic properties, leading to a superior OER electrocatalytic performance. Analogously, singleatom strategy can also be recognized as spatially confinement and has been demonstrated to be greatly effective to fabricate superb OER electrocatalysts [156, 157].
|
Download:
|
| Fig. 5. Tailored nanostructure for OER electrocatalysis. (a) Spatial-confine growth of NiFe LDH on N-doped graphene framework. Reproduced with permission [155]. Copyright 2015, John Wiley and Sons. (b) 3D mesoporous van der Waals heterostructure with curved N-MoS2 nanosheets intimately merged on porous graphene surface. Reproduced with permission [158]. Copyright 2017, John Wiley and Sons. | |
In addition to spatially confinement, nano-sized heterostrucure construction between conductive materials and active compounds is revealed to be another fascinating strategy to construct highperformance OER catalysts. For example, Zhang and co-workers proposed a 3D mesoporous van der Waals heterostructure (G@N-MoS2) with curved N-MoS2 nanosheets intimately merged on electron highway, strongly coupled interface and boosted mass transfer process, G@N-MoS2 delivers an enhanced electrocatalytic performance in OER, as well as ORR and HER.
3.2.3. Self-supporting electrodeTo thoroughly demonstrate the full potential of regulated electronic structure and tailored nanostructure in practical energy applications, the construction of self-supporting electrode material is highly desired. Electrodepositing active compounds on macroscopic conductive framework has been proved to be an effective strategy.
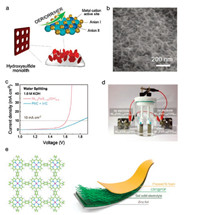
Wang et al. proposed an anion-regulated hydroxysulfide monolith material (Ni1.9FeS1.09(OH)4.6) in OER, ORR, and HER electrocatalysis (Fig. 6a) [159]. A NiFe LDH layer is in-situ grown on Ni foam substrates with a short electrodeposition duration in advance, followed by a controllable sulfurization with the obtained material immersed in concentrated S2- solution for a designed duration at room temperature. The moderate shift compared to that of deeply sulfurized sample in high-resolution Ni 2p spectra indicates a controllable anion regulation for the constructed hydroxysulfide. Finally, with ultrathin NiFe hydroxysulfide nanosheets interconnected and vertically in-situ grown on Ni foam substrates (Fig. 6b), the hydroxysulfide monolith is constructed and affords multifunctional modifications: (1) regulated electronic structure via controllable incorporation of sulfur; (2) tailored nanostructure: short electrodeposition duration renders a nanosized NiFe LDH. Further, Ni foam as substrates well uniformly disperse and strongly couple with the NiFe LDH nanosheets, leading to fully exposed active sites, reduced interface resistance, interconnected electron highway, and hierarchical porous channel; (3) macroscopic electrode feature: with the freestanding nature, the constructed hydroxysulfide monolith can be directly used as electrodes and is robust enough to undergo the crush of continuously generated bubbles during electrocatalysis. Synthetically contributed by the regulated electronic structure in atom scale, material structure in nanoscale, and favorable electrode feature in macroscale, Ni1.9FeS1.09(OH)4.6 exhibit superior electrocatalytic performance in zinc-air batteries as air electrodes, and water splitting units as both anode and cathode (Fig. 6c). Powered by the two zinc-air batteries driven by Ni1.9FeS1.09(OH)4.6 and connected serially, bulky O2 and H2 bubbles are continuously generated on both constructed electrodes when electrochemically water splitting (Fig. 6d).
|
Download:
|
| Fig. 6. Self-supporting electrode materials for oxygen electrocatalysis. (a) Schematic of constructed Ni1.9FeS1.09(OH)4.6 hydroxysulfide monolith. (b) TEM image of Ni1.9FeS1.09(OH)4.6. (c) LSV curves of Ni1.9FeS1.09(OH)4.6 and precious metal for water splitting evaluation. (d) The photo of self-powered water splitting unit, of which Ni1.9FeS1.09(OH)4.6 monolith are served as air electrode in zinc-air batteries, and both anode and cathode in water-splitting unit. Reproduced with permission [159]. Copyright 2018, John Wiley and Sons. (e) Schematic of Co-based POF (left) and flexible zinc-air battery with the constructed CNT@POF as air electrode. Reproduced with permission [160]. Copyright 2018, Royal Society of Chemistry. | |
In addition to the in-situ growth of active components on macroscopic substrates, self-assembly of active composites is also an effective strategy in free-standing electrode fabrication. Taken porphyrin covalent organic framework (POF) as example (Fig. 6e), Li et al. prepared Co based POF with the exist of carbon nanotubes in the synthetic system. As a result, ultrathin POF layer are intimately coated on the nanotube surface and self-assemble into a free-standing electrode (CNT@POF) [160]. Contributed to the high intrinsic activity of POF composites and excellent extrinsic characters, both the flexible and aqueous zinc-air batteries with CNT@POF as air electrodes present superior performance.
4. Conclusions and perspectivesBoth ORR and OER electrocatalysis has been strongly investigated with the regards in both fundamental mechanism and practical applications, due to its importance for future energy scenario. Herein, we reviewed recent progress of material design and synthesis for OER and ORR, respectively, in the respects of electronic structure regulation, nanostructure tailor, and macroscopic electrode construction. The challenging but significant directions are still needed to be considered as follows:
(1) Electronic structure: The types of active sites are generally complicated on an actual electrocatalysts. The identification of the activity origin and in-depth understanding of the relationship between the different-type active sites are of great benefit to guide electronic structure regulation. The combination of experiments and theoretical analysis is believed to be effective to address the issues. Moreover, a model material system with unique active site type should be fabricated to identify actual active sites for OER and ORR electrocatalysis, respectively.
(2) Nanostructure. The strain effects in oxygen electrocatalysis need to be sufficiently propped, especially for the nanomaterials with curved morphology. Besides, the role of interface need to be further investigated in catalyzing OER and ORR, including that between active components and conductive substrates.
(3) Macroscopic electrode. The properties of metal anodes play a major role on the electrocatalytic performance of OER and ORR on the cathodes of metal-air batteries. A deeper understanding of the impacts is required. Besides, the reactant of OH- for OER is from the electrolyte in the metal-air battery inside. In contrast, the ORR reactant of O2 comes from outside air. This distinction should be greatly considered during electrode design and cell fabrication.
The concepts of energy chemistry and material design presented in this review facilitate to the fabrication of advanced energy materials in OER and ORR electrocatalysis with enhanced reactivity, favorable durability, and high selectivity. Furthermore, it is also instructive for other gas-involved electrocatalytic reactions, such as nitrogen reduction, CO2 reduction, and hydrogen peroxide production.
AcknowledgmentThis work was supported by National Key Research and Development Program (No. 2016YFA0202500), the Natural Scientific Foundation of China (Nos. 21676160, 21776019 and 21825501), the CAS Key Laboratory of Carbon Materials (No. KLCMKFJJ1701), and 111 Project (No. B12015P). The authors thank Cheng Tang, HaoFan Wang, and Bo-Quan Li for insightful discussion.
| [1] |
W. Zhang, W.Z. Lai, R. Cao, Chem. Rev. 117 (2017) 3717-3797. DOI:10.1021/acs.chemrev.6b00299 |
| [2] |
A.G.L. Borthwick, Engineering 2 (2016) 69-78. DOI:10.1016/J.ENG.2016.01.011 |
| [3] |
D.F. Yan, Y.X. Li, J. Huo, et al., Adv. Mater. 29 (2017) 1606459. DOI:10.1002/adma.v29.48 |
| [4] |
B. Wang, C. Han, Q. Zhang, et al., Energ. Fuel 29 (2015) 5701-5713. DOI:10.1021/acs.energyfuels.5b01280 |
| [5] |
X.Q. Zhang, X.B. Cheng, Q. Zhang, J. Energy Chem. 25 (2016) 967-984. DOI:10.1016/j.jechem.2016.11.003 |
| [6] |
J. Masa, P. Weide, D. Peeters, et al., Adv. Energy Mater. 6 (2016) 1502313. DOI:10.1002/aenm.201502313 |
| [7] |
C.C. McCrory, S. Jung, I.M. Ferrer, et al., J. Am. Chem. Soc. 137 (2015) 4347-4357. DOI:10.1021/ja510442p |
| [8] |
X. Jia, Y. Zhao, G. Chen, et al., Adv. Energy Mater. 6 (2016) 1502585. DOI:10.1002/aenm.201502585 |
| [9] |
T. Liu, Q. Liu, A.M. Asiri, Y. Luo, X. Sun, Chem. Commun. 51 (2015) 16683-16686. DOI:10.1039/C5CC06892D |
| [10] |
L. Qu, Y. Liu, J.B. Baek, L. Dai, ACS Nano 4 (2010) 1321-1326. DOI:10.1021/nn901850u |
| [11] |
M. Lefèvre, E. Proietti, F. Jaouen, J.P. Dodelet, Science 324 (2009) 71-74. DOI:10.1126/science.1170051 |
| [12] |
J. Suntivich, H.A. Gasteiger, N. Yabuuchi, et al., Nat. Chem. 3 (2011) 546. DOI:10.1038/nchem.1069 |
| [13] |
J. Fu, Z.P. Cano, M.G. Park, et al., Adv. Mater. 29 (2017) 1604685. DOI:10.1002/adma.201604685 |
| [14] |
J.S. Lee, S.T. Kim, R. Cao, et al., Adv. Energy Mater. 1 (2011) 34-50. DOI:10.1002/aenm.201000010 |
| [15] |
R. Cao, J.S. Lee, M. Liu, J. Cho, Adv. Energy Mater. 2 (2012) 816-829. DOI:10.1002/aenm.201200013 |
| [16] |
Y. Li, H. Dai, Chem. Soc. Rev. 43 (2014) 5257-5275. DOI:10.1039/C4CS00015C |
| [17] |
P. Gu, M. Zheng, Q. Zhao, et al., J. Mater. Chem. A 5 (2017) 7651-7666. DOI:10.1039/C7TA01693J |
| [18] |
Y. Li, M. Gong, Y. Liang, et al., Nat. Commun. 4 (2013) 1805. DOI:10.1038/ncomms2812 |
| [19] |
G. Zhao, K. Rui, S.X. Dou, W. Sun, Adv. Funct. Mater. 28 (2018) 1803291. DOI:10.1002/adfm.v28.43 |
| [20] |
J.A. Turner, Science 305 (2004) 972-974. DOI:10.1126/science.1103197 |
| [21] |
C. Tang, M.M. Titirici, Q. Zhang, J. Energy Chem. 26 (2017) 1077-1093. DOI:10.1016/j.jechem.2017.08.008 |
| [22] |
X.X. Huang, Y.Z. Wang, W. Li, Y.L. Hou, Sci. China Chem. 60 (2017) 1494-1507. DOI:10.1007/s11426-017-9153-6 |
| [23] |
W. Xia, A. Mahmood, Z.B. Liang, R.Q. Zou, S.J. Guo, Angew. Chem. Int. Ed. 55 (2016) 2650-2676. DOI:10.1002/anie.201504830 |
| [24] |
Y. Liang, Y. Li, H. Wang, et al., Nat. Mater. 10 (2011) 780. DOI:10.1038/nmat3087 |
| [25] |
F. Cheng, J. Chen, Chem. Soc. Rev. 41 (2012) 2172-2192. DOI:10.1039/c1cs15228a |
| [26] |
D.U. Lee, B.J. Kim, Z. Chen, J. Mater. Chem. A 1 (2013) 4754-4762. DOI:10.1039/c3ta01402a |
| [27] |
T. Reier, M. Oezaslan, P. Strasser, ACS Catal. 2 (2012) 1765-1772. DOI:10.1021/cs3003098 |
| [28] |
H.A. Gasteiger, S.S. Kocha, B. Sompalli, F.T. Wagner, Appl. Catal. B 56 (2005) 9-35. DOI:10.1016/j.apcatb.2004.06.021 |
| [29] |
N.T. Suen, S.F. Hung, Q. Quan, et al., Chem. Soc. Rev. 46 (2017) 337-365. DOI:10.1039/C6CS00328A |
| [30] |
A. Li, Y. Sun, T. Yao, H. Han, Chem.-Eur. J. 24 (2018) 18334-18355. DOI:10.1002/chem.v24.69 |
| [31] |
Y. Peng, B. Lu, S. Chen, Adv. Mater. 30 (2018) 1801995. DOI:10.1002/adma.v30.48 |
| [32] |
S.K. Singh, K. Takeyasu, J. Nakamura, Adv. Mater. (2018) 1804297. DOI:10.1002/adma.201804297 |
| [33] |
S. Wang, Z. Teng, C. Wang, G. Wang, ChemSusChem 11 (2018) 2267-2295. DOI:10.1002/cssc.v11.14 |
| [34] |
G.R. Cai, W. Zhang, L. Jiao, S.H. Yu, H.L. Jiang, Chem 2 (2017) 791-802. DOI:10.1016/j.chempr.2017.04.016 |
| [35] |
Y.L. Zhu, W. Zhou, Z.P. Shao, Small 13 (2017) 1603793. DOI:10.1002/smll.v13.12 |
| [36] |
Y. Jiao, Y. Zheng, M. Jaroniec, S.Z. Qiao, Chem. Soc. Rev. 44 (2015) 2060-2086. DOI:10.1039/C4CS00470A |
| [37] |
C. Tang, H.F. Wang, Q. Zhang, Acc. Chem. Res. 51 (2018) 881-889. DOI:10.1021/acs.accounts.7b00616 |
| [38] |
Y. Zheng, Y. Jiao, S.Z. Qiao, Adv. Mater. 27 (2015) 5372-5378. DOI:10.1002/adma.201500821 |
| [39] |
T. Asefa, Acc. Chem. Res. 49 (2016) 1873-1883. DOI:10.1021/acs.accounts.6b00317 |
| [40] |
Y.P. Zhu, C. Guo, Y. Zheng, S.Z. Qiao, Acc. Chem. Res. 50 (2017) 915-923. DOI:10.1021/acs.accounts.6b00635 |
| [41] |
Y. Zhao, K. Watanabe, K. Hashimoto, J. Am. Chem. Soc. 134 (2012) 19528-19531. DOI:10.1021/ja3085934 |
| [42] |
Y.P. Zhu, Y.P. Liu, T.Z. Ren, Z.Y. Yuan, Adv. Funct. Mater. 25 (2015) 7337-7347. DOI:10.1002/adfm.v25.47 |
| [43] |
J. Tian, Q. Liu, N. Cheng, A.M. Asiri, X. Sun, Angew. Chem. Int. Ed. 53 (2014) 9577-9581. DOI:10.1002/anie.201403842 |
| [44] |
Y. Li, H. Wang, L. Xie, et al., J. Am. Chem. Soc. 133 (2011) 7296-7299. DOI:10.1021/ja201269b |
| [45] |
B. Hinnemann, P.G. Moses, J. Bonde, et al., J. Am. Chem. Soc. 127 (2005) 5308-5309. DOI:10.1021/ja0504690 |
| [46] |
M.A. Lukowski, A.S. Daniel, F. Meng, et al., J. Am. Chem. Soc. 135 (2013) 10274-10277. DOI:10.1021/ja404523s |
| [47] |
C. Cheng, S.S.A. Shah, T. Najam, et al., J. Energy Chem. 26 (2017) 1245-1251. DOI:10.1016/j.jechem.2017.09.028 |
| [48] |
S.Q. Lu, Z.B. Zhuang, Sci. China Mater. 59 (2016) 217-238. DOI:10.1007/s40843-016-0127-9 |
| [49] |
L. Peng, J. Wang, Y. Nie, et al., ACS Catal. 7 (2017) 8184-8191. DOI:10.1021/acscatal.7b01971 |
| [50] |
D. Bao, Q. Zhang, F.L. Meng, et al., Adv. Mater. 29 (2017) 1604799. DOI:10.1002/adma.v29.3 |
| [51] |
X. Cui, C. Tang, Q. Zhang, Adv. Energy Mater. 8 (2018) 1800369. DOI:10.1002/aenm.v8.22 |
| [52] |
F. Li, L. Chen, G.P. Knowles, D.R. MacFarlane, J. Zhang, Angew. Chem. Int. Ed. 56 (2017) 505-509. DOI:10.1002/anie.201608279 |
| [53] |
Y. Jiao, Y. Zheng, P. Chen, M. Jaroniec, S.Z. Qiao, J. Am. Chem. Soc. 139 (2017) 18093-18100. DOI:10.1021/jacs.7b10817 |
| [54] |
X.H. Guo, Y.P. Zhu, T.Y. Ma, J. Energy Chem. 26 (2017) 1107-1116. DOI:10.1016/j.jechem.2017.09.012 |
| [55] |
M. Zhou, H.L. Wang, S. Guo, Chem. Soc. Rev. 45 (2016) 1273-1307. DOI:10.1039/C5CS00414D |
| [56] |
H.W. Liang, Z.Y. Wu, L.F. Chen, C. Li, S.H. Yu, Nano Energy 11 (2015) 366-376. DOI:10.1016/j.nanoen.2014.11.008 |
| [57] |
X. Wang, P. Sebastian, M.A. Smit, H. Yang, S. Gamboa, J. Power Sources 124 (2003) 278-284. DOI:10.1016/S0378-7753(03)00737-7 |
| [58] |
H.C. Yang, J. Liang, Z.X. Wang, B.G. An, F. Li, New Carbon Mater. 31 (2016) 243-263. |
| [59] |
Q. Xue, G.R. Xu, R.D. Mao, et al., J. Energy Chem. 26 (2017) 1153-1159. DOI:10.1016/j.jechem.2017.06.007 |
| [60] |
Y. Nie, L. Li, Z. Wei, Chem. Soc. Rev. 44 (2015) 2168-2201. DOI:10.1039/C4CS00484A |
| [61] |
J.S. Spendelow, A. Wieckowski, Phys.Chem. Chem. Phys. 9 (2007) 2654-2675. DOI:10.1039/b703315j |
| [62] |
P. Christensen, A. Hamnett, D. Linares-Moya, Phys. Chem. Chem. Phys. 13 (2011) 5206-5214. DOI:10.1039/c0cp02365e |
| [63] |
L. Jörissen, J. Power Sources 155 (2006) 23-32. DOI:10.1016/j.jpowsour.2005.07.038 |
| [64] |
P.S. Ruvinskiy, A. Bonnefont, C. Pham-Huu, E.R. Savinova, Langmuir 27 (2011) 9018-9027. DOI:10.1021/la2006343 |
| [65] |
A. Schneider, L. Colmenares, Y. Seidel, et al., Phys. Chem. Chem. Phys. 10 (2008) 1931-1943. DOI:10.1039/b719775f |
| [66] |
D. Strmcnik, K. Kodama, D. Van der Vliet, et al., Nat. Chem. 1 (2009) 466. DOI:10.1038/nchem.330 |
| [67] |
T. Zhang, A.B. Anderson, Electrochim. Acta 53 (2007) 982-989. DOI:10.1016/j.electacta.2007.08.014 |
| [68] |
A.B. Anderson, J. Roques, S. Mukerjee, et al., J. Phys. Chem. B 109 (2005) 1198-1203. DOI:10.1021/jp047468z |
| [69] |
T.V. Albu, A.B. Anderson, Electrochim. Acta 46 (2001) 3001-3013. DOI:10.1016/S0013-4686(01)00515-1 |
| [70] |
A.B. Anderson, T.V. Albu, J. Electrochem. Soc. 147 (2000) 4229-4238. DOI:10.1149/1.1394046 |
| [71] |
A.B. Anderson, T.V. Albu, Electrochem. Commun. 1 (1999) 203-206. DOI:10.1016/S1388-2481(99)00039-9 |
| [72] |
Y. Sha, T.H. Yu, B.V. Merinov, P. Shirvanian, W.A. Goddard Ⅲ, J. Phys. Chem. Lett. 2 (2011) 572-576. DOI:10.1021/jz101753e |
| [73] |
J.K. Nørskov, J. Rossmeisl, A. Logadottir, et al., J. Phys. Chem. B 108 (2004) 17886-17892. DOI:10.1021/jp047349j |
| [74] |
V. Tripković, E. Skúlason, S. Siahrostami, J.K. Nørskov, J. Rossmeisl, Electrochim. Acta 55 (2010) 7975-7981. DOI:10.1016/j.electacta.2010.02.056 |
| [75] |
V. Stamenkovic, B.S. Mun, K.J. Mayrhofer, et al., Angew. Chem. Int. Ed. 45 (2006) 2897-2901. |
| [76] |
J. Wang, Z.X. Wu, L.L. Han, et al., Chin. Chem. Lett. 27 (2016) 597-601. DOI:10.1016/j.cclet.2016.03.011 |
| [77] |
B.B. Huang, L. Peng, F.F. Yang, Y.C. Liu, Z.L. Xie, J. Energy Chem. 26 (2017) 712-718. DOI:10.1016/j.jechem.2017.03.016 |
| [78] |
L. Zhong, C. Tang, B. Wang, et al., New Carbon Mater. 32 (2017) 509-516. DOI:10.1016/S1872-5805(17)60136-7 |
| [79] |
M. Li, Z.W. Liu, F. Wang, J.J. Xuan, J. Energy Chem. 26 (2017) 422-427. DOI:10.1016/j.jechem.2017.01.004 |
| [80] |
J.J. Xu, C.H. Xiao, S.J. Ding, Chin. Chem. Lett. 28 (2017) 748-754. DOI:10.1016/j.cclet.2016.12.006 |
| [81] |
D. Wang, Z.Y. Wang, Q.Q. Zhan, L.M. Dai, et al., Engineering 3 (2017) 402-408. DOI:10.1016/J.ENG.2017.03.014 |
| [82] |
L.X. Li, H.W. Zhao, T.Y. Xing, et al., New Carbon Mater. 32 (2017) 419-426. |
| [83] |
K. Gong, F. Du, Z. Xia, M. Durstock, L. Dai, Science 323 (2009) 760-764. DOI:10.1126/science.1168049 |
| [84] |
G.L. Tian, Q. Zhang, B. Zhang, et al., Adv. Funct. Mater. 24 (2014) 5956-5961. DOI:10.1002/adfm.201401264 |
| [85] |
H.B. Yang, J. Miao, S.F. Hung, et al., Sci. Adv. 2 (2016) 1501122. DOI:10.1126/sciadv.1501122 |
| [86] |
C.H. Choi, S.H. Park, S.I. Woo, ACS Nano 6 (2012) 7084-7091. DOI:10.1021/nn3021234 |
| [87] |
C. Tang, H.F. Wang, X. Chen, et al., Adv. Mater. 28 (2016) 6845-6851. DOI:10.1002/adma.201601406 |
| [88] |
Y. Jiang, L. Yang, T. Sun, et al., ACS Catal. 5 (2015) 6707-6712. DOI:10.1021/acscatal.5b01835 |
| [89] |
L. Tao, Q. Wang, S. Dou, et al., Chem. Commun. 52 (2016) 2764-2767. DOI:10.1039/C5CC09173J |
| [90] |
Y. Jia, L. Zhang, A. Du, et al., Adv. Mater. 28 (2016) 9532-9538. DOI:10.1002/adma.201602912 |
| [91] |
X. Yan, Y. Jia, X. Yao, Chem. Soc. Rev. 47 (2018) 7628-7658. DOI:10.1039/C7CS00690J |
| [92] |
C. Tang, Q. Zhang, Adv. Mater. 29 (2017) 1604103. DOI:10.1002/adma.201604103 |
| [93] |
Y. Liang, H. Wang, J. Zhou, et al., J. Am. Chem. Soc. 134 (2012) 3517-3523. DOI:10.1021/ja210924t |
| [94] |
H. Wu, T. Peng, Z.K. Kou, et al., J. Energy Chem. 26 (2017) 1160-1167. DOI:10.1016/j.jechem.2017.08.012 |
| [95] |
H. Wang, Y. Liang, Y. Li, H. Dai, Angew. Chem. Int. Ed. 50 (2011) 10969-10972. DOI:10.1002/anie.v50.46 |
| [96] |
N. Alonso-Vante, H. Tributsch, O. Solorza-Feria, Electrochim. Acta 40 (1995) 567-576. DOI:10.1016/0013-4686(94)00377-D |
| [97] |
M.R. Gao, J. Jiang, S.H. Yu, Small 8 (2012) 13-27. DOI:10.1002/smll.201101573 |
| [98] |
H. Yuan, L. Kong, T. Li, Q. Zhang, Chin. Chem. Lett. 28 (2017) 2180-2194. DOI:10.1016/j.cclet.2017.11.038 |
| [99] |
H. Yin, C. Zhang, F. Liu, Y. Hou, Adv. Funct. Mater. 24 (2014) 2930-2937. DOI:10.1002/adfm.v24.20 |
| [100] |
C.W. Tsai, M.H. Tu, C.J. Chen, et al., RSC Adv. 1 (2011) 1349-1357. DOI:10.1039/c1ra00373a |
| [101] |
K. Chen, X. Huang, C. Wan, H. Liu, Chem. Commun. 51 (2015) 7891-7894. DOI:10.1039/C5CC02028J |
| [102] |
V.V. Doan-Nguyen, S. Zhang, E.B. Trigg, et al., ACS Nano 9 (2015) 8108-8115. DOI:10.1021/acsnano.5b02191 |
| [103] |
H. Yang, Y. Zhang, F. Hu, Q. Wang, Nano Lett. 15 (2015) 7616-7620. DOI:10.1021/acs.nanolett.5b03446 |
| [104] |
V.M. Bau, X.J. Bo, L.P. Guo, J. Energy Chem. 26 (2017) 63-71. DOI:10.1016/j.jechem.2016.07.005 |
| [105] |
S. Li, B. Li, L. Ma, J. Yang, H.X. Xu, Chin. Chem. Lett. 28 (2017) 2159-2163. DOI:10.1016/j.cclet.2017.08.029 |
| [106] |
Y.F. Ye, F. Cai, C.C. Yan, et al., J. Energy Chem. 26 (2017) 1174-1180. DOI:10.1016/j.jechem.2017.06.013 |
| [107] |
J.N. Guo, M.Y. Ning, Z.H. Xiang, J. Energy Chem. 26 (2017) 1168-1173. DOI:10.1016/j.jechem.2017.09.004 |
| [108] |
J. Wang, F. Ciucci, Small 13 (2017) 1604103. DOI:10.1002/smll.v13.16 |
| [109] |
H.F. Wang, C. Tang, B.Q. Li, Q. Zhang, Inorg. Chem. Front. 5 (2018) 521-534. |
| [110] |
B. Xiong, L. Chen, J. Shi, ACS Catal. 8 (2018) 3688-3707. DOI:10.1021/acscatal.7b04286 |
| [111] |
H.F. Wang, C. Tang, B. Wang, B.Q. Li, Q. Zhang, Adv. Mater. 29 (2017) 1702327. DOI:10.1002/adma.201702327 |
| [112] |
H.F. Wang, C. Tang, X. Zhu, Q. Zhang, J. Mater. Chem. A 4 (2016) 3379-3385. DOI:10.1039/C5TA09327A |
| [113] |
J. Su, R. Ge, Y. Dong, F. Hao, L. Chen, J. Mater. Chem. A 6 (2018) 14025-14042. DOI:10.1039/C8TA04064H |
| [114] |
P. Yin, T. Yao, Y. Wu, et al., Angew. Chem. Int. Ed. 55 (2016) 10800-10805. DOI:10.1002/anie.201604802 |
| [115] |
C. Tang, B. Wang, H.F. Wang, Q. Zhang, Adv. Mater. 29 (2017) 1703185. DOI:10.1002/adma.v29.37 |
| [116] |
H.F. Wang, R. Chen, J. Feng, et al., ChemElectroChem 5 (2018) 1786-1804. DOI:10.1002/celc.v5.14 |
| [117] |
Y. Wang, W. Huang, C. Si, et al., Nano Res. 9 (2016) 3781-3794. DOI:10.1007/s12274-016-1248-x |
| [118] |
H.F. Wang, C. Tang, B. Wang, et al., Energy Storage Mater. 15 (2018) 124-130. |
| [119] |
D.J. Yang, L.J. Zhang, X.C. Yan, X.D. Yao, Small Methods 1 (2017) 1770123. |
| [120] |
Y.C. Tu, D.H. Deng, X.H. Bao, J. Energy Chem. 25 (2016) 957-966. DOI:10.1016/j.jechem.2016.10.012 |
| [121] |
W. Chen, Y.F. Gong, J.H. Liu, Chin. Chem. Lett. 28 (2017) 709-718. DOI:10.1016/j.cclet.2016.10.023 |
| [122] |
H.H. Wu, X.L. Jiang, Y.F. Ye, et al., J. Energy Chem. 26 (2017) 1181-1186. DOI:10.1016/j.jechem.2017.09.022 |
| [123] |
K. Dang, T. Wang, C.C. Li, et al., Engineering 3 (2017) 285-289. DOI:10.1016/J.ENG.2017.03.005 |
| [124] |
X. Wang, A. Vasileff, Y. Jiao, Y. Zheng, S.Z. Qiao, Adv. Mater. 30 (2018) 1803625. |
| [125] |
L. Zhou, M.F. Shao, M. Wei, X. Duan, J. Energy Chem. 26 (2017) 1094-1106. DOI:10.1016/j.jechem.2017.09.015 |
| [126] |
Z.F. Chen, H.B. Zhao, J.J. Zhang, J.Q. Xu, Sci. China Mater. 60 (2017) 119-130. DOI:10.1007/s40843-016-5134-5 |
| [127] |
Z.Q. Zhou, N. Mahmood, Y.C. Zhang, et al., J. Energy Chem. 26 (2017) 1223-1230. DOI:10.1016/j.jechem.2017.07.021 |
| [128] |
D.Y. Guo, F.F. Chen, W. Zhang, R. Cao, Sci. Bull. 62 (2017) 626-632. DOI:10.1016/j.scib.2017.03.027 |
| [129] |
L. Yao, H.X. Zhong, C.W. Deng, X.F. Li, H.M. Zhang, J. Energy Chem. 25 (2016) 153-157. DOI:10.1016/j.jechem.2015.11.013 |
| [130] |
W. Zhang, Y.Z. Wu, J. Qi, M.X. Chen, R. Cao, Adv. Energy Mater. 7 (2017) 1602547. DOI:10.1002/aenm.201602547 |
| [131] |
B.Q. Li, C. Tang, H.F. Wang, X.L. Zhu, Q. Zhang, Sci. Adv. 2 (2016) e1600495. DOI:10.1126/sciadv.1600495 |
| [132] |
E. Pizzolato, S. Scaramuzza, F. Carraro, et al., J. Energy Chem. 25 (2016) 246-250. DOI:10.1016/j.jechem.2015.12.004 |
| [133] |
C. Tang, H.F. Wang, X.L. Zhu, B.Q. Li, Q. Zhang, Part. Part. Syst. Char. 33 (2016) 473-486. DOI:10.1002/ppsc.v33.8 |
| [134] |
S. Wan, J. Qi, W. Zhang, et al., Adv. Mater. 29 (2017) 1700286. DOI:10.1002/adma.v29.28 |
| [135] |
S.J. Deng, S.H. Shen, Y. Zhong, et al., J. Energy Chem. 26 (2017) 1203-1209. DOI:10.1016/j.jechem.2017.10.015 |
| [136] |
X.L. Zhao, W. Zhang, R. Cao, J. Energy Chem. 26 (2017) 1210-1216. DOI:10.1016/j.jechem.2017.08.014 |
| [137] |
Q.L. Zhu, Q. Xu, Chem 1 (2016) 220-245. DOI:10.1016/j.chempr.2016.07.005 |
| [138] |
N. Cheng, Q. Liu, A.M. Asiri, W. Xing, X. Sun, J. Mater. Chem. A 3 (2015) 23207-23212. DOI:10.1039/C5TA06788J |
| [139] |
C. Tang, H.F. Wang, H.S. Wang, F. Wei, Q. Zhang, J. Mater. Chem. A 4 (2016) 3210-3216. DOI:10.1039/C6TA00328A |
| [140] |
B.Q. Li, Z.J. Xia, B. Zhang, et al., Nat. Commun. 8 (2017) 934. DOI:10.1038/s41467-017-01053-x |
| [141] |
P. Cai, J. Huang, J. Chen, Z. Wen, Angew. Chem. Int. Ed. 56 (2017) 4858-4861. DOI:10.1002/anie.201701280 |
| [142] |
J. Duan, S. Chen, A. Vasileff, S.Z. Qiao, ACS Nano 10 (2016) 8738-8745. DOI:10.1021/acsnano.6b04252 |
| [143] |
B.Q. Li, S.Y. Zhang, C. Tang, X. Cui, Q. Zhang, Small 13 (2017) 1700610. DOI:10.1002/smll.v13.25 |
| [144] |
M. Gong, H. Dai, Nano Res. 8 (2015) 23-39. DOI:10.1007/s12274-014-0591-z |
| [145] |
Z. Lu, W. Xu, W. Zhu, et al., Chem. Commun. 50 (2014) 6479-6482. DOI:10.1039/C4CC01625D |
| [146] |
M.W. Louie, A.T. Bell, J. Am. Chem. Soc. 135 (2013) 12329-12337. DOI:10.1021/ja405351s |
| [147] |
D. Friebel, M.W. Louie, M. Bajdich, et al., J. Am. Chem. Soc. 137 (2015) 1305-1313. DOI:10.1021/ja511559d |
| [148] |
J. Suntivich, K.J. May, H.A. Gasteiger, J.B. Goodenough, Y. Shao-Horn, Science 334 (2011) 1383-1385. DOI:10.1126/science.1212858 |
| [149] |
O. Mabayoje, A. Shoola, B.R. Wygant, C.B. Mullins, ACS Energy Lett. 1 (2016) 195-201. DOI:10.1021/acsenergylett.6b00084 |
| [150] |
B. Konkena, J. Masa, A.J. Botz, et al., ACS Catal. 7 (2016) 229-237. |
| [151] |
L.A. Stern, L. Feng, F. Song, X. Hu, Energy Environ. Sci. 8 (2015) 2347-2351. DOI:10.1039/C5EE01155H |
| [152] |
T. Wang, G. Nam, Y. Jin, et al., Adv. Mater. 30 (2018) 1800757. DOI:10.1002/adma.201800757 |
| [153] |
X. Zhu, C. Tang, H.F. Wang, et al., J. Mater. Chem. A 3 (2015) 24540-24546. DOI:10.1039/C5TA08019C |
| [154] |
J.T. Ren, Z.P. Hu, C. Chen, Y.P. Liu, Z.Y. Yuan, J. Energy Chem. 26 (2017) 1196-1202. DOI:10.1016/j.jechem.2017.07.016 |
| [155] |
C. Tang, H.S. Wang, H.F. Wang, et al., Adv. Mater. 27 (2015) 4516-4522. DOI:10.1002/adma.v27.30 |
| [156] |
X. Li, P. Cui, W. Zhong, et al., Chem. Commun. 52 (2016) 13233-13236. DOI:10.1039/C6CC07049C |
| [157] |
S. Dou, C.L. Dong, Z. Hu, et al., Adv. Funct. Mater. 27 (2017) 1702546. DOI:10.1002/adfm.v27.36 |
| [158] |
C. Tang, L. Zhong, B. Zhang, H.F. Wang, Q. Zhang, Adv. Mater. 30 (2018) 1705110. DOI:10.1002/adma.201705110 |
| [159] |
B. Wang, C. Tang, H.F. Wang, et al., Small Methods 22222222 (2018) 1800055. |
| [160] |
B.Q. Li, S.Y. Zhang, B. Wang, et al., Energy Environ. Sci. 11 (2018) 1723-1729. DOI:10.1039/C8EE00977E |
 2018, Vol. 29
2018, Vol. 29 

