b Department of Chemistry, Modern College of Arts and Sciences, Shanxi Normal University, Linfen 041000, China
Since 1971, the Mizoroki-Heck reaction [1-3] between aryl halides and olefins is one of the most efficient methods for the C-C bond formation and it has been widely used in synthesis of pharmaceuticals [4], agrochemicals [5], and natural products [6]. In the past few years, the Mizoroki-Heck reactions were successfully achieved with different metal catalysts such as Pd [7-9], Rh [10-12], Cu [13, 14], Ni [15, 16] and so on. Initially, the Mizoroki-Heck cross-coupling reaction using palladium complexes with various phosphorous ligands [17-21] as catalyst was investigated. Bulky electron-rich phosphine-based and phosphorus-containing palladacycles exhibited good catalytic activity for the Mizoroki-Heck reaction [22, 23]. In 2010, Diebold and coworker reported palladium complexes based on polystyrenesupported phosphine ligands as reusable catalysts for the Mizoroki-Heck reaction [24]. In 2015, palladium complexes bearing meta-terarylphosphine ligands was synthesized and used as catalyst for the Mizoroki-Heck reaction of (hetero)aryl bromides and olefins by Tay's groups [25]. However, most phosphine-based palladium complexes have some drawbacks of expensive, poisonous, air-and moisture-sensitive which limited their application in many fields [26-28]. Therefore more attention was paid on palladium complexes with nitrogen-based ligands [29-31]. For example, N-heterocyclic carbene-based palladium complexes [32-35] and pincer palladium(Ⅱ) complexes [36-38] showed good catalytic performance for the Mizoroki-Heck reaction. Beyond that, dinuclear palladium complexes with rotaxane were employed in ring-closure Mizoroki-Heck reaction by Suzaki's groups in 2011 [39]. At the same year, palladium complexes with anilidooxazolinate [40] or N-[1-alkylpyridin-4(1H)-ylidene]amides ligands [41] were developed as efficient catalyst for the Mizoroki-Heck reaction. In 2012, Keske and co-worker documented the synthesis of 1, 2, 3-triazol-5-ylidene mesoionic carbinebased PEPPSI palladium complexes and their catalytic activity in Mizoroki-Heck reaction was investigated [42]. Although great progress had been made in this field, the design of palladium complexes containing novel ligands and their catalytic application in the Mizoroki-Heck reaction is therefore a crucial target.
In view of the application value of palladium(Ⅱ) complexes in organic synthesis, we try to develop new heterocycles as efficient ligands for preparation of palladium complexes. We found that the synthesis of 1H-1, 2, 4-diazaphospholes containing low-coordinated phosphorous and nitrogen atoms was early reported in 1984 [43]. However, the catalytic application of metal complexes with 1H-1, 2, 4-diazaphospholes is rarely studied in spite of its good coordinating properties to metal. Recently we have accidentally synthesized a series of phosphorus-coordinated palladium(Ⅱ) complexes (Fig. 1) derived from 3, 5-disubstituted-1H-1, 2, 4-diazaphospholes {H[3, 5-R2dp] (R = H, i-Pr, Ph, t-Bu)} as unique heteroatom-containing monophosphine ligands and explored their catalytic activity in Suzuki-Miyaura cross-coupling reactions [44]. The structure of the catalyst was confirmed by X-ray singlecrystal diffraction of palladium(Ⅱ) complexes (Ⅳ) [44]. In consideration of our continuing interest on catalytic application of these new palladium(Ⅱ) complexes, we herein report catalytic performance of novel phosphorus-coordinated palladium(Ⅱ) complexes for Mizoroki-Heck reaction of aryl halide with electron-deficient olefins.
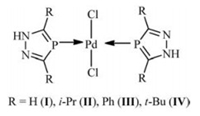
|
Download:
|
| Fig. 1. Palladium complexes Ⅰ-Ⅳ. | |
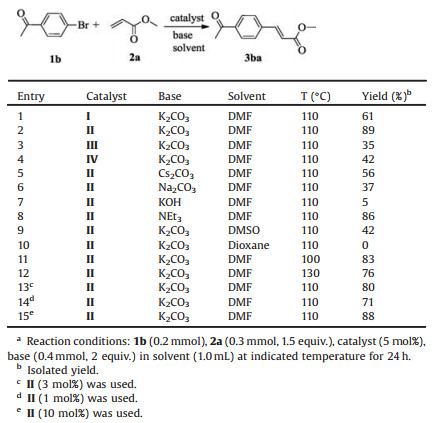
Firstly, the catalytic activities of palladium complexes Ⅰ–Ⅳ were examined by using 4-bromoacetophenone (1b) and methyl acrylate (2a) as the standard substrates (detail experimental procedure and characterization data of the products 3, please see Supporting information). When we used palladium complex (5 mol%) as catalyst, K2CO3 as base, DMF as solvent to perform the model reaction at 110 ℃ for 24 h, the desired product 3ba was obtained in moderate to good yield (Table 1, entries 1-4). These results demonstrated that four novel phosphorus-coordinated palladium complexes could catalyze Mizoroki-Heck reaction of 1b with 2a. Among them, complex Ⅱ was obviously the best catalyst for this reaction. Next, we studied the effect of different bases on the model reaction. When K2CO3 was switched to Cs2CO3, Na2CO3 or KOH, the products 3ba was isolated in lower yields (Table 1, entries 5-7). We also tried to use Et3N as organic base to perform the reaction and the 86% yield was afforded (Table 1, entry 8). So K2CO3 was the best choice. When the reaction of 1b with 2a performed in DMSO or dioxane, the yield of 3ba obviously decreased (Table 1, entries 9 and 10). This indicated that DMF should be the best solvent. Then the effect of temperature on the reaction was also investigated. Changing the reaction temperature to 100 ℃ or 130 ℃, the yield of 3ba had a slight decrease (Table 1, entries 11 and 12). Finally, the Mizoroki-Heck coupling reaction of 1b with 2a was carried out in the presence of 3 mol% and 1 mol% palladium catalyst, providing the product 3ba in 80% and 71% yields respectively (Table 1, entries 13 and 14). However, no improved yield was observed when 10 mol% palladium complex Ⅱ was used as catalyst (Table 1, entry 15). Thus the optimized conditions were established for exploring substrate scope of this Mizoroki-Heck reaction: palladium complex Ⅱ (5mol%) as catalyst, K2CO3 (2 equiv.) as base, and 1b/2a = 1:1.5 in DMF (1 mL) at 110 ℃ for 24 h.
|
|
Table 1 Optimization of reaction conditions. a |
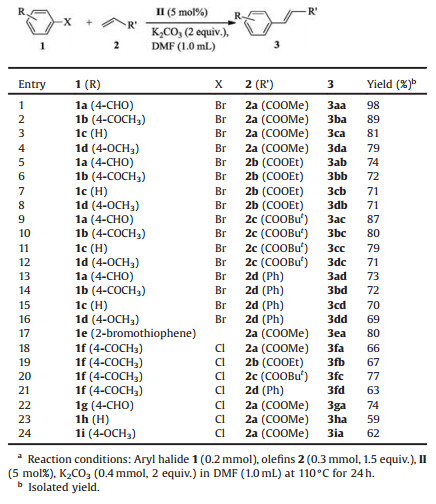
With optimal reaction conditions in hand, the reactions of different aryl bromide with methyl acrylate (2a) were firstly tested to evaluate the substrate scope of this Mizoroki-Heck reaction. As shown in Table 2, the substrates with electron-withdrawing groups such as 4-formyl (1a) and 4-acetyl (1b) were tolerated and the desired products were afforded in 97% and 89% yields (Table 2, entries 1 and 2). Comparatively, the substrates with no substituent (1c) or electron-donating groups such as 4-methoxy (1d) reacted with methyl acrylate (2a) to furnish the products in 81% and 79% yields, showing a lower reactivity (Table 2, entries 3 and 4). Then we explored the reactivity of other acrylate with respect to the ester group. When ethyl acrylate (2b) was used as substrate to react with different aryl bromide (1a-d), the corresponding products were isolated in 71%-74% yields (Table 2, entries 5-8). The results illustrate that the activity of ethyl acrylate (2b) was lower than methyl acrylate (2a). However, tert-butyl acrylate (2c) was further chosen as an olefin source and reacted with 1a-d, leading to the desired products in higher yields (Table 2, entries 9-12). Subsequently, styrene (2d) was selected as coupling partner and provided the corresponding products in 69%-73% yields (Table 2, entries 13-16). These experimental results demonstrated that the substituents on olefin substrates affected this MizorokiHeck reaction activity, which may be attributed to the electron density change of carbon-carbon double bonds and the steric hindrance of ester group on acrylate. Finally, the Mizoroki-Heck reaction of 2-bromothiophene (1e) with methyl acrylate (2a) was carried out and the product (3ea) was isolated in 80% yield, indicating that the heteroaryl bromide could be tolerated as good coupling partner (Table 2, entry 17).
|
|
Table 2 Substrate scope for Mizoroki-Heck reactions.a |
In addition, the activity of aryl chloride was also tested. To our delight, We found that the cross-coupling reaction of 4-chloroacetophenone (1e) with olefins derivatives such as 2a, 2b, 2c, 2d proceeded well, giving the desired product in moderate to good yields (Table 2, entries 18-21). Then several different aryl chlorides such as 4-chlorobenzaldehyde (1g), chlorobenzene (1h) and 4-chloroanisole (1i) reacted with 2a to furnish the desired products in good yields (Table 2, entries 22-24), which indicated that aryl chlorides were also suitable substrates in the reaction.
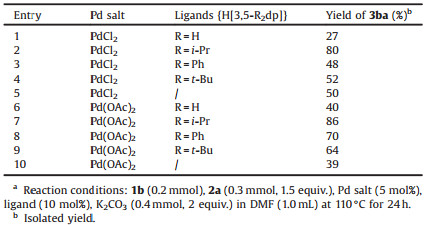
In order to illustrate the role of these monophosphine ligands in catalysis, control experiments using Pd salt as the catalyst under the standard conditions were performed. We used different monophosphine ligands in combination with PdCl2 or Pd(OAc)2 for the Mizoroki-Heck coupling reaction between 4-bromoacetophenone (1b) and methyl acrylate (2a). As shown in Table 3, the substituents on the ligands obviously affected the yields of the product 3ba (Table 3, entries 1-4 and 6-9). Among them, H[3, 5-i-Pr2dp] exhibited higher efficiency than other three ligands, which might be attributed to electronic effects and steric hindrance of substituents. Compared with H[3, 5-R2dp] (R = H, Ph, t-Bu), H[3, 5-i-Pr2dp] was more suitable for this coupling reaction. Furthermore, we found that the combination of H[3, 5-i-Pr2dp] with Pd(OAc)2 was better catalytic system than those with PdCl2 (Table 3, entry 7 vs. entry 2). When no ligands were used, the yields of 3ba significantly decreased (Table 3, entries 5 and 10). These results demonstrated that the ligands were crucial for this transformation.
|
|
Table 3 Control experiments for the role of monophosphine ligands.a |
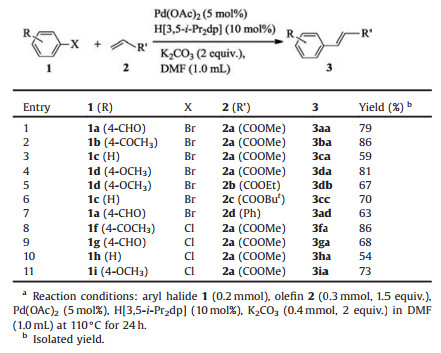
Thus we employed Pd(OAc)2 (5 mol%) and H[3, 5-i-Pr2dp] (10 mol%) as combined catalyst for the Mizoroki-Heck reactions. As summarized in Table 4, the reaction of several aryl bromide such as 1a, 1b, 1c and 1d with methyl acrylate (2a) performed well and offered the desired products in moderate to good yields (Table 4, entries 1-4). When the coupling reaction between 4-bromoanisole (1d) and ethyl acrylate (2b) was carried out under combined catalyst system, the product (3db) was isolated in 67% yield (Table 4, entry 5). The coupling of bromobenzene (1c) with tertbutyl acrylate (2c) also took place and led to the product (3cc) in 70% yield (Table 4, entry 6). We also observed that 4-bromobenzaldehyde (1a) reacted with styrene (2d) to provide the product (3ad) in 63% yield (Table 4, entry 7). These data showed that the different aryl bromide and olefin substrates could be tolerated under this catalytic system. More importantly, the reaction of aryl chlorides such as 1f, 1g, 1h, 1i and 1j with methyl acrylate (2a) also furnished the corresponding product in 54%-86% yield (Table 4, entries 8-11). Therefore we believe that this alternative catalytic system might be more attractive and convenient for application of Mizoroki-Heck reaction in organic synthesis.
|
|
Table 4 The Mizoroki-Heck reaction of several aryl halides and olefins catalyzed by Pd (OAc)2 and H[3, 5-i-Pr2dp].a |
In conclusion, we have developed Mizoroki-Heck crosscoupling reaction of aryl halides and electron-deficient olefins using novel phosphorus-coordinated palladium (Ⅱ) complexes derived from 3, 5-disubstituted-1H-1, 2, 4-diazaphospholes as efficient catalyst. The experiment results demonstrated that the reaction of aryl halide bearing different groups (-CHO, -COCH3, -OCH3, 2-thienyl) with electron-deficient olefin derivatives performed well and high yields(up to 98%) were obtained under optimal conditions. The role of monophosphine ligands in catalysis was manifested by control experiments. Furthermore, the Mizoroki-Heck reaction of different substrates were achieved under Pd (OAc)2 and H[3, 5-i-Pr2dp] combined systems, providing the direct and convenient methods for C-C bond formation in organic synthesis.
AcknowledgmentsFinancial support from Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2015157), Teaching Reform Project of Shanxi Normal University (No. SD2014JGKT-52) and Undergraduate Training Programs for Innovation and Entrepreneurship of Modern College of Arts and Sciences, Shanxi Normal University (No. WL2015CXCY-YJ-26) are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.08.004.
| [1] |
G.T. Crisp, Chem. Soc. Rev. 27 (1998) 427-436. DOI:10.1039/a827427z |
| [2] |
I.P. Beletskaya, A.V. Cheprakov, Chem. Rev. 100 (2000) 3009-3066. DOI:10.1021/cr9903048 |
| [3] |
J.L. Bras, J. Muzart, Chem. Rev. 111 (2011) 1170-1214. DOI:10.1021/cr100209d |
| [4] |
I. Shinkai, A.O. King, R.D. Larsen, Pure Appl. Chem. 66 (1994) 1551-1556. DOI:10.1351/pac199466071551 |
| [5] |
J.G.D. Vries, Can. J. Chem. 79 (2001) 1086-1092. DOI:10.1139/v01-033 |
| [6] |
L.E. Overman, D.J. Ricca, V.D. Tran, J. Am. Chem. Soc. 115 (1993) 2042-2044. DOI:10.1021/ja00058a064 |
| [7] |
N.J. Whitcombe, K.K. Hii, S.E. Gibson, Tetrahedron 57 (2001) 7449-7476. DOI:10.1016/S0040-4020(01)00665-2 |
| [8] |
A.D. Meijere, F.E. Meyer, Chem Angew., Int. Ed. Engl. 33 (1995) 2379-2411. DOI:10.1002/(ISSN)1521-3773 |
| [9] |
T. Mino, T. Koizumi, S. Suzuki, et al., Eur. J. Org. Chem. 2012 (2012) 678-680. |
| [10] |
K. Fagnou, M. Lautens, Chem. Rev. 103 (2003) 169-196. DOI:10.1021/cr020007u |
| [11] |
D.A. Colby, R.G. Bergman, J.A. Ellman, Chem. Rev. 110 (2010) 624-655. DOI:10.1021/cr900005n |
| [12] |
S. Tanaka, K. Itami, K. Sunahara, G. Tatsuta, A. Mori, Chem. Commun. 51 (2015) 1949-1952. DOI:10.1039/C4CC09306B |
| [13] |
J. Wang, C. Liu, J. Yuan, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 2256-2259. DOI:10.1002/anie.201208920 |
| [14] |
T. Nishikata, Y. Noda, R. Fujimoto, T. Sakashita, J. Am. Chem. Soc. 135 (2013) 16372-16375. DOI:10.1021/ja409661n |
| [15] |
E.A. Standley, T.F. Jamison, J. Am. Chem. Soc. 135 (2013) 1585-1592. DOI:10.1021/ja3116718 |
| [16] |
S.Z. Tasker, A.C. Gutierrez, T.F. Jamison, Angew. Chem. Int. Ed. 53 (2014) 1858-1861. DOI:10.1002/anie.201308391 |
| [17] |
V.P.W. Böhm, W.A. Herrmann, Chem-Eur. J. 6 (2000) 1017-1025. DOI:10.1002/(ISSN)1521-3765 |
| [18] |
J.P. Stambuli, S.R. Stauffer, K.H. Shaughnessy, J.F. Hartwig, J. Am. Chem. Soc. 123 (2001) 2677-2678. DOI:10.1021/ja0058435 |
| [19] |
A.F. Littke, G.C. Fu, J. Am. Chem. Soc. 123 (2001) 6989-7000. DOI:10.1021/ja010988c |
| [20] |
W.A. Herrmann, C. Brossmer, O. Felek, et al., Angew. Chem. Int. Ed. Engl. 34 (1995) 1844-1848. DOI:10.1002/(ISSN)1521-3773 |
| [21] |
M. Ohff, A. Ohff, M.E. Boom, D. Milstein, J. Am. Chem. Soc. 119 (1997) 11687-11688. DOI:10.1021/ja9729692 |
| [22] |
M. Portnoy, Y. Ben-David, D. Milstein, Organometallics 12 (1993) 4734-4735. DOI:10.1021/om00036a008 |
| [23] |
G.C. Fu, Acc. Chem. Res. 41 (2008) 1555-1564. DOI:10.1021/ar800148f |
| [24] |
C. Diebold, S. Schweizer, J. Becht, C.L. Drian, Org. Biomol. Chem. 8 (2010) 4834-4836. DOI:10.1039/c0ob00523a |
| [25] |
D.W. Tay, H. Jong, Y.H. Lim, et al., J. Org. Chem. 80 (2015) 4054-4063. DOI:10.1021/acs.joc.5b00386 |
| [26] |
X.Q. Zhang, Y.P. Qiu, B. Rao, M.M. Luo, Organometallics 28 (2009) 3093-3099. DOI:10.1021/om8011695 |
| [27] |
B.P. Morgan, G.A. Galdamez, R.J. Gilliard, R.C. Smith, Dalton Trans. 2009 (2009) 2020-2028. |
| [28] |
Y.B. Zhou, Z.X. Xi, W.Z. Chen, D.Q. Wang, Organometallics 27 (2008) 5911-5920. DOI:10.1021/om800711g |
| [29] |
X. Gai, R. Grigg, M.I. Ramzan, et al., Chem. Commun. 2000 (2000) 2053-2054. |
| [30] |
F.E. Hahn, M.C. Jahnke, Angew. Chem. Int. Ed. 47 (2008) 3122-3172. DOI:10.1002/(ISSN)1521-3773 |
| [31] |
E.A.B. Kantchev, C.J. O'Brien, M.G. Organ, Angew. Chem. Int. Ed. 46 (2007) 2768-2813. DOI:10.1002/(ISSN)1521-3773 |
| [32] |
A. Poulain, A. Neels, M. Albrecht, Eur. J. Inorg. Chem. 2009 (2009) 1871-1881. |
| [33] |
C. Allolio, T. Strassner, J. Org. Chem. 79 (2014) 12096-12105. DOI:10.1021/jo501897s |
| [34] |
D. Yuan, Q. Teng, H.V. Huynh, Organometallics 33 (2014) 1794-1800. DOI:10.1021/om500140g |
| [35] |
D. Guest, V.H.M. Silva, A.P.L. Batista, et al., Organometallics 34 (2015) 2463-2470. DOI:10.1021/om5012038 |
| [36] |
W.J. Sommer, K. Yu, J.S. Sears, et al., Organometallic 24 (2005) 4351-4361. DOI:10.1021/om048992v |
| [37] |
L. Yang, X. Zhang, P. Mao, et al., RSC Adv. 5 (2015) 25723-25729. DOI:10.1039/C5RA01706H |
| [38] |
S. Sobhani, Z. Zeraatkar, F. Zarifi, New J. Chem. 39 (2015) 7076-7085. DOI:10.1039/C5NJ00344J |
| [39] |
Y. Suzaki, K. Shimada, E. Chihara, et al., Org. Lett. 13 (2011) 3774-3777. DOI:10.1021/ol201357b |
| [40] |
K.F. Peng, C.T. Chen, Eur. J. Inorg. Chem. 2011 (2011) 5182-5195. DOI:10.1002/ejic.v2011.33 |
| [41] |
P.D.W. Boyd, L.J. Wright, M.N. Zafar, Inorg. Chem. 50 (2011) 10522-10524. DOI:10.1021/ic201527s |
| [42] |
E.C. Keske, O.V. Zenkina, R. Wang, C.M. Crudden, Organometallics 31 (2012) 6215-6221. |
| [43] |
A. Schmidpeter, A. Willhalm, Angew. Chem. Int. Ed. 23 (1984) 903-904. DOI:10.1002/(ISSN)1521-3773 |
| [44] |
X.F. Jia, F. Zhao, Inorg. Chim. Acta 461 (2017) 145-149. DOI:10.1016/j.ica.2017.02.020 |
 2018, Vol. 29
2018, Vol. 29