α-Diazo carbonyl compounds [1] are a potential source of amino alcohols and acids. α-Diazo carbonyl compounds are generally prepared by the azido transfer reaction of carbonyl compounds [2]. Recently the synthesis of α-diazo carbonyl compounds has been widely explored, such as α-diazo-β-hydroxy esters which are involved in many reactions in organic chemistry. The most straightforward synthesis of α-diazo carbonyl compounds involves the condensation of aldehydes and acyldiazomethanes. This is generally carried out by using strong bases, such as butylithium [3], lithium diisopropylamide (LDA) [4], sodium hydride (NaH) [5], potassium hydroxide (KOH) [6], t-BuOK [7], TBAOH [8], DBU [9], Mg/La mixed oxide [10], NAP-MgO [11], Bu2Mg [12]. As well, some acids, such as ZnEt2[13], ZnMe2[14], PhCOOH [15], Ti(OiPr)4[16], Zr(OtBu)4 [2d], Sc(OTf)3[17] are utilized into this coupling as catalysts. However, some of these methods involve the use of very strong bases, expensive catalysts, prolonged reaction time and vigorous reaction conditions, which afford the low yields of the products. Moreover, the use of strong bases may not be compatible with certain functional groups in the substrates. From the viewpoints above, the development of less expensive, environmentally benign, and easily handled promoters for aldol reaction of acyldiazomethane with aldehydes to form α-diazo-β-hydroxy esters is still highly desirable.
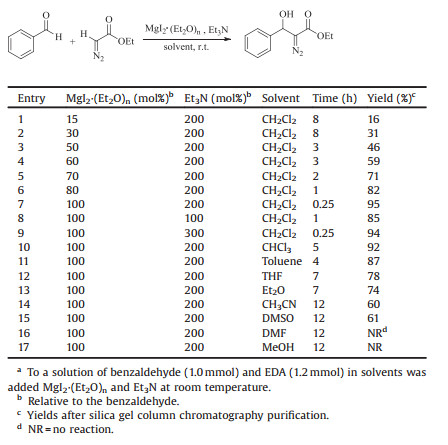
Magnesium (Ⅱ) species is widely used as Lewis acid promoter in various functional transformations and C-C bond-forming reactions due to the high electrophilicity of Mg2+ ion and its good coordination to Lewis basic oxygen and nitrogen atom [18]. Among them, magnesium halides are most frequently used. Herein, in the continuation of our research field [19], we will wish to report an efficient and facile method for the synthesis of α-diazo-β-hydroxy esters by the coupling of aldehydes with acyldiazomethane promoted by MgI2·(Et2O)n in the presence of DIPEA in CH2Cl2 at room temperature (Scheme 1).

|
Download:
|
| Scheme 1. MgI2·(Et2O)n-promoted aldol reaction of acyldiazomethane with various aldehydes. | |
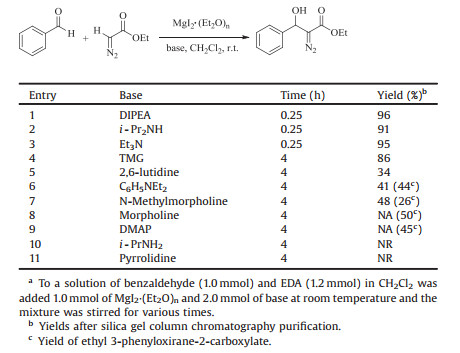
We initiated to optimize the reaction conditions by the condensation of benzaldehyde with ethyl diazoacetate (EDA) as a model reaction and the results are summarized in Table 1. No product formation was observed by using the sole MgI2·(Et2O)n or Et3N, respectively. The reaction stoichiometry was checked by varying the amounts of MgI2·(Et2O)n used. As shown in Table 1, the yields of ethyl 3-hydroxy-2-diazo-3-phenyl propionate are improved by increasing the amount of MgI2·(Et2O)n (Table 1, entries 2–7) and 1.0 equiv. of MgI2·(Et2O)n was sufficient. In addition, the amount of Et3N has also effects on the reaction conversion and yield. 2.0 equiv. of Et3N was enough although good yield was also afforded with 3.0 equiv. of Et3N (Table 1, entries 7–9). Furthermore, we explored the use of a range of solvents. It is evident that the excellent yield was given using CH2Cl2 as solvent and good yields are afforded in CHCl3 and toluene, respectively (Table 1, entries 10, 11). Moderate yields of the product were isolated in THF and Et2O (Table 1, entries 12, 13), while low yields were given in CH3CN and DMSO (Table 1, entries 14, 15). No reactions were occured in DMF and MeOH (Table 1, entries 16, 17). As well, a variety of Lewis acids, such as ZnCl2, ZnI2, Al(OiPr)3, TiCl4, FeCl3, Mg(ClO4)2, MgCl2 and MgBr2 were compared under parallel reaction conditions (100 mol% of Lewis acid and 200 mol% of Et3N) in the condensation of benzaldehyde with EDA in CH2Cl2. Only ZnCl2 as a promoter could give the desired product in moderate yield (71%). ZnI2, Al(OiPr)3, TiCl4, FeCl3, Mg(ClO4)2, MgCl2 and MgBr2 are practically inert to this aldol reaction.
|
|
Table 1 Screening the reaction parameters for the condensation of benzaldehyde with EDA.a |
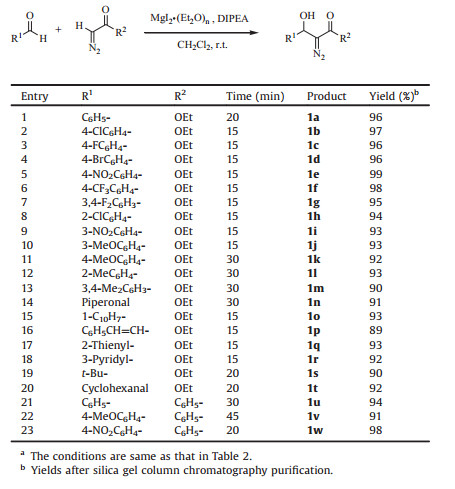
Of various the bases screened, DIPEA, Et3N and iPr2NH gave the excellent yields, respectively (Table 2, entries 1–3). Good yield was obtained in the presence of TMG (Table 2, entry 4). 2, 6-Lutidine gave very low yield. It is worthy to be noted that the desired aldol product was yielded in the presence of N, N-diethyl aniline and N-methylmorpholine, respectively, which was accompanied by formation of ethyl 3-phenyloxirane-2-carboxylate (Table 2, entries 6, 7). Furthermore, ethyl 3-phenyloxirane-2-carboxylate was exclusively afforded in the presence of morpholine and DMAP, respectively (Table 2, entries 8, 9). No reactions occurred using iPrNH2, and pyrrolidine (Table 2, entries 10, 11). So DIPEA was chosen to be the optimal base due to its more stability and convenient workup.
|
|
Table 2 Optimization of base in the condensation of benzaldehyde with EDA catalyzed by MgI2·(Et2O)n.a |
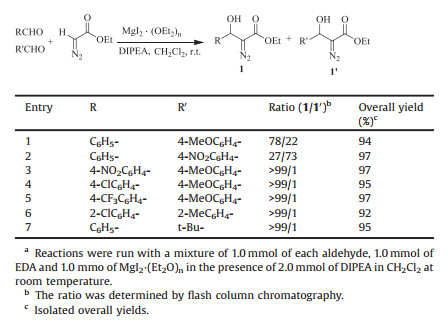
Encouraged by this optimizing reaction conditions, we chose a variety of structurally divergent aldehydes possessing a wide range of functional groups to understand the scope and generality of this MgI2·(Et2O)n-promoted aldol-type condensation to form β-hydroxy-α-diazo carbonyl compounds. The detailed procedures are deposited in Supporting information. The results are summarized in Table 3. A variety of substrates, including aromatic, heteroaromatic and aliphatic aldehydes, smoothly underwent condensation with EDA to afford the corresponding β-hydroxy-α-diazo carbonyl compounds in a short time (15–30 min). Nearly quantitative yields were obtained with aromatic aldehydes possessing electron-withdrawing groups at the paraposition (Table 3, entries 2–6) and excellent yields were afforded with ortho-and meta-substituted aromatic aldehydes (Table 3, entries 7–10). Aromatic aldehydes with electron-donating groups also afforded high yields of the desired products (Table 3, entries 11–14), in contrast to TABOH [6], DBU [8], Mg/La mixed oxide [9], and NAP-MgO [10] reported earlier which gave poor yields. 1-Naphthaldehyde, which contains a highly conjugated plane, seems to be effective, and gave the corresponding aldol adduct in 93% yield (Table 3, entry 15). Moreover, α, β-unsaturated aldehyde such as cinnamaldehyde also gave good yield of 1, 2-addition product in a short time (Table 3, entry 16). Heteroaromatic aldehydes such as pyridine-3-carboxaldehyde, thiophene-2-carboxaldehyde gave high yields (Table 3, entries 17, 18). As well, the aliphatic aldehydes with the bulkier substituents such as tert-butyl and cyclohexyl groups gave good yields (Table 3, entries 19, 20). Gratifyingly, the reactivity of benzoyldiazomethane (R = Ph) toward the aldehydes bearing electron-withdrawing groups and electron-donating groups is similar compared to that of EDA under the identical condition (Table 3, entries 21–23), which afforded the desired products in excellent yields. However, the condensation of aliphatic and aromatic ketones, such as cyclohexanone and acetophenone, with EDA were also found to be unsuccessful. All the characterization of products and copies of 1H NMR and 13C NMR spectra are put in Supporting information.
|
|
Table 3 MgI2·(Et2O)n-promoted aldol condensation of acyldiazomethane to aldehydes.a |
Next, we investigated the reaction of dicarboxaldehyde with EDA. In this reaction, 2.5 equivalents of EDA were required in order to have a complete conversion of dicarboxaldehyde. Aromatic dicarboxaldehyde such as 1, 4-phthalaldehyde was examined. The reaction exclusively produced bis-aldol adduct 1x in 91% yield (Eq. (1)).
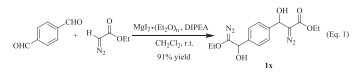
|
(1) |
The delicate chemoselectivity of aldol-type condensation was evaluated by crossover experiments of various aldehydes with EDA. MgI2·(Et2O)n shows high levels of aromatic aldehydes discrimination in the competitive reactions with EDA (Table 4). Firstly, MgI2·(Et2O)n can uniquely recognize the delicate difference in electronic effect involved in aromatic aldehydes. p-Anisaldehyde is less reactive than benzaldehyde and the aldol product of benzaldehyde was predominately afforded (Table 4, entry 1). Similarly, the aldol product of 4-nitrobenzaldehyde was mainly obtained over benzaldehyde. In crossover reactions of p-anisaldehyde with 4-nitrobenzaldehyde, 4-chlorobenzaldehyde or 4-trifluoromethylbenzaldehyde, respectively, the reaction exclusively gave the aldol product of 4-nitrobenzaldehyde, 4-chlorobenzaldehyde or 4-trifluoromethyl-benzaldehyde (Table 4, entries 3–5). More significantly, MgI2·(Et2O)n shows the remarkable preference for benzaldehyde over pivalaldehyde (Table 4, entry 7). These results suggest that the relative reactivity of aromatic aldehydes in the MgI2·(Et2O)n-promoted process is determined almost solely by eletrophilicity of carbonyl group of aromatic aldehydes.
|
|
Table 4 Crossover aldol reaction of aldehydes with EDA.a |
In summary, we have developed a facile and efficient method for the direct ester aldol condensation between acyldiazomethanes and aromatic, heteroaromatic, aliphatic, and α, β-unsaturated aldehydes promoted by MgI2·(Et2O)n at room temperature. The broad substrate scope, simple operation, high chemoselectivity, and mild condition make this a powerful method. This methodology may find widespread use in organic synthesis for the preparation of β-hydroxy-α-diazo carbonyl compounds. Further investigation is in progress in our laboratories to study stereoselectivity, and to attempt the preparation of chiral units which might function as important synthetic targets in drugs and natural products.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21372203 and 21272076) for the financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2017.04.019.
| [1] |
M. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis With Diazo Compounds: From Cyclopropanes To Ylides, WilevInter-Science, New York, 1998 p. 652.
|
| [2] |
(a) M. Regitz, G. Maas, Diazo Compounds. Properties and Synthesis, Academic Press, Orlando, FL, 1986 p. 596; (b) O. A. Kruglaya, N. S. Vyazankin, Russ. Chem. Rev. 49(1980) 679-705; (c) C. J. Moody, C. N. Mortt, Synthesis (1998) 1039-1042; (d) W. Yao, J. Wang, Org. Lett. 5(2003) 1527-1530. |
| [3] |
(a) U. SchÖllkopf, H. Frasnelli, D. Hoppe, Angew. Chem. Int. Ed. 9(1970) 301-302; (b) U. SchÖllkopf, B. Banhidai, H. Frasnelli, R. Meyer, H. Beckhaus, Liebigs Ann. Chem. 11(1975) 1767-1783. |
| [4] |
(a) R. Pellicciari, B. Natalini, J. Chem. Soc. Perkin Trans. Ⅰ 16(1977) 1882-1884; (b) R. Pellicciari, B. Natalini, B. M. Sadeghpour, et al., J. Am. Chem. Soc. 118(1996) 1-12; (c) C. J. Moody, R. J. Taylor, Tetrahedron Lett. 28(1987) 5351-5352. |
| [5] |
N. Jiang, Z. Qu, J. Wang, Org. Lett. 3(2001) 2989-2992. DOI:10.1021/ol016324p |
| [6] |
(a) E. Wenkert, C. A. McPherson, J. Am. Chem. Soc. 94(1972) 8084-8090; (b) T. L. Burkoth, Tetrahedron Lett. 10(1969) 5042-5049; (c) N. F. Woolsey, M. H. Khalil, J. Org. Chem. 37(1972) 2405-2408. |
| [7] |
B. Sreedhar, V. Balasubrahmanyam, C. Sridhar, Catal. Commun. 6(2005) 517-519. DOI:10.1016/j.catcom.2005.04.019 |
| [8] |
R. Varala, R. Enugala, S. Nuvula, S.R. Adapa, Tetrahedron Lett. 47(2006) 877-880. DOI:10.1016/j.tetlet.2005.12.005 |
| [9] |
N. Jiang, J. Wang, Tetrahedron Lett. 43(2002) 1285-1287. DOI:10.1016/S0040-4039(01)02375-9 |
| [10] |
M.L. Kantham, V. Balasubrahmanyam, K.B. Kumar, G.F. Venkanna, F. Figueras, Adv. Synth. Catal. 349(2007) 1887-1890. DOI:10.1002/(ISSN)1615-4169 |
| [11] |
M.L. Kantham, L. Chakrapani, T. Ramani, Tetrahedron Lett. 48(2007) 6121-6123. DOI:10.1016/j.tetlet.2007.06.141 |
| [12] |
(a) B. M. Trost, S. Malhotra, B. Fried, J. Am. Chem. Soc. 131(2009) 1674-1675; (b) B. M. Trost, S. Malhotra, P. Koschker, P. Ellerbrock, J. Am. Chem. Soc. 134(2012) 2075-2084. |
| [13] |
H. Cui, L. Wang, L. Yang, et al., Tetrahedron 67(2011) 8470-8476. DOI:10.1016/j.tet.2011.09.009 |
| [14] |
F. Benfatti, S. Yilmaz, P.G. Cozzia, Adv. Synth. Catal. 351(2009) 1763-1767. DOI:10.1002/adsc.200900435 |
| [15] |
P.R. Krishna, Y.L. Prapurna, M. Alivelu, Eur. J. Org. Chem.(2011), 5089-5095. |
| [16] |
W. Wang, K. Shen, X.L. Hu, et al., Synlett(2009), 1655-1658. |
| [17] |
F. Wang, X. Liu, Y. Zhang, L. Lin, X. Feng, Chem. Commun.(2009), 7297-7299. |
| [18] |
X.X. Zhang, W.D. Li, Chin. J. Org. Chem. 23(2003) 1185-1197. |
| [19] |
(a) X. X. Zhang, J. C. Shi, Tetrahedron 67(2011) 898-903; (b) X. X. Zhang, G. D. Weng, Y. D. Zhang, P. C. Li, Tetrahedron 71(2015) 2595-2602. |
 2018, Vol. 29
2018, Vol. 29