b State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China
Uncontrollable delivery method causes the low utilization rates of pesticide, which in turn led to many adverse effects such as increased dosage, high toxicity and environmental pollution [1]. Therefore, new technologies are needed for controlled release of pesticide molecules. Controlled release system can enhance bioavailability, prolong length of activity and reduce the usage amount of pesticides [1]. Microencapsulation and nanotechnology are previously developed for slow-release of pesticides [2, 3]. Recently, the photo-triggered technology provides an alternative strategy for better controlled release in which the release process can be remotely, temporally and spatially regulated [4].
The light-dependent release technology takes advantage of a kind of molecules called photocage that can undergo cleavage at the presence of certain wavelength of light [4, 5]. This technology has broad applications in controlled release of pharmaceuticals as well as agrichemicals [4-6]. This process is triggered by light which can provide invasive, clean and spatiotemporal control over the release. A photocaged release system is composed of a photocage and a bioactive fragment [4, 5]. The well-studied photocages include o-nitrobenzyl, coumarin-4-ylmethyl and p-hydroxylphenacyl. Coumarin (Cou) derivatives are the mostly-used photocages because of their easy preparation, adjustable irradiation wavelength, fast release rates and low intrinsic toxicity [6].
Spirotetramat is a keto-enol insecticide with excellent efficacy against sucking insects [7]. It acts on insect acetyl-CoA carboxylase that can interfere with lipid biosynthesis [8]. Spirotetramat has good systematic properties with both phloem and xylem mobility [7]. After administration on plant, spirotetramat can easily be degraded to its active enol form named as spirotetramat-enol (SE) [9-11]. Photorelease of agrochemicals mainly focuses on the herbicides [12], sex pheromone [13] and plant growth regulators [14], such as 2, 4-D [15, 16], salicylic acid [17] and trehalose-6- phosphate [18]. Recently, we reported coumarin-caged fipronil that enabled success release of insecticide fipronil both in vivo and in vitro [19]. Inspired by above descriptions, we herein described a photorelease system for conditionally releasing active spirotetramat-enol by linking SE with coumarin photocage.
Spirotetramat is a proinsecticide. It can be hydrolyzed in plant tissues to insecticidally active enol metabolite SE [10]. SE has the similar mode of action with that of spirotetramat that can inhibit acetyl-CoA carboxylases partially purified from Myzus persicae, Spodoptera frugiperda and Tetranychus urticae [8]. Most importantly, SE has good physiochemical properties that permitting its movement both upwards and downwards [10]. Therefore, we can envision that designing a SE releasing system is feasible to maintain high activity and excellent systematic properties of spirotetramat. Taking absorption wavelength into account, 7-diethylamino substituted coumarin was used here as a phototrigger as it has the biologically benign absorption region. The free hydroxyl group in SE also provide a position for attachment of phototriggered protection group.
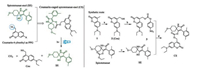
The synthetic route for coumarin caged SE (denoted as CS) was depicted in Fig. 1. Instruments, detailed procedure and analysis data can be found in Supporting information. Coumarin derivative 3 was prepared according to previously-reported procedure [19]. SE was synthesized by hydrolysis of spirotetramat. Finally, coupling of intermediate 3 with SE catalyzed by N, N-Diisopropylethylamine (DIPEA) afforded the caged product CS.
|
Download:
|
| Fig. 1. Molecular design of photoresponsive coumarin-caged spirotetramat-enol and its synthetic route. Reagents and condition: (a) 1. SeO2, Ar, p-xylene, reflux, 53 h; 2. NaBH4, CH3OH, r.t., 4 h, 26%. (b) p-nitrophenyl chloroformate, DIPEA, Ar, dry dichloromethane, r.t., 20 h, 50%. (c) NaOH, methanol, H2O, r.t., 47%. (d) DIPEA, Ar, dry DMF (N, N-dimethylformamide), r.t., 48 h, 32%. | |
With coumarin-triggered SE in hand, we firstly investigated its photophysical properties. The maximum absorption and emission wavelength of CS are 395 nm and 485 nm, respectively (Table S1 in Supporting information). The Stokes shift is 90 nm and fluorescence quantum yield is around 0.11. CS has two obvious absorption bands centered at 395 nm and 250 nm which corresponded to the absorption of coumarin and SE, respectively (Fig. 2).

|
Download:
|
| Fig. 2. (A) UV–vis absorption of CS at regular intervals of irradiation in MeOH/H2O (50/50) (5.6 ×10-5 mol/L). (B) UV–vis absorption of SE. (C) UV–vis absorption of Cou. | |
To investigate the phototriggered SE release ability, the solution of CS was irradiated under blue light (420 nm, LED) and the UVvisible spectra changes were recorded at time intervals. Obvious spectra changes were observed, with the increase of absorption at 250 nm corresponding to SE. The intensity of absorption at 395 nm also increased along with the wavelength hypochromatically shifting to 388 nm. The above observations indicate that photolysis occurred when CS was subjected to irradiation.
To further identify the occurrence of photocleavage, the photorelease process was monitored by ultra performance liquid chromatography (UPLC) (Fig. S1 in Supporting information). The sample was analyzed every 10 min of irradiation. The overlay chromatogram indicated gradual decrease of peak at tR = 7.10 min which is corresponding to caged compound CS, suggesting the photolysis of CS. Appearance of two new peaks at tR = 4.60 min and 5.95 min related to cleaved product SE and coumarin derivative 2, respectively. Trim amount of photolysis byproducts also generated upon irradiation whose chemical structures were not identified here. Same trend were also observed when CS was exposed to sunlight (Fig. S1).
The time-dependent release of SE upon blue light irradiation was also studied by plotting UPLC peak area of SE versus differentirradiation times (Fig. 3). The photorelease approaches saturation at about 20 min and 100% SE was regenerated. By switching the light source on and off, the release process can be initiated or stopped, indicating that release of SE can only be induced by light.

|
Download:
|
| Fig. 3. Photo-controlled release of SE irradiated by blue light. (A) Time-dependent release of SE upon blue light (420 nm) irradiation. (B) The release process can be turned on and off by switching the light on and off. | |
After successful identification of photorelease of SE, we then studied its insecticidal effects against cowpea aphids (Aphis craccivora) (Table 1). The bioassay against cowpea aphids was carried out according to our previously reported procedure [20, 21]. All compounds were dissolved in dimethyl sulfoxide (DMSO) as stock solutions. Various concentrations of test compounds were prepared by serial dilution of stock solutions with distilled water. The plant leaves of horsebean with about 50 apterous adults were dipped in corresponding chemical solutions containing Triton X-100 (0.1 mg/L) for 5 s and the excess solutions were sucked out with filter paper. Then the burgeons were positioned in the conditioned room ((25 ±1) ℃). Water with Triton X-100 (0.1 mg/L) was used as control. Three sets of experiments were performed for each compound. The first group was held in the dark throughout the trials. The second group was irradiated for 1 h with a LED lamp (420 nm), and then returned to darkness for 24 h incubation. The third group was irradiated by sunlight (September, Shanghai) for 1 h, and also returned to darkness for 24 h incubation. Controls were treated similarly. The mortality rates were calculated 24 h after treatment. Each treatment had three repetitions and the data were subjected to probit analysis.
|
|
Table 1 Insecticidal activity of CS, SE, Cou against cowpea aphids (Aphis craccivora). |
CS alone in the dark has low insecticidal activity with LC50 value more than 1 mmol/L. Upon irradiation, the activity increased significantly with LC50 approaching 0.11 mmol/L. No significant activity difference was observed for SE before and after irradiation. SE has higher activity than CS plus blue light. Light alone do not have any obvious activity to aphids. The above results indicated that efficient light-triggered release of SE was achieved using coumarin as a cage.
In summary, a photo-triggered release system was synthesized by covalently linking of coumarin photocage with spirotetramatenol. The system can efficiently release insecticidal SE upon irradiation of sunlight or blue light. The compound has the lighttriggered insecticidal activity again cowpea aphid. This provides a new strategy for controlled release of SE, which would enable precise and spatiotemporal control over insecticidal activity.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21472046, 21372079), Science and Technology Commission of Shanghai Municipality (No. 16391902300) and the Fundamental Research Funds for the Central Universities (No. 222201718004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.01.025.
| [1] |
M. Kah, T. Hofmann, Environ. Int. 63 (2014) 224-235. DOI:10.1016/j.envint.2013.11.015 |
| [2] |
D. Yang, G. Li, X. Yan, H. Yuan, J. Agric. Food Chem. 62 (2014) 10632-10637. DOI:10.1021/jf502537x |
| [3] |
D. Wibowo, C. Zhao, B.C. Peters, A.P.J. Middelberg, J. Agric. Food Chem. 62 (2014) 12504-12511. DOI:10.1021/jf504455x |
| [4] |
P. Klan, T. Šolomek, C.G. Bochet, et al., Chem. Rev. 113 (2013) 119-191. DOI:10.1021/cr300177k |
| [5] |
C. Brieke, F. Rohrbach, A. Gottschalk, et al., Angew. Chem. Int. Ed. 51 (2012) 8446-8476. DOI:10.1002/anie.201202134 |
| [6] |
C. Bao, L. Zhu, Q. Lin, H. Tian, Adv. Mater. 27 (2015) 1647-1662. DOI:10.1002/adma.201403783 |
| [7] |
P. Lümmen, J. Khajehali, K. Luther, et al., Insect Biochem. Mol. Biol. 55 (2014) 1-8. DOI:10.1016/j.ibmb.2014.09.010 |
| [8] |
T. Peng, Y. Pan, C. Yang, et al., Pestic. Biochem. Physiol. 126 (2016) 64-69. DOI:10.1016/j.pestbp.2015.07.008 |
| [9] |
X. Chen, Z. Meng, Y. Zhang, et al., Environ. Sci. Pollut. Res. 23 (2016) 1-10. DOI:10.1007/s11356-015-5714-x |
| [10] |
J. Cheng, X. He, Z. Wang, et al., Pest Manag. Sci. 69 (2013) 1121-1130. DOI:10.1002/ps.2013.69.issue-10 |
| [11] |
P. Jeschke, Pest Manag. Sci. 72 (2016) 210-225. DOI:10.1002/ps.2016.72.issue-2 |
| [12] |
P. Stloukal, P. Kucharczyk, V. Sedlarik, et al., J. Agric. Food Chem. 60 (2012) 4111-4119. DOI:10.1021/jf300521j |
| [13] |
S. Atta, M. Ikbal, N. Boda, et al., Photoch. Photobio. Sci. 12 (2013) 393-403. DOI:10.1039/C2PP25118C |
| [14] |
S. Atta, M. Ikbal, A. Kumar, et al., J. Photoch. Photobio. B 111 (2012) 39-49. DOI:10.1016/j.jphotobiol.2012.03.008 |
| [15] |
S. Atta, M. Bera, T. Chattopadhyay, et al., RSC Adv. 5 (2015) 86990-86996. DOI:10.1039/C5RA17121K |
| [16] |
S. Atta, A. Paul, R. Banerjee, et al., RSC Adv. 5 (2015) 99968-99975. DOI:10.1039/C5RA18944F |
| [17] |
S. Barman, S.K. Mukhopadhyay, K.K. Behara, et al., ACS Appl. Mater. Inter. 6 (2014) 7045-7054. DOI:10.1021/am500965n |
| [18] |
C.A. Griffiths, R. Sagar, Y. Geng, et al., Nature 540 (2016) 574-592. DOI:10.1038/nature20591 |
| [19] |
Z. Gao, P. Yuan, D. Wang, et al., Bioorg. Med. Chem. Lett. 27 (2017) 2528-2535. DOI:10.1016/j.bmcl.2017.03.091 |
| [20] |
Y. Zhang, Y. Feng, Z. Li, et al., Chin. Chem. Lett. 28 (2017) 1228-1231. DOI:10.1016/j.cclet.2017.04.003 |
| [21] |
Q. Hou, Y. Jing, X. Shao, Chin. Chem. Lett. 28 (2017) 1723-1726. DOI:10.1016/j.cclet.2017.05.016 |
 2018, Vol. 29
2018, Vol. 29