b Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c Institute of Functional Nano and Soft Materials, Soochow University, Suzhou 215123, China
Phosphorescent OLEDs employing heavy transition metal complexes as emitters have drawn considerable attention for their intrinsic ability to simultaneously harvest both 25% singlet exicitons and 75% triplet excitons to facilitate a theoretical internal quantum efficiency of 100% [1]. Up to date, the peak external quantum efficiencies of green, red and blue PHOLED can be up to 30% under a luminance lower than 100 cd/m2, illustrating their potential application in next-generation lighting and displaying [2, 3]. However, the high efficiency is not the only criterion to evaluate device performance, higher operating lifetime, lower voltage, and importantly, reduced efficiency roll-off at high brightness should also be fulfilled to minimize the power consumption and realize practical application [4].
The charge accumulation and exciton diffusion at the EML interface are two crucial factors leading to the fast decrease of efficiency. For PHOLED, the triplet excitons with long emission lifetime and diffusion lengths [5] were more inclined to collide with other excitons resulting in disadvantageous nonradiative transition and thus lower optical efficiency. With increasing the driving voltage, the current density and triplet density becomes higher leading to triplet-triplet annihilation (TTA) and tripletpolaron quenching (TPQ) [6], which consume the triplet excitons rapidly and give rise to efficiency decline in PHOLED. To tackle this problem, some feasible solutions from three aspects had been taken: 1) optimize the device architecture [7], such as reduce the device layers [8], broaden the recombination zone [9] and modify the emissive architecture [10]; 2) develope novel phosphorescence complex to balance charge carriers in the emission layer (EML) [11]; 3) explore novel host materials or design novel cohost system. For example, Kim et al. [12] achieved a 10% roll-off of current efficiency at a luminance of 20000 cd/m2 in green PHOLED with Ir (ppy)3 doped in a TCTA: TPBi mixed host. Ma et al. [13] designed an oligomer-like host material to allow the suppression of TTA and TPQ by separated chemical channels. Liu et al. [14] demonstrated a novel phosphorescent material with bipolar charge transporting ability to host efficient deep-red PHOLED. Recently, Cheng and Chu [15], Liao and Jiang [16], and our group [17] independently reported the utilization of TADF molecules as host material for PHOLED to enable full singlet and triplet energy transfer to emitter as well as decrease the device efficiency roll-off by virtue of efficient upconversion from triplet to singlet.
With our persistent enthusiasm on TADF materials, in this work, we designed and synthesized a novel host material exhibiting TADF behavior, DMBFTX, by introducing two fluorene groups into the thioxanthone (TX) core. The fluorene group with methyl substitution was selected for its relatively high triplet energy level of 2.89 eV, weak electron-donating property to form D-A-D triads and steric hindrance to prevent the host self-aggregation [18]. And the thioxanthone core with a relatively small singlet-triplet energy gap (ΔEST) lower than 0.3 eV is favorable for the RISC from lowest triplet state T1 to lowest singlet state S1 [17]. Thus, the DMBFTX molecule was anticipated to possess a smaller singlet-triplet energy gap and TADF property by connecting TX acceptor and fluorene donor through a certain twisting angle [19]. The synthesis and characterization of DMBFTX, including its photophysical and electrochemical properties, thermal stability property, transient photoluminescence property, and electroluminescence (EL) performance were well performed. The developed TADF host DMBETX displayed the best performance with a CIE colour purity of (0.68, 0.32), maximum quantum efficiencies of 12.9% and EQE roll-off as low as 38.8% at 10000 cd/m2.
DMBFTX was synthesized by Suzuki coupling reaction of brominated TX and fluorene boric acid as outlined in Scheme S1 in Supporting information. The HOMO and LUMO of DMBFTX were determined to be 5.78 and 2.93 eV from the cyclic voltammetrical analysis (Fig. S3 in Supporting information) and UV–vis absorption edge [20]. DMBFTX exhibited good thermal stability with a high decomposition temperature (Td: corresponding to 5 wt% mass loss) of 334.5 ℃ and a high glass transition temperature (Tg) of 133.9 ℃ (Fig. S4). Fig. 1a and Table 1 display the absorption and photoluminescence (PL) spectra in CH2Cl2 solution, neat film at 298 K and phosphorescence spectrum in frozen 2-MeTHF at 77 K. The absorption peak around 275 nm and 325 nm was assigned to the TX-centered n–π* transition and fluorene-centered π–π* transition, respectively. Meanwhile, the weak shoulder peak with small molar extinction coefficient at ~400 nm was attributed to the intermolecular charge transfer transition from fluorene to TX. A narrow FWHM (full width at half maximum) of 48 nm with maximum centered at 439 nm was observed in the PL spectra in CH2Cl2 solution, while the emission peak of neat film is red-shifted slightly to 456 nm implying the diminished or disappeared intermolecular interaction for aggregated molecules. The triplet energy (ET) of DMBFTX is estimated to be 2.46 eV from highestenergy phosphorescence peak, which is lower than that of sky-blue phosphor FIrpic (~2.65 eV) [20] (Table 1). Moreover, the ΔEST of 0.26 eV between S1 and T1 is evaluated from the peak wavelength of fluorescence and phosphorescence indicating its potential to convert excitons from T1 to S1 state via RISC process.

|
Download:
|
| Fig. 1. (a) Absorption, fluorescence spectra in solutions and neat film of DMBFTX at room temperature and phosphorescence spectrum in 2-methyl tetrahydrofuran at 77 K. (b) The overlap between absorption spectra of (piq)2Ir(acac) and PL spectrum of DMBFTX neat film. (c) Temperature dependent transient PL characteristics of mCP: 5±1 wt% DMBFTX film (100 nm) detected at 463 nm. | |
|
|
Table 1 Property summary of DMBFTX and mCP. |
To verify the TADF property of DMBFTX in solid states, we fabricated a doped film of mCP: 5±1 wt% DMBFTX to investigate its transient PL property under different temperatures. Conventional host matrix mCP was chosen for its high singlet energy of 3.57 eV and triplet energy of 2.90 eV to prevent exiton quenching and enable efficient energy transfer. The PL spectrum of the doped film was slightly red-shifted to 463 nm compared with that in CH2Cl2 solution and no emission observed from mCP reflecting the complete energy transfer from mCP matrix to DMBFTX. Intriguingly, it appeared a new peak centered at 524 nm by the side of the fluorescence emission of 463 nm when the temperature decreased below 150 K (Fig. S5a in Supporting information). The intensity of the new peak at 524 nm increased as the temperature decreased. We attributed the new band to the phosphorescence from its identical peak position and analogical spectra shape as the phosphorescence spectra detected from FL4600. As shown in Fig. 1c, the transient PL curve at 200 ~ 300 K can be fitted biexponentially to the value of time. For example, it can be fitted to a prompt component with a transient decay time of 0.42 ns and a delayed component of 23.06 μs at 300 K. On the other hand, an increase in the proportion of delayed component with temperatures up was clearly observed (τ200 < τ250 < τ300). We also noticed that the spectra of the delayed component collected from 10 μs to 200 μs after excitation agreed well with the prompt component as shown in Fig. S5b in Supporting information. Judging from the temperature effect and the perfect fluorescence spectra coincidence, we assigned the delayed component to thermally activated delayed fluorescence, analogous to other typical TADF materials [21-25]. Accordingly, the delayed and the prompt component are both from the excited S1 radiation, the only difference is that the former emission generated from the T1-S1 reverse ISC and then excited S1 radiation, and thus a comparatively longer lifetime.
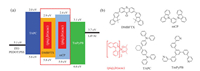
The PL quantum yield of DMBFTX neat film is evaluated to be 23.7% by the integrating sphere, not suitable to serve as TADF emitter. In this study, we focus on studying the performance of DMBFTX as host for red phosphorescent OLEDs. As shown in Fig. 1b, the PL emission of DMBFTX was well overlapped with UV– vis absorption of red phosphor (piq)2Ir(acac) implying efficient Förster energy transfer from DMBFTX to the red phosphor. Accordingly, we fabricated devices A and B by employing DMBFTX and mCP as the host material with a configuration of ITO/PEDOT: PSS/TAPC (15 nm)/DMBFTX: 5±1 wt% (piq)2Ir(acac) (device A) or mCP: 5±1 wt% (piq)2Ir(acac) (device B)/TmPyPb (55 nm)/LiF (0.9 nm)/Al (100 nm) as shown in Fig. 2, in which ITO and LiF/Al are the anode and cathode, TAPC (1, 1-bis[4-[N, N'-di(p-tolyl) amino]phenyl]cyclohexane) is the hole-transporting layer, TmPyPB (1, 3, 5-tri[(3-pyridyl)-phen-3-yl]benzene) is the electron-transporting and hole-blocking layer. And mCP was selected as the reference host for its suitable HOMO-LUMO energy, higher S1 and T1 energy, but larger singlet-triplet splitting.
|
Download:
|
| Fig. 2. (a) Energy level diagrams of device A and device B. (b) Related molecular structures. | |
Fig. 3 represents the current density–voltage-luminance (J-V-L) and external quantum efficiency-voltage-luminance (EQE-L) of the PHOLEDs. The turn-on voltage is 4.3 and 4.5 V for device A and B, respectively. They emitted red light with peaks centered at 625 and 632 nm with color coordinates of (0.68, 0.32). Although the electron injection barrier at the interface between the EML and ETL is different for the two devices, the efficient energy transfer is reflected by the nearly overlapped peak position (633 nm for device A and 623 nm for device B) and the approximately equal PLQY (photoluminescent quantum yields) for the dopant films (51.3% for DMBFTX: 5±1 wt% (piq)2Ir(acac) and 47.6% for mCP: 5±1 wt% (piq)2Ir(acac)). The highest luminances are 22674 cd/m2 at 16.5 V and 27425 cd/m2 at 13.9 V for device A and B, respectively. The device A afforded a maximum current efficiency of 9.3 cd/A, a maximum power efficiency of 6.0 lm/W, and a maximum external quantum efficiency of 12.9% without any light out-coupling enhancement. The maximum current efficiency, power efficiency and maximum external quantum efficiency of device B, was estimated to be 7.8 cd/A, 4.0 lm/W and 12.5%, respectively. By contrast, the titled host displays competitive or even preponderant performance compared with commercially available mCP in sensitizing the red (piq)2Ir(acac) phosphor. It is noteworthy that the device A showed lower efficiency roll-off under high current density and luminescence. For device A, the EQE can be up to 10.4% with low efficiency roll-off of 19.4% at the luminescence up to 1000 cd/m2 and 7.9% with an efficiency roll-off of 38.8% at the luminescence up to 10 000 cd/m2. However, for device B, the EQE is only 6.3% with an efficiency roll-off up to 49.6% at the luminescence up to 1000 cd/m2 and 3.6% with an efficiency rolloff of 71.2% at the luminescence up to 10 000 cd/m2. From the previous research, the efficiency roll-off is mainly resulted from either the TTA mode or the TPQ mechanism for PHOLED [6], which implies that the abundent triplet exciton is primarily responsible for the conventional efficiency decline. In our previous work [26], the cooperative effect of the reverse ISC from triplet to singlet on the host and rapid Förster energy transfer process from host to guest reduced the density of free triplet excitons on the host, and thereby diminishing the efficiency roll-off. To further illustrate the influence of the RISC from the T1 to S1 on the energy transfer from host to guest, the PL spectrum and PLQYof the two different dopant films [DMBFTX: 5±1 wt% (piq)2Ir(acac) and mCP: 5±1 wt% (piq)2Ir(acac)] in vacuum and exposure to air were investigated. Since the concentration of the dopant is as low as 5±1 wt% and the number of adjacent host and guest is few, the Dexter energy transfer could be neglected. As shown in Fig. S7 in Supporting information, the emission of the doped film is from the T1 of the phosphor via Förster energy transfer (FRET) from host. The PLQY of DMBFTX: 5±1 wt% (piq)2Ir(acac) is 51.3% in vacuum, and decreased to 34.8% and 23.1% after exposure in air for 30 and 60 min. While the reduction of PLQY on the mCP: 5±1% (piq)2Ir (acac) was comparatively small (47.6%, 38.5% and 31.4%). In the above mentioned mechanism, the process susceptible by the oxygen concentration was marked as red line in Fig. S7. The main differences between the two films are the RISC from T1 to S1 and subsequently FRET for DMBFTX host, but direct FRET for mCP host. The distinction of PLQY versus the air exposure time indirectly illustrated that RISC is closely related to the energy transfer between host and guest. In this study, the compound DMBFTX is a TADF host material with small ΔEST, which is composed of TX and fluorene moiety. Consequently, we ascribe the reduced efficiency roll-off of PHOLED to the evacuation of the triplet population on the DMBFTX host, resulting from the fast RISC from T1 to S1. In fact, the efficiency roll-off for DMBFTX based PHOLED (device A) is actually weakened when compared to mCP with no TADF and RISC, which indirectly reflecting the superiority of TADF host in reducing the efficiency decline for PHOLED. Further investigation of the direct proof related to the detailed energy transfer process of this TADF host is underway.

|
Download:
|
| Fig. 3. (a) Current density–voltage–luminance characteristics. (b) The EL spectra of device A and device B. (c) The EQE–luminance characteristics of device A and device B. | |
In conclusion, we have designed and synthesized a novel TADF host material, DMBFTX, for efficiency red PHOLED with low efficiency roll-off at high luminance. The performance further proves that our strategy of employing the TADF host material with TX moiety to reduce efficiency roll-off in PHOLED is validated and suggests the potential of applying small ΔEST host materials for high-performance PHOLEDs.
AcknowledgmentsThis study was supported by National Natural Science Foundation of China (No. 61605158), the Science and Technology Department of Shaanxi Province (No. 2016JQ2028), and the Education Department of Shaanxi Province (No. 16JK1790).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.07.025.
| [1] |
M.A. Baldo, M.E. Thompson, S.R. Forrest, Nature 403 (2000) 750-753. DOI:10.1038/35001541 |
| [2] |
K. Udagawa, H. Sasabe, C. Cai, et al., Adv. Mater. 26 (2014) 5062-5066. DOI:10.1002/adma.201401621 |
| [3] |
D.R. Lee, B.S. Kim, C.W. Lee, et al., ACS Appl. Mater. Inter. 7 (2015) 9625-9629. DOI:10.1021/acsami.5b01220 |
| [4] |
C. Murawski, K. Leo, M.C. Gather, Adv. Mater. 25 (2013) 6801-6827. DOI:10.1002/adma.v25.47 |
| [5] |
M.A. Baldo, C. Adachi, S.R. Forrest, Phys. Rev. B 62 (2000) 10967-10977. DOI:10.1103/PhysRevB.62.10967 |
| [6] |
N. Chopra, J. Lee, Y. Zheng, et al., ACS Appl. Mater. Inter. 1 (2009) 1169-1172. DOI:10.1021/am900228b |
| [7] |
R. Wang, L.H. Xu, Y.Q. Li, et al., Adv. Opt. Mater. 3 (2015) 203-210. DOI:10.1002/adom.201400391 |
| [8] |
X. Zhou, D.S. Qin, M. Pfeiffer, et al., Appl. Phys. Lett. 81 (2002) 4070-4072. DOI:10.1063/1.1522495 |
| [9] |
L. Duan, D.Q. Zhang, K.W. Wu, et al., Adv. Funct. Mater. 21 (2011) 3540-3545. DOI:10.1002/adfm.201100943 |
| [10] |
N.C. Erickson, R.J. Holmes, Adv. Funct. Mater. 24 (2014) 6074-6080. DOI:10.1002/adfm.201401009 |
| [11] |
J.S. Wang, X.J. Xu, Y. Tian, et al., Synthetic Met. 197 (2014) 90-98. DOI:10.1016/j.synthmet.2014.08.015 |
| [12] |
S.H. Kim, J. Jang, K.S. Yook, et al., Appl. Phys. Lett. 92 (2008) 023513. DOI:10.1063/1.2836270 |
| [13] |
C.M. Han, L.P. Zhu, J. Li, et al., Adv. Mater. 26 (2014) 7070-7077. DOI:10.1002/adma.201400710 |
| [14] |
Y.S. Feng, P. Li, X.M. Zhuang, et al., Chem. Commun. 51 (2015) 12544-12547. DOI:10.1039/C5CC04297F |
| [15] |
C.C. Lin, M.J. Huang, M.J. Chiu, et al., Chem. Mater. 29 (2017) 1527-1537. DOI:10.1021/acs.chemmater.6b03979 |
| [16] |
Y. Liu, F. Liang, Y. Yuan, et al., Chem. Commun. 52 (2016) 8149-8151. DOI:10.1039/C6CC02856J |
| [17] |
H. Wang, L.Q. Meng, X.X. Shen, et al., Adv. Mater. 27 (2015) 4041-4047. DOI:10.1002/adma.201501373 |
| [18] |
P.I. Shih, C.H. Chien, C.Y. Chuang, et al., J. Mater. Chem. 17 (2007) 1692-1698. DOI:10.1039/b616043c |
| [19] |
Q.S. Zhang, H. Kuwabara, W.J. Potscavage, et al., J. Am. Chem. Soc. 136 (2014) 18070-18081. DOI:10.1021/ja510144h |
| [20] |
J.J. Huang, M.K. Leung, T.L. Chiu, et al., Org. Lett. 16 (2014) 5398-5401. DOI:10.1021/ol502602t |
| [21] |
H. Uoyama, K. Goushi, K. Shizu, et al., Nature 492 (2012) 234-238. DOI:10.1038/nature11687 |
| [22] |
V. Jankus, C.J. Chiang, F. Dias, et al., Adv. Mater. 25 (2013) 1455-1459. DOI:10.1002/adma.v25.10 |
| [23] |
D.D. Zhang, L. Duan, D.Q. Zhang, et al., Org. Electron. 14 (2013) 260-266. DOI:10.1016/j.orgel.2012.11.003 |
| [24] |
Y. Tao, K. Yuan, T. Chen, et al., Adv. Mater. 26 (2014) 7931-7958. DOI:10.1002/adma.v26.47 |
| [25] |
K. Liu, Z. Chen, J. Qing, et al., Adv. Mater. 27 (2015) 7079-7085. DOI:10.1002/adma.201502897 |
| [26] |
H. Wang, L.S. Xie, Q. Peng, et al., Adv. Mater. 26 (2014) 5198-5204. DOI:10.1002/adma.201401393 |
 2018, Vol. 29
2018, Vol. 29 



