b Beijing Center Physical and Chemical Analysis, Beijing 100089, China
Over the past decade, the development of miniature mass spectrometers [1, 2] has enabled the application of conventional mass spectrometry (MS) techniques from laboratory into different on-field analysis areas, ranging from space exploration to personal usage [3-9]. Up to now, nearly all kinds of miniaturized mass analyzers have been tested with quite small sizes even at micrometer level [10-12]. Among the present mass analyzers, ion trap is one of the most suitable candidates for a miniature mass spectrometer [13-19], since it is compact-in-size and could work at higher pressures. The miniaturization of mass analyzer as an individual component, ion trap for example, could help in terms of lowering voltage requirement. Nevertheless, the miniaturization of mass spectrometers is a complex systematic procedure, involving the miniaturized design of many components, including mass analyzer, ion optics, electronic and vacuum system [1, 2]. As one of the most critical issues during MS miniaturization, the requirement of a high vacuum environment is needed for high resolution, but is contradictory to high sensitivity (typically realized by high gas flow rate) and low-power-consumption. Therefore, the design of vacuum and atmospheric pressure interface (API) is one of the key challenges, which largely determine the size and power consumption of a MS system, as well as its analytical performances. Generally speaking, there have been three types of APIs developed for miniature mass spectrometers, namely membrane inlet (MI) [20, 21], discontinuous atmospheric pressure interface (DAPI) [22-24] and continuous atmospheric pressure interface (CAPI) [25]. Using different APIs, many miniature instruments have been developed in the recent ten years by either academic laboratories or commercial companies.
The recent development of miniature ion trap mass spectrometer systems in the last ten years will be reviewed in this paper. These instruments will be categorized into three groups based on different APIs, which are MI, DAPI and CAPI. We would also focus on personal handheld mini MS system, and not including MS systems that are small enough to be field-able but too large to be hand-portable.
2. Miniature mass spectrometers with membrane inlets (MIs)MIs use gas semi-permeable membrane to enable the entry of selected gas molecules into the mass spectrometer. This interface is a simple approach for sample-introduction in MS and has been widely used in durable analysis of volatile gases, particularly atmospheric gases [26-28]. Capable of limiting gas conductance through a membrane inherently, a MI is helpful to maintain a high vacuum environment of the mass spectrometer even by coupling with small vacuum pumps, which simplify the miniaturization of mass spectrometers. Therefore, this interface has been the first API adopted for mini MS systems. Utilizing the semi-permeability of a membrane, the MI allows selectively specific gas molecules to pass through the membrane into vacuum chamber for MS analysis, while blocking the most of gas outside the vacuum [20, 21]. However, a MI has no ability to transfer generous ions from atmosphere into vacuum, and the mass spectrometer with this interface has to integrate an in-vacuum ionization source for the ionization of gas introduced inside vacuum chamber, for example electron ionization (EI) [29] and glow discharge ionization source (GDEI) [30]. In practice, miniature mass spectrometers using MIs can only analyze volatile or gaseous samples, which restricts the application broaden of miniature MS in some degrees. Even so, MIs have contributed largely to the miniaturization of mass spectrometers and also been incorporated in a handful of mini MS systems, including Mini 10 developed by Purdue University and some other commercialized ones from a variety of companies.
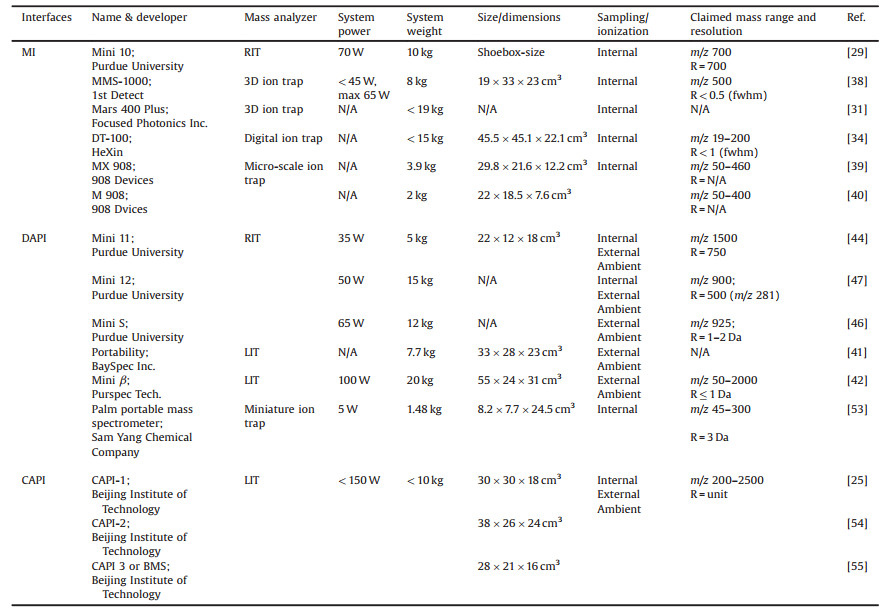
Mini 10 is a handheld mass spectrometer developed by Purdue University in 2006 [29]. This miniature instrument uses a MI as API (Fig. 1a), whose performance was also optimized and demonstrated in Mini 10 platform by Christian Janfelt [21]. This inlet enables the adoption of a miniature and ruggedized pumping system enough to meet the vacuum requirements. The packaged Mini 10, as shown in Fig. 1b, is as small as a shoebox in size, 10 kg in weight, and consumes less than 70 W running on batteries. A single-stage vacuum manifold is designed for Mini 10, where mass analyzer, ionization source and electron multiplier are located (shown in Fig. 1c). Considering its simplified geometry and high trapping capacity, a rectilinear ion trap (RIT) [15] is used as the mass analyzer in Mini 10, as a replacement of a cylindrical ion trap (CIT) used in an earlier instrument [30]. Either a filament electron ionization source or glow discharge electron ionization source was tested. Unit mass resolution at m/z 100 and a mass range over m/z 500 have been achieved by Mini 10 for analyses of gas and volatile samples. A limit of detection (LOD) of ~50 ppb was also achieved as shown in Fig. 1d.

|
Download:
|
| Fig. 1. (a) PDMS inlet used in Mini 10 mass spectrometer. Copied with permission [20]. Copyright 2002, American Chemical Society. (b) Photograph of Mini 10. (c) The components assembled in the vacuum manifold. (d) The calibration curve for volatile sample. Copied with permission [29]. Copyright 2006, American Chemical Society. | |
MMS 1000 (Fig. 2a) is a commercialized miniature mass spectrometer based on the MI, and has been developed by 1st Detect (Webster, United States) [32]. A heated PDMS membrane is used for the sample introduction and it can be heated up to 80 ℃. The overall dimensions and weight of the system are 19 × 33 × 23 cm3 and 8 kg, respectively, with no needs for external pumps or carrier gases. MMS 1000 is equipped with an EI source for gas analyses, and its mass range covers from 45 Da to 400 Da. LOD of 500 ppb can be achieved when using MI directly, while 0.5 ppb when using a pre-concentrator to accumulate for 30 s. Benefited from the capabilities of a CIT used as mass analyzer, MMS 1000 is able to perform tandem MS for the accurate identification of gas molecules using air as the collision buffer gas. This miniature mass spectrometer gives a unit resolution for mass analysis with a typical scan rate of 1 spectrum per second.

|
Download:
|
| Fig. 2. Alternative miniature mass spectrometers from commercial companies: (a) MMS 1000 by 1st Detect Inc. Reproduced with permission [38]. Copyright 2008, 1st Detect. (b) M 908 by 908 Devices Inc. Reproduced with permission [39, 40]). Copyright 2012, 908 Devices. (c) Mars 400 Plus by Focused Photonics Inc. Reproduced with permission [31]. Copyright 2010, Focused Photonics Inc. (d) DT 100 by Guangzhou HeXin Inc. Reproduced with permission [34]. Copyright 2012, Guangzhou HeXin Inc. (e) Portability by BaySpec Inc. Reproduced with permission [41]. Copyright 2009, BaySpec Inc. (f) Mini β by PURSPEC Technologies Inc. Reproduced with permission [42]. Copyright 2017, Purspec Tech. Among these instruments, a-d are based on MI, e and f are based on DAPI. | |
M 908 and MX 908 (Fig. 2b) are two types of handheld mass spectrometers developed by 908 Devices (Boston, United States), which are designed for near-trace vapors analyses in civilian, federal and military fields [33, 34]. The two instruments can rapid detect explosives, chemical warfare agents (CWA), toxic industrial chemicals or toxic industrial materials (TIC/TIM), simulant precursors and other emerging threats with sensitivity of lowmid ppm. Taking advantages of high-pressure mass spectrometry (HPMS) technique using a micro-scale ion trap [35], both devices can work without the need for high vacuum or additional gas supply, and are small enough to be held in hands for operation. Based on MIs, these two mass spectrometers introduce gas vapors continuously for direct analysis, as well as solids and liquids via thermal desorption swabs. Both systems are powered by rechargeable batteries and can be operated continuously for over 2 h of MX 908 and 4 h of M 908. The analysis results are displayed on a user interface with adjustable backlit display for direct sunlight and nighttime conditions. MX 908 has dimensions of 29.8 × 21.6 ×12.2 cm3 and a weight of 3.9 kg including batteries, and those of M 908 are 22 ×18.5 × 7.6 cm3 and 2 kg. In terms of mass range, 50-460 Da and 55-400 Da can be achieved respectively for MX 908 and M 908.
Mars 400 plus (Fig. 2c) is a portable GC-MS developed by Focused Photonics Inc. (Hangzhou, China) in 2012 [31]. Equipped with battery and carrier gas supply, this instrument is less than 19 kg in weight and can be carried by one person for on-field applications. Mars 400 plus incorporates a 3D ion trap and a patented internal ionization source [32]. By combining low thermal mass gas chromatograph [33], the mass spectrometer has the capability of analyzing complex samples with high specificity and high sensitivity, which make it suitable to detect volatile and semi-volatile organic compounds in air, water, soil and solid wastes.
Latest in 2017, Guangzhou Hexin Instrument Inc. (Guangzhou, China) released a miniature mass spectrometer, DT-100 (Fig. 2d) [34]. This mass spectrometer has been announced to be the first mini MS using a digital ion trap as the mass analyzer since its integration into the conventional lab-scale mass spectrometers. The linear ion trap (LIT) is driven by a digital square waveform developed by Ding and coworkers [35-37]. DT-100 does also use a PDMS MI for sample introduction and an ultraviolet (UV) [34] single photon ionization source in vacuum. The instrument has the capability of qualitative and quantitative analysis for over 300 VOCs, whose masses range from 19 Da to 652 Da, and offers a unit resolution of full width at half maxima (fwhm) < 1 Da. As a highly integrated miniature system, this mass spectrometer is < 15 kg in total weight with dimensions of 45.5 × 45.1 ×22.1 cm3, and it is powered by internal or external batteries.
3. Miniature mass spectrometers with DAPIEven MIs have significantly promoted the miniaturization of mass spectrometers, some of its inherent features have limited the general use of this interface. A membrane has strong chemical discrimination and its permeability can be easily affected by either its porosity or temperature. The miniature mass spectrometers with MIs is not able to couple ambient ionization sources for analyses of non-volatile samples in atmospheric pressure environment.
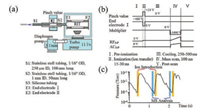
To overcome the drawbacks of MI based miniature mass spectrometers, a DAPI was developed by Purdue university in 2008 [22, 23]. DAPI breaks the pumping speed barrier in MS and enables the coupling of atmospheric pressure ionization source to a miniature mass spectrometer with limited pumping capacity. As shown in Fig. 3a, DAPI typically consists of two metal capillaries connected with a pinch valve, which directly separate the ambient pressure environment from vacuum region. The interface is coupled to a single stage vacuum chamber, without ion optical elements and differential pumping stages. The pinch valve is opened for a short time by an electronically controlled pulse voltage (~24 V), allowing ions generated in ambient environment to be introduced into the mass spectrometer for analysis. At the end of the pulse, the valve closes to let the pressure in mass analyzer region to drop back to a lower pressure and ion cooling and mass analysis are performed in succession. A typical electronic control signal function is shown in Fig. 3b, and vacuum pressure in the manifold during scanning is presented in Fig. 3c. To further improve the ion transmission efficiency of DAPI, another discontinuous introduction system, a pulsed pinhole atmospheric pressure interface (PP-API) has also been developed [43]. Different from the construction of DAPI, PP-API consists of a PEEK (polyetheretherketone) or Teflon ball affixed to the end of a telescopic rod, which is pushed into or pulled out of the aperture in vacuum manifold by a solenoid valve, either sealing the vacuum region or allowing for ion introduction, respectively.
|
Download:
|
| Fig. 3. (a) The schematic of a miniature mass spectrometer using a DAPI. (b) Scan function for mass analysis for DAPI interfaced mass spectrometers. (c) Manifold pressure during DAPI switching on and off, with an open time of 20 ms and a closed time of 850 ms. Copied with permission [24]. Copyright 2008, American Chemical Society. | |
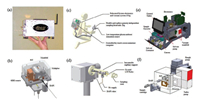
Since tested and demonstrated in Mini 10 platform, DAPI has been implemented into some mini MS systems, of which Mini 11 is the first integrated one at a system level (Fig. 4a) [44]. Similar to Mini 10, Mini 11 is also a single-stage vacuum chamber mass spectrometer, but with a DAPI instead of a MI for sampling. Optimized from Mini 10, the total weight and power consumption of Mini 11 are further lowered to 5 kg with batteries and < 35 W, respectively. The dimensions of Mini 11 are 22 ×12 ×18 cm3. This mini MS also uses a RIT as mass analyzer, and can be controlled by wireless. Benefited by the DAPI, Mini 11 is compatible with kinds of ambient ionization sources, including electrospray ionization, atmospheric pressure chemical ionization, and desorption electrospray ionization for different sample analyses [44]. Of course, Mini 11 is also designed to have capability of coupling internal ionization sources, such as glow discharge ionization source (GDEI) and synchronized discharge ionization (SDI) [44, 45].
|
Download:
|
| Fig. 4. Miniature mass spectrometers with DAPI. (a) Photograph and (b) 3D assembly of Mini 11. Copied with permission [44]. Copyright 2008, American Chemical Society. (c) Instrumental construction and (d) vacuum system of Mini 12. Copied with permission [47]. Copyright 2014, American Chemical Society. (e) Mini S and (f) the handheld LTP integrated with Mini S. Copied with permission [46]. Copyright 2014, American Chemical Society. | |
Following developments of the Mini 10 and Mini 11 platforms, another two miniature mass spectrometers have been developed, one is a wearable backpack system (Mini S) [46] and the other is a stand-alone single-unit instrument (Mini 12) [47, 48]. Both mass spectrometers have similar components, including mechanical and turbo pumps, DAPI, and RITs. Mini S (Fig. 4e) is an instrument fully contained in a wearable backpack (10 kg) integrated with a handheld LTP (low temperature plasma) ionization source (Fig. 4f) for direct surface sampling and analysis [46, 49, 50]. This mini system has a power consumption of 65 W in average, and can be operated autonomously for 1.5 h under battery power. The mass range is up to around 925 Da with a mass resolution of 1-2 Da (fwhm). Multiple stages of tandem MS can be performed to identify individual compounds in complex mixtures in both positive and negative ion modes. As a bench-top instrument developed at nearly the same time as Mini S, Mini 12 (Figs. 4c and d) is designed as a self-contained system to produce quantitative results for unprocessed samples including nonvolatile analytes. This instrument has an integrated sample loading system designed as a consumable cartridge, using paper spray ionization source [51]. It also has a user interface which can interpret analytical results onto a display screen. Mini 12 can perform tandem MS function and has a mass resolution of 0.6 Da (fwhm) and a mass range of up to m/z 900. The limit of quantitation (LOQ) of the system is around 7.5 ng/mL and relative stanfard deviations is below 10%.
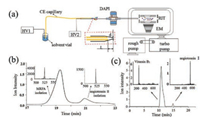
Based on DAPI and RIT, a miniature mass spectrometer coupling with a simplified capillary electrophoresis (CE) has been developed by Beijing Institute of Technology (Fig. 5) [52]. In this system, an electrical driven miniaturized CE device was coupled with a typical DAPI mini MS system by a sheath liquid interface. The interface consists of a glass capillary for nano-liter electro spray and a CE capillary inserted inside. The mini CE-MS device is able to separate singly charged MRFA and doubly charged angiotensin Ⅱ (Fig. 5b), which have the same nominal m/z of 524. In addition, the system was reported to have capability of avoiding charge competition effects in the nano-ESI source (Fig. 5c).
|
Download:
|
| Fig. 5. Miniature capillary electrophoresis MS (mini CE-MS). (a) Schematic structure of mini CE-MS. (b) CE chromatograph of MRFA and Angiotensin Ⅱ, with the same nominal m/z. (c) CE chromatograph of vitamin b1 and Angiotensin Ⅱ. Copied with permission [52]. Copyright 2015, American Chemical Society. | |
In terms of commercial instruments based on DAPI developed by Purdue University, there have been two systems appeared up to now. The first one is the PortabilityTM transportable mass spectrometer (Fig. 2e) designed by BaySpec Inc. (San Jose, U.S. A.) [41], and the second is Mini β (Fig. 2f) released by PURSPEC Technologies Inc. (Beijing, China) in 2017 [42]. Both instruments use LITs and can perform tandem mass spectrometry (MS/MS) for the ion structure identification. Portability offers ppb-level detection sensitivity and the whole system powered batteries is 7.7 kg in weight and around 33 × 28 × 23 cm3 in dimensions. Mini β has dimensions of 55 × 24 × 31 cm3, a weight of 20 kg and a power consumption of 100 W. LOD of 5 ng/mL can be achieved for Mini β, with a mass range of 50-2000 Da and resolution of better than unit (fwhm < 1 Da). The dynamic range of Mini β is more than 3 orders of magnitude, and its scan rate can be extended to 50, 000 Da/s.
In addition to the two commercialized miniature mass spectrometers mentioned above, one palm portable mass spectrometer (PPMS) was also developed by Sam Yang Chemical Company in 2008 [53]. This PPMS has a pulsed interface for sample introduction, which is similar to DAPI. Different from the other miniature mass spectrometers with typical combinations of a mechanical pump and a turbo pump, the PPMS uses an ion-getter pump to serve for high vacuum. A miniaturized ion trap is used as the mass analyzer in this system and LODs of 6 ppm for toluene and 22 ppm for dimethyl methylphosphonate (DMMP) have been achieved.
This device can be operated with average battery power of 5 W, and has a weight of 1.48 kg and a size of 1.54 L (8.2 × 7.7 × 24.5 cm3).
4. Miniature mass spectrometers with CAPICAPI has been widely used in conventional lab-scale mass spectrometers. It is robust, stable, and easy to couple with various atmosphere ionization sources. To highest the efficiency of ion transfer, CAPI is usually designed to have a relatively high gas flow rate and complicated ion guided lens, which need large pumps and complicated electronic system with high power consumption. Therefore, it is difficult to apply a CAPI into mini MS systems. There have not emerged miniature mass spectrometers with CAPI until 2015, when the first CAPI interfaced miniature mass spectrometer was developed by Wei Xu and coworkers in Beijing Institute of Technology [25]. In the first generation of mini MS with a CAPI, a differential pumping system with a two-stage vacuum manifold was designed. The first vacuum chamber was connected to atmosphere with a stainless steel capillary (0.25 mm in inner diameter, 20 cm in length), and to the second vacuum chamber via a pinhole. At the second vacuum chamber located a hyperbolic LIT and electron multiplier. A turbo pump (10 L/s, Hipace 10, Pfeiffer Inc., Germany) and a mechanical pump (50 L/min, SVF-E0-50, Scroll Tech Inc., China) were used to pump the vacuum chambers, whose final pressures are maintained at ~6 Torr and ~6 m Torr, respectively. The whole instrument is around 30 × 30 × 18 cm3 in dimensions (Fig. 6a), consumes less than 150 W and weighs less than 10 kg. Benefited by the CAPI, this mini MS gives excellent reproducibility and stability (RSD < 7%, Fig. 6c) while coupling with a variety of ambient ionization sources for volatile and nonvolatile sample analyses. Additionally, the CAPI improves the analysis speed of mini MS to a rate of 5 Hz. That is to say, the mini MS can analyze 5 samples within 1 s, accomplishing full mass scan followed by tandem MS. This first generation has low ppm limits of detection and unit mass resolution for mass analysis. Based on the first generation instrument with CAPI, a vacuum plasma ionization source has also been developed and integrated into this system, enhancing the detection sensitivity for gas or volatile samples to a LOD of ppb level [25].
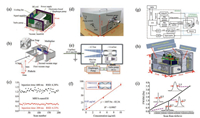
|
Download:
|
| Fig. 6. Miniature mass spectrometers with CAPI from the first generation to the third one. (a-c) The first generation. Copied with permission [25]. Copyright 2015, Royal Society of Chemistry. (d-f) The second generation integrated with a mini ion funnel. Copied with permission [54]. Copyright 2017, American Chemical Society. (g-i) The third generation using sinusoidal frequency scanning technique. Copied with permission [55]. Copyright 2017, American Chemical Society. | |
Demonstrated in the first generation mini MS, CAPI largely improves robustness, stability, and scan speed of the instrument system, but it also results in limited ion transfer efficiency. To solve the problem, a miniature ion funnel was designed and integrated into the system, appeared as the second generation of the mini MS with CPAI (Figs. 6d-f) [54]. In terms of instrumental structure, the capillary in this generation is pulled away from the pinhole skimmer to allow the placement of the ion funnel in the first vacuum stage, and the skimmer is also isolated from the vacuum chamber. Additionally, the infrastructure of the second generation instrument was redesigned to improve system portability, with a compact size of 38 × 26 × 24 cm3. Integration of the mini ion funnel increases ion transfer efficiency by more than 10 times, while the background pressure in the ion trap region was also lowered by ~2 times. Consequently, sensitivity and mass resolution of the second generation miniature mass spectrometer are improved by ~20 times and nearly 2 times, respectively.
The third generation of the CAPI based instrument is a brick-size miniature mass spectrometer (BMS) with smaller dimensions of 28 × 21 ×16 cm3 (Figs. 6g-i) [55]. Instead of the conventional voltage ramping method or digital square waveforms [35], this mass spectrometer employs sinusoidal frequency scanning waveform to drive the LIT. This new technique could not only reduce the size and power consumption of the system, but also improve its analytical performances, especially in terms of mass range and resolution. Similar to the two generations built earlier, this BMS can also couple with both internal ionization sources and various ambient ionization sources. The detection sensitivity was demonstrated using in-vacuum plasma ionization source with a LOD of 20 ppbv for volatile samples. The mass range of 100-2000 Da was achieved at normal scan mode, with a mass resolution of fwhm = 0.2 Da measured at m/z 128 (Fig. 6i).
5. ConclusionsAs important components in mass spectrometers, API and mass analyzer play indispensable roles for the miniaturization of mass spectrometers. Taking advantages of capabilities of ion traps, amounts of miniature ion trap mass spectrometers have been available based on different APIs (Table 1). Different types of APIs and ion traps lead these miniature systems to have variable features, including instrumental power, weight, dimensions as well as analytical performances. Relatively speaking, MI based mini-MS typically has superiorities in system size and power consumption, but limitations to the sensitive analysis for nonvolatile samples. These problems are resolved by miniature mass spectrometers with DAPI, which can couple with ambient ionization sources for different analytes. However, a DAPI system usually have low stability and analysis speed with a duty cycle of only 1%, determined by the pulsed sampling introduction using a mechanical valve. CAPI with a miniature differential pumping system enables the realization of continuous sampling introduction into miniature mass spectrometers. The CAPI based miniature mass spectrometers not only have adequate performance, but have been proved to possess high stability and rapid analysis speed for analysis of complex samples using ambient ionization sources. This type of miniature mass spectrometers has promising potential to become one powerful device for the in-situ analysis, with robustness, stability and high analysis speed.
|
|
Table 1 Overview of miniature ion trap mass spectrometers. |
In future, miniature mass spectrometers are expected to be much more portable with higher analytical performances. As one of the trends for MS, miniature mass spectrometers have shown strong superiorities in in-situ and online analysis applications. Therefore, we have evident reasons to believe in that miniature mass spectrometers will soon become a major widely used tool in the analytical fields, especially the areas with interests in point-of-care, online, and on-site measurements.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21475010, 61635003), Beijing Natural Science Foundation (No. 16L00065) and State Key Laboratory Explosion Science and Technology (No. YBKT16-17).
| [1] |
D.T. Snyder, C.J. Pulliam, Z. Ouyang, R.G. Cooks, Anal. Chem. 88 (2016) 2-29. DOI:10.1021/acs.analchem.5b03070 |
| [2] |
Z. Ouyang, R.G. Cooks, Ann. Rev. Anal. Chem. 2 (2009) 187-214. DOI:10.1146/annurev-anchem-060908-155229 |
| [3] |
P.A. Smith, C.J. Lepage, M. Lukacs, et al., Int. J. Mass Spectrom. 295 (2010) 113-118. DOI:10.1016/j.ijms.2010.03.001 |
| [4] |
U.V. Zahn, K.H. Fricke, D.M. Hunten, et al., J. Geophys. Res.-Space Phys. 85 (1980) 7829-7840. DOI:10.1029/JA085iA13p07829 |
| [5] |
H.B. Niemann, S.K. Atreya, S.J. Bauer, et al., Space Sci. Rev. 104 (2002) 553-591. DOI:10.1023/A:1023680305259 |
| [6] |
L. Li, T.C. Chen, Y. Ren, et al., Anal. Chem. 86 (2014) 2909-2916. DOI:10.1021/ac403766c |
| [7] |
R.G. Cooks, N.E. Manicke, A.L. Dill, et al., Faraday Disscuss. 149 (2011) 247-267. DOI:10.1039/C005327A |
| [8] |
G. Huang, L. Gao, J. Duncan, et al., J. Am. Soc. Mass Spectr. 21 (2010) 132-135. DOI:10.1016/j.jasms.2009.09.018 |
| [9] |
P.I. Hendricks, J.K. Dalgleish, J.T. Shelley, et al., Anal. Chem. 86 (2014) 2900. |
| [10] |
Y. Peng, D.E. Austin, Trac. Trend. Anal. Chem. 30 (2011) 1560-1567. DOI:10.1016/j.trac.2011.07.003 |
| [11] |
Z. Ouyang, R.J. Noll, R.G. Cooks, Anal. Chem. 81 (2009) 2421-2425. DOI:10.1021/ac900292w |
| [12] |
R.R.A. Syms, S. Wright, J. Micromech. Microeng. 26 (2016) 023001. DOI:10.1088/0960-1317/26/2/023001 |
| [13] |
Y. Tian, J. Higgs, A. Li, et al., J. Mass Spectrom. 49 (2014) 233-240. DOI:10.1002/jms.3343 |
| [14] |
G.C. StaffordJr, P.E. Kelley, J.E.P. Syka, et al., Int. J. Mass. Spectrom. 60 (1984) 85-98. DOI:10.1016/0168-1176(84)80077-4 |
| [15] |
Z. Ouyang, G. Wu, Y. Song, et al., Anal.Chem. 76 (2004) 4595-4605. DOI:10.1021/ac049420n |
| [16] |
N. Taylor, D.E. Austin, Int. J. Mass Spectrom. 321- 322 (2012) 25-32. |
| [17] |
D.E. Austin, M. Wang, S.E. Tolley, et al., Anal. Chem. 79 (2007) 2927. DOI:10.1021/ac062155g |
| [18] |
J.M. Wells, E.R. Badman, R.G. Cooks, Anal. Chem. 70 (1998) 438-444. DOI:10.1021/ac971198h |
| [19] |
Y. Tian, J. Higgs, A. Li, et al., J. Mass Spectrom. 49 (2014) 233-240. DOI:10.1002/jms.3343 |
| [20] |
L.S. Riter, Y. Peng, R.J. Noll, et al., Anal. Chem. 74 (2002) 6154-6162. DOI:10.1021/ac0204956 |
| [21] |
C. Janfelt, R. Graesboll, F.R. Lauritsen, Int. J. Mass Spectrom. 276 (2008) 17-23. DOI:10.1016/j.ijms.2008.06.019 |
| [22] |
W. Xu, N. Charipar, M.A. Kirleis, et al., Anal. Chem. 82 (2010) 6584-6592. DOI:10.1021/ac101002t |
| [23] |
L. Gao, R.G. Cooks, Z. Ouyang, Anal. Chem. 80 (2008) 4026-4032. DOI:10.1021/ac800014v |
| [24] |
L. Gao, R.G. Cooks, Z. Ouyang, Anal. Chem. 80 (2008) 4026-4032. DOI:10.1021/ac800014v |
| [25] |
Y. Zhai, Y. Feng, Y. Wei, et al., Analyst 140 (2015) 3406-3414. DOI:10.1039/C5AN00462D |
| [26] |
V. Lopez-Avila, J. Benedicto, H. Prest, S. Bauer, Spectrosc.-Int. J. 14 (1999) 40-41. |
| [27] |
N. Srinivasan, R.C. Johnson, N. Kasthurikrishnan, et al., Anal. Chim. Acta 350 (1997) 257-271. DOI:10.1016/S0003-2670(97)00212-2 |
| [28] |
N.G. Davey, E.T. Krogh, C.G. Gill, Trac. Trends Anal. Chem. 30 (2011) 1477-1485. DOI:10.1016/j.trac.2011.05.003 |
| [29] |
L. Gao, Q. Song, G.E. Patterson, et al., Anal. Chem. 78 (2006) 5994-6002. DOI:10.1021/ac061144k |
| [30] |
L. Gao, Q. Song, R.J. Noll, et al., J. Mass Spectrom. 42 (2007) 675-680. DOI:10.1002/jms.1201 |
| [31] |
Mars400, in: http://www.fpi-inc.com/product_info.php?80/14.
|
| [32] |
L. Liu, M. Wang, W. Wu, et al., CN101777482A, 2010.
|
| [33] |
K.M. Sloan, R.V. Mustacich, B.A. Eckenrode, Field Anal. Chem. Technol. 5 (2001) 288-301. DOI:10.1002/fact.10011 |
| [34] | |
| [35] |
D. Li, M. Sudakov, F.L. Brancia, et al., J. Mass Spectrom. 39 (2004) 471-484. DOI:10.1002/jms.637 |
| [36] |
L. Ding, M. Sudakov, S. Kumashiro, Int. J. Mass Spectrom. 221 (2002) 117-138. DOI:10.1016/S1387-3806(02)00921-1 |
| [37] |
L. Ding, S. Kumashiro, Rapid Commun. Mass Spectrom. 20 (2006) 3-8. DOI:10.1002/rcm.2253 |
| [38] |
MMS-1000, in: http://www.1stdetect.com/products/mms-1000/.
|
| [39] |
MX908, in: http://908devices.com/products/mx908/.
|
| [40] |
M908, in: http://908devices.com/products/m908/.
|
| [41] |
Portability, in: http://www.bayspec.com/spectroscopy/portable-massspectrometer/.
|
| [42] |
Minib, in: http://www.purspec.cn/products.html.
|
| [43] |
Y. Wei, C. Bian, Z. Ouyang, W. Xu, Rapid Commun. Mass Spectrom. 29 (2015) 701-706. DOI:10.1002/rcm.7140 |
| [44] |
L. Gao, A. Sugiarto, J.D. Harper, et al., Anal. Chem. 80 (2008) 7198-7205. DOI:10.1021/ac801275x |
| [45] |
T.C. Chen, Z. Ouyang, Anal. Chem. 85 (2013) 1767-1772. DOI:10.1021/ac303112d |
| [46] |
P.I. Hendricks, J.K. Dalgleish, J.T. Shelley, et al., Anal. Chem. 86 (2014) 2900-2908. DOI:10.1021/ac403765x |
| [47] |
L. Li, T.C. Chen, Y. Ren, et al., Anal. Chem. 86 (2014) 2909-2916. DOI:10.1021/ac403766c |
| [48] |
A.E. Kirby, N.M. Lafreniere, B. Seale, et al., Anal. Chem. 86 (2014) 6121-6129. DOI:10.1021/ac5012969 |
| [49] |
J.D. Harper, N.A. Charipar, C.C. Mulligan, et al., Anal. Chem. 80 (2008) 9097-9104. DOI:10.1021/ac801641a |
| [50] |
H. Wang, J. Liu, R.G. Cooks, Z. Ouyang, Angew. Chem. 49 (2010) 877. DOI:10.1002/anie.200906314 |
| [51] |
J.J. Liu, H. Wang, N.E. Manicke, et al., Anal. Chem. 82 (2010) 2463. DOI:10.1021/ac902854g |
| [52] |
M. He, Z. Xue, Y. Zhang, et al., Anal. Chem. 87 (2015) 2236-2241. DOI:10.1021/ac504868w |
| [53] |
M. Yang, T.Y. Kim, H.C. Hwang, et al., J. Am. Soc. Mass. Spectrom. 19 (2008) 1442-1448. DOI:10.1016/j.jasms.2008.05.011 |
| [54] |
Y. Zhai, X. Zhang, H. Xu, et al., Anal. Chem. 89 (2017) 4177-4183. DOI:10.1021/acs.analchem.7b00195 |
| [55] |
T. Jiang, H. Zhang, Y. Tang, et al., Anal. Chem. 89 (2017) 5578-5584. DOI:10.1021/acs.analchem.7b00719 |
 2018, Vol. 29
2018, Vol. 29