Cucurbit[n]uril, abbreviated as CBn, is the condensation product of glycoluril and formaldehyde in acid aqueous solution [1]. Due to a rigid hydrophobic cavity of low polarity accessible through two open polar portals rimmed with carbonyl groups [2, 3], CBn could be used as a macrocyclic polydentate ligand to coordinate with various metal ions, e.g., alkali and alkaline earth metals [4, 5], transition metals [6], lanthanides [7, 8] and uranyl [9, 10]. Besides, CBn can coordinate with metals through secondsphere hydrogen bonding interactions [11, 12]. Therefore, CBn could be used to isolate kinetically labile lanthanide(Ⅲ) complexes and to remove uranium [13, 14] from aqueous solution efficiently. Now, CBn-based coordination chemistry becomes an important area in CBn chemistry.
Thorium is a very important nuclear fuel element. With respect to the Th4+-CBn based complexes, there are only a few reports [12, 15]. Samsonenko et al. [15] got a single crystal [{Th(H2O)5Cl}2(C36H36N24O12)]Cl6·13H2O in hydrochloric acid. The coordination number of Th4+ is nine and one CB6 molecule serves as a hexadentate ligand coordinated with two thorium cations. Thuery [12] obtained a complex [Th(NO3)(H2O)8][(ReO4)(CB6)](ReO4)2·3H2O by the reaction between thorium(Ⅳ) nitrate and CB6 in the presence of perrhenic acid by hydrothermal method. In the complex, one Th4+ cation coordinates with eight monodentate water molecules and one bidentate nitrate ion. Then, one [Th(NO3)(H2O)8]3+ cation is located close to each CB6 portal, and the water ligands are hydrogen-bonded to carbonyl groups, i.e., CB6 acts as a second-sphere ligand. It is worthwhile that nitric acid is always used in the extraction of thorium from natural ores and spent nuclear fuel. Thus, the interaction between CB6 and Th4+ in nitric acid is of great importance. However, to the best of our knowledge, there is seldom report in the literature.
Herein, we report the synthesis and crystal structure of a new supramolecular adduct of thorium nitrate and macrocyclic cavitand CB6 in HNO3 aqueous solutions, which is helpful for understanding the extraction mechanism of Th4+ from nitric acid solution.
Cucurbit[6]uril (CB6) was synthesized according to the reference [16]. All other reagents were purchased from commercial sources and used without further purification. The organic element analysis was performed on an elemental analyzer, vario EL (Elementar Analyse systeme GmbH, Germany). FT-IR spectrum of the complex was recorded on a NICOLET iN10 MX spectrometer. The thermogravimetric analysis (TGA) was measured on a Q600 SDT thermoanalyzer under air atmosphere with the temperature ranging from room temperature to 700 ℃ at a heating rate of 10 ℃/min. Powder X-ray diffraction (PXRD) data was measured on a DMAX-2400 diffractometer Cu Kα radiation (λ = 1.5406 Å) and the simulated data was carried out by the crystal analytic software 'Mercury'. A suitable crystal was selected and was measured on a Bruker APEX-Ⅱ CCD diffractometer, where monochromated MoKα (λ = 0.71073 Å) radiation was used. The crystal was kept at 300 K during data collection. The structure was solved with the SIR2004 structure solution program using Direct Methods and refined with the olex2.
In a glass vessel, 11 mg CB6 and 5 mLwater were added. After increasing the temperature to 80 ℃, HNO3 was added with stirring until CB6 was dissolved. Th(NO3)4 (ten-folded of CB6) aqueous solution was added. The mixed solution was left in the room temperature. Over a period of 3 days, the X-ray quality crystal was obtained from the solution. Caution! Thorium is a radioactive element. Suitable precautions and protection should be taken, and all operations should follow the criteria while handling the thorium ion in the experiment. According to the result of X-ray diffraction, the components of the crystal is [Th(NO3)2(H2O)6](CB6) (NO3)2·2H2O (ThC36H52N28O32). There exist Q peaks resulting from solvent molecules in the cavity of the CB6, which is highly disorder with the shielding of CB6. It is difficult to ascertain the orientation of the water molecules in the cavity of CB6. After three water molecules were added into a crystal cell, i.e., [Th(NO3)2(H2O)6] (CB6)(NO3)2·5H2O, the result of elemental analysis was identical to the theoretical value. Elemental analysis: calcd. (%) for [Th (NO3)2(H2O)6](CB6)(NO3)2·5H2O: C, 25.81%; H, 3.49%; N, 23.41%; found (%): C, 25.55%; H, 3.09%; N, 23.78%.
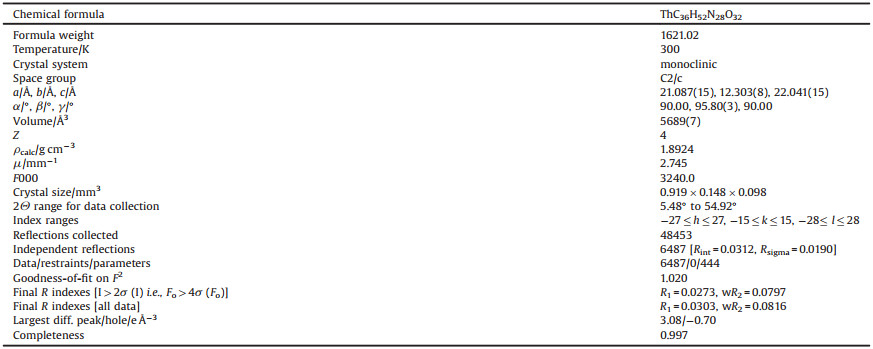
The structure of [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O is presented in Fig. 1A. Single crystal analysis revealed that the central Th4+ cation is coordinated with six monodentate water molecules and two bidentate nitrates. The two bidentate nitrates coordinate with Th4+ in a symmetrical way. While the six oxygen atoms of water, molecules are coordinated to Th4+ asymmetrically. Meanwhile, four water ligands adjoin CB6, while the other two water ligands coordinate with Th4+ on the opposite position, which connect with other NO3- ions via hydrogen bonding. There is no direct bonding between CB6 and Th4+. However, one [Th (NO3)2(H2O)6]2+cation is located close to one portal of CB6, and water ligands in a suitable position can bond to the carbonyl groups of CB6 through hydrogen bonding. From the selected bond lengths data, the average bond distances of Th-O3(H) and Th-O1(H) are 2.469(3) and 2.426(3) Å, respectively. The bond length of Th-O2(H) is 2.457(3) Å, while those of Th-O4(N14) and Th-O5(N14) are 2.642 (3) and 2.546(3) Å, respectively. There also exist two free water molecules and two NO3- ions. Meanwhile, every water molecule connected one free NO3- and carbonyl groups of CB6 through hydrogen bonding.
|
Download:
|
| Fig. 1. (A) Ellipsoid representation of the molecular structure of [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O. Selected bond lengths: Th[1-O12.426(3); Th1-O2 2.457(3); Th1-O3 2.469 (3); Th1-O4 2.642(3); Th1-O5 2.546(3) Å. (B) Packing of [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O molecules in the crystal structure, viewed along b direction. | |
The coordination interaction between Cl- and Th4+ is weak in aqueous solution, so CB6 acts as a hexadentate ligand coordinated to Th4+ ion in HCl solutions [15]. However, in the presence of NO3-, which could coordinate with Th4+ more strongly, the bonding cannot be formed between Th4+ and carbonyl in HReO4 [12] and HNO3 solutions, the thorium cation is prone to coordinate with nitrate ion and water molecules, while CB6 serves as a secondsphere ligand bonding to thorium through hydrogen bonding. The differences of the ligand affinity can lead to the formation of Th4+-CB6 based crystals with different coordination modes and structures. In addition, with respect to Th(NO3)4·4H2O crystal, the Th4+ ion is coordinated by four bidentate nitrate ions and four monodentate water molecules, and the coordination number of Th4+ is 12 [17], obviously different from the CB6-based crystals. In other words, the addition of CB6 could affect the coordination interaction between Th4+ and NO3- although CB6 only act as a second-sphere ligand. This will be helpful for synthesizing the CBn based crystals with different structures and components.
The crystal data and structure refinement of the Th4+-CB6 based complex are shown in Table 1. The Th4+-CB6 complex is a monoclinic system with space group C2/c. In the crystal lattice (Fig. 1B), the [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O molecules arranged to tubular structure along b direction. The crystal was also investigated by powder X-ray diffraction. In the XRD pattern of the crystal, there appear ten main peaks at the 2θ values of 8.08°, 14.43°, 14.80°, 15.00°, 15.43°, 18.21°, 22.21°, 24.36°, 26.64° and 28.78°, agree well with the simulated result (Fig. 2). This means that the purity of the crystal is high enough.
|
|
Table 1 Crystal data and structure refinement of [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O. |

|
Download:
|
| Fig. 2. Simulated and experimental PXRD result of single crystal [Th(NO3)2(H2O)6](CB6)(NO3)2·2H2O. | |
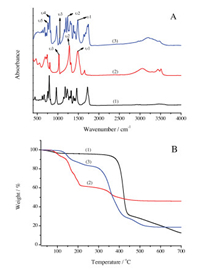
The infrared spectra of Th(NO3)4·4H2O and Th4+-CB6 complex contain some typical features of water and nitrate (Fig. 3A). The asymmetric stretching vibrations (υ1), the symmetric stretching vibrations (υ2) and the N—O stretching vibrations (υ3) of the NO3- in Th(NO3)4·4H2O appear at 1479, 1307 and 1047 cm-1, respectively, indicating the presence of bidentate nitrates [18, 19]. The Th4+-CB6 complex also contains similar vibrations, which means the existence of bidentate nitrates. Besides, vibrations at 806 and 759 cm-1 of the Th4+-CB6 complex can be assigned to the bending out-of-plane (υ4) and in-of-plane (υ5) of NO3-, respectively. Compared with the crystal of Th(NO3)4·4H2O, the IR spectrum of water in the Th4+-CB6 based single crystal gets more broader.The vibrations of the carbonyl groups in CB6 appear at 1747 cm-1 (weak peak) and 1723 cm-1 (Fig. S1 in Supporting information), while the vibrations of the carbonyl groups in Th4+-CB6 complex are slightly red shifted to 1742 and 1720 cm-1, respectively. Besides the vibration frequency of the C=O in Th4+-CB6 complex partly shifts down to a new peak at 1675 cm-1 compared with CB6 due to the splitting of the carbonyl groups of CB6. This also means that there exist two kinds of carbonyl groups with different environments in the Th4+-CB6 complex and the formation of hydrogen bonding.
|
Download:
|
| Fig. 3. IR spectra (A) and TGA curves (B) of CB6 (1), Th(NO3)4·4H2O (2) and [Th (NO3)2(H2O)6](CB6)(NO3)2·2H2O (3). | |
In order to assess the thermal stabilities and the phase behaviors of the precursors as well as the Th4+-CB6 complex, the decomposition temperature was determined using TGA (Fig. 3B). CB6 starts to decompose at 360 ℃. The weight percent of the Th4+-CB6 complex decrease by 9.86% at 167 ℃, which is attributed to the loss of the water molecules [20]. The complex decreased to 83.27% at 253 ℃ due to the decomposition of NO3- [20]. The last stage is mainly the decomposition of CB6 of the crystal. The possible final residue of the crystal is thorium dioxide [20].
In summary, the single crystal [Th(NO3)2(H2O)6](CB6) (NO3)2·2H2O was synthesized and characterized. The single crystal is a second-sphere complex of [Th(NO3)2(H2O)6]2+ with CB6 through hydrogen bongding. Meanwhile, the thorium ion is coordinated by six water molecules and two bidentate nitrates, and two NO3- act as the counterion anions. Besides, there are also two free water molecules in the crystal system. The infrared spectrum of the compound exhibited some typical features of water molecules and bidentate nitrates of the complex. The TGA curve of the crystal shows a three-step decomposition process. The weight loss of the crystal is mainly the loss of water molecules, and then the removal of nitrogen oxides and carbon dioxides at high temperature. Eventually, the crystal decomposed to thorium dioxide. The structural differences of thorium with CB6 in hydrochloric acid, perrhenic acid and nitric acid aqueous solutions can be helpful to understand the differences of coordination abilities of Cl-, ReO4- and NO3-. Besides, the formation of Th4+-CB6 based crystal can contribute to explore the recycle and separation of thorium in HNO3 system.
AcknowledgmentsThis work was supported by Science Challenge Project (No. TZ2016004) and the National Natural Science Foundation of China (No. 91226112). Ms Zhixian Wang is acknowledged for the elemental analyses. Dr. Nengdong Wang and Dr. Lei Mei are acknowledged for single-crystal X-ray analyses measurements. The authors also thank Prof. Wenxiong Zhang, Prof. Zheming Wang, and Miss Xueling Qiao for their help in the crystal structure analyses and discussion.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.09.030.
| [1] |
S.J. Barrow, S. Kasera, M.J. Rowland, J. del Barrio, O.A. Scherman, Chem. Rev. 115 (2015) 12320-12406. DOI:10.1021/acs.chemrev.5b00341 |
| [2] |
W.L. Mock, Top. Curr. Chem. 175 (1995) 1-24. DOI:10.1007/3-540-58800-0 |
| [3] |
J. Kim, I.S. Jung, S.Y. Kim, et al., J. Am. Chem. Soc. 122 (2000) 540-541. DOI:10.1021/ja993376p |
| [4] |
Y.M. Jeon, H. Kim, D. Whang, K. Kim, J. Am. Chem. Soc. 118 (1996) 9790-9791. DOI:10.1021/ja962071x |
| [5] |
O.A. Gerasko, A.V. Virovets, D.G. Samsonenko, et al., Russ. Chem. Bull. 52 (2003) 585-593. DOI:10.1023/A:1023942303190 |
| [6] |
J.X. Liu, C.H. Dong, L.S. Long, R.B. Huang, L.S. Zheng, Dalton Trans. 37 (2009) 7344-7346. |
| [7] |
W. Zhao, C. Wang, Y. Zhang, et al., New J. Chem. 40 (2016) 2763-2767. DOI:10.1039/C5NJ00964B |
| [8] |
Y. Zhao, L.L. Liang, K. Chen, et al., Crystengcomm. 15 (2013) 7987-7998. DOI:10.1039/c3ce40568k |
| [9] |
L. Mei, L. Wang, C.M. Liu, et al., Chem.-Eur. J. 21 (2015) 10226-10235. DOI:10.1002/chem.201500343 |
| [10] |
P. Thuery, Cryst. Growth Des. 8 (2008) 4132-4143. DOI:10.1021/cg800594m |
| [11] |
B.A. Blight, K.A. Van Noortwyk, J.A. Wisner, M.C. Jennings, Angew Chem. Int. Ed. 44 (2005) 1499-1504. DOI:10.1002/(ISSN)1521-3773 |
| [12] |
P. Thuery, Eur J. Inorg. Chem.(2015), 2037-2040. |
| [13] |
L. Shao, X.F. Wang, Y.M. Ren, et al., Chem. Eng. J. 286 (2016) 311-319. DOI:10.1016/j.cej.2015.10.062 |
| [14] |
L. Shao, J.R. Zhong, Y.M. Ren, H. Tang, X.F. Wang, J. Radioanal, Nucl. Chem. 311 (2017) 627-635. DOI:10.1007/s10967-016-5067-z |
| [15] |
D.G. Samsonenko, M.N. Sokolov, O.A. Gerasko, et al., Russ. Chem. Bull. 52 (2003) 2132-2139. DOI:10.1023/B:RUCB.0000011868.74173.8d |
| [16] |
W.A. Freeman, W.L. Mock, N.Y. Shih, J. Am. Chem. Soc. 103 (1981) 7367-7368. DOI:10.1021/ja00414a070 |
| [17] |
P. Charpin, G. Chevrier, M. Lance, et al., Acta Cryst. C 43 (1987) 1239-1241. DOI:10.1107/S010827018709231X |
| [18] |
W. Levason, E.H. Newman, M. Webster, Polyhedron 19 (2000) 2697-2705. DOI:10.1016/S0277-5387(00)00588-X |
| [19] |
C.D. Deng, Y.J. Qiao, Q.D. Chen, X.H. Shen, Chin. Chem. Lett. 28 (2017) 19-23. DOI:10.1016/j.cclet.2016.06.020 |
| [20] |
G.A.M. Hussein, H.M. Ismail, Colloid Surf. A-Physicochem. Eng. 99 (1995) 129-139. DOI:10.1016/0927-7757(95)03089-V |
 2018, Vol. 29
2018, Vol. 29